User login
Acellular Dermal Matrix in Rotator Cuff Surgery
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
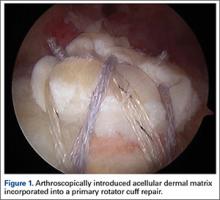
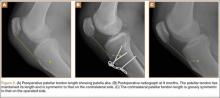
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
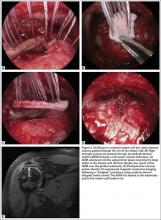
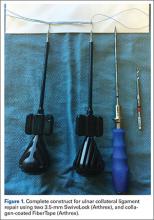
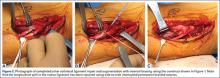
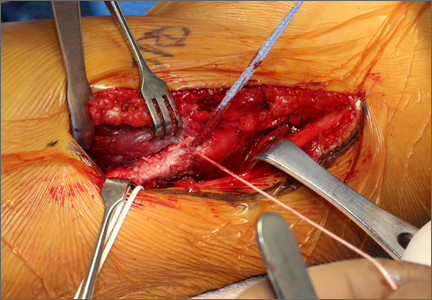
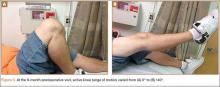
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
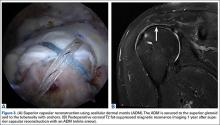
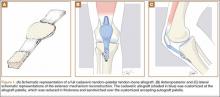
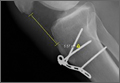
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
Platelet-rich plasma (PRP) is a refined product of autologous blood with a platelet concentration greater than that of whole blood. It is prepared via plasmapheresis utilizing a 2-stage centrifugation process and more than 40 commercially available systems are marketed to concentrate whole blood to PRP.1 It is rich in biologic factors (growth factors, cytokines, proteins, cellular components) essential to the body’s response to injury. For this reason, it was first used in oromaxillofacial surgery in the 1950s, but its effects on the musculoskeletal system have yet to be clearly elucidated.2 However, this lack of clarity has not deterred its widespread use among orthopedic surgeons. In this review, we aim to delineate the current understanding of PRP and its proven effectiveness in the treatment of rotator cuff tears, knee osteoarthritis, ulnar collateral ligament (UCL) tears, lateral epicondylitis, hamstring injuries, and Achilles tendinopathy.
Rotator Cuff Tears
Rotator cuff tears are one of the most common etiologies for shoulder pain and disability. The incidence continues to increase with the active aging population.3 Rotator cuff tears treated with arthroscopic repair have exhibited satisfactory pain relief and functional outcomes.4-7 Despite advances in fixation techniques, the quality and speed of tendon-to-bone healing remains unpredictable, with repaired tendons exhibiting inferior mechanical properties that are susceptible to re-tear.8-10
Numerous studies have investigated PRP application during arthroscopic rotator cuff repair (RCR) in an attempt to enhance and accelerate the repair process.11-15 However, wide variability exists among protocols of how and when PRP is utilized to augment the repair. Warth and colleagues16 performed a meta-analysis of 11 Level I/II studies evaluating RCR with PRP augmentation. With regards to clinical outcome scores, they found no significant difference in pre- and postoperative American Shoulder and Elbow Surgeons (ASES), Constant, Disability of the Arm, Shoulder and Hand (DASH), or visual analog scale (VAS) pain scores between those patients with or without PRP augmentation. However, they did note a significant increase in Constant scores when PRP was delivered to the tendon-bone interface rather than over the surface of the repair site. There was no significant difference in structural outcomes (evaluated by magnetic resonance imaging [MRI] re-tear rates) between those RCRs with and without PRP augmentation, except in those tears >3 cm in anterior-posterior length using double-row technique, with the PRP group exhibiting a significantly decreased re-tear rate (25.9% vs 57.1%).16 Zhao and colleagues17 reported similar results in a meta-analysis of 8 randomized controlled trials, exhibiting no significant differences in clinical outcome scores or re-tear rates after RCR with and without PRP augmentation. Overall, most studies have failed to demonstrate a significant benefit with regards to re-tear rates or shoulder-specific outcomes with the addition of PRP during arthroscopic RCR.
Knee Osteoarthritis
Osteoarthritis is the most common musculoskeletal disorder, with an estimated prevalence of 10% of the world’s population age 60 years and older.18 The knee is commonly symptomatic, resulting in pain, disability, and significant healthcare costs. Novel biologic, nonoperative therapies, including intra-articular viscosupplementation and PRP injections, have been proposed to treat the early stages of osteoarthritis to provide symptomatic relief and delay surgical intervention.
A multitude of studies have been performed investigating the effects of PRP on knee osteoarthritis, revealing mixed results.19-22 Campbell and colleagues23 published a 2015 systematic review of 3 overlapping meta-analyses comparing the outcomes of intra-articular injection of PRP vs control (hyaluronic acid [HA] or placebo) in 3278 knees. They reported a significant improvement in patient outcome scores for the PRP group when compared to control from 2 to 12 months after injection, but due to significant differences within the included studies, the ideal number of injections or time intervals between injections remains unclear. Meheux and colleagues24 reported a 2016 systematic review including 6 studies (817 knees) comparing PRP and HA injections. They demonstrated significantly better improvements in Western Ontario and McMaster Universities Arthritis Index (WOMAC) outcome scores with PRP vs HA injections at 3 and 12 months postinjection. Similarly, Smith25 conducted a Food and Drug Administration-sanctioned, randomized, double-blind, placebo-controlled clinical trial investigating the effects of intra-articular leukocyte-poor autologous conditioned plasma (ACP) in 30 patients. He reported an improvement in the ACP treatment group WOMAC scores by 78% compared to 7% improvement in the placebo group after 12 months. Despite the heterogeneity amongst studies, the majority of published data suggests better symptomatic relief in patients with early knee degenerative changes, and use of PRP may be considered in this population.
Ulnar Collateral Ligament Injuries
The anterior band of the UCL of the elbow provides stability to valgus stress. Overhead, high-velocity throwing athletes may cause repetitive injury to the UCL, resulting in partial or complete tears of the ligament. This may result in medial elbow pain, as well as decreased throwing velocity and accuracy. Athletes with complete UCL tears have few nonoperative treatment options and generally, operative treatment with UCL reconstruction is recommended for those athletes desiring to return to sport. However, it remains unclear how to definitively treat athletes with partial UCL tears. Recently, there has been an interest in treating these injuries with PRP in conjunction with physical therapy to facilitate a more predictable outcome.
Podesta and colleagues26 published a case series of 34 athletes with MRI-diagnosed partial UCL tears who underwent ultrasound-guided UCL injections and physical therapy. At an average follow-up of 70 weeks, they reported an average return to play (RTP) of 12 weeks, with significant improvements in Kerlan-Jobe Orthopaedic Clinic (KJOC) and DASH outcome scores, and decreased dynamic ulnohumeral joint widening to valgus stress on ultrasound. Most athletes (30/34) returned to their previous level of play, and 1 patient underwent subsequent UCL reconstruction. This study demonstrates that PRP may be used in conjunction with physical therapy and an interval throwing program for the treatment of partial UCL tears, but without a comparison control group, more studies are necessary to delineate the role of PRP in this population.
Lateral Elbow Epicondylitis
Lateral elbow epicondylitis, also known as “tennis elbow,” is thought to be caused by repetitive wrist extension and is more likely to present in patients with various comorbidities such as rotator cuff pathology or a history of smoking.27-29 The condition typically presents as radiating pain centered about the lateral epicondyle. Annual incidence ranges from 0.34% to 3%, with the most recent large-scale, population-based study estimating that nearly 1 million individuals in the United States develop lateral elbow epicondylitis each year.30 For the majority of patients, symptoms resolve after 6 to 12 months of various nonoperative or minimally invasive treatments.31-33 Those who develop chronic symptoms (>12 months) may benefit from surgical intervention.34 The use of PRP has become a contentious topic of debate in treating lateral epicondylitis. Its use and efficacy have been empirically examined and compared among more traditional treatments.35-37
In a small case-series of 6 patients, contrast-enhanced ultrasound imaging was utilized to demonstrate that PRP injection therapy may induce vascularization of the myotendinous junction of the common extensor tendon up to 6 months following injection.38 These physiologic changes may precede observable clinical improvements. Brklijac and colleagues39 prospectively followed 34 patients who had refractory symptoms despite conservative treatment and elected to undergo injection with PRP. At a mean follow-up of 26 weeks, 88.2% of the patients demonstrated improvements on their Oxford Elbow Score (OES). While potentially promising, case series lack large sample sizes, longitudinal analysis, and adequate control groups for comparative analyses of treatments, thereby increasing the likelihood of unintended selection bias.
Randomized controlled trials have demonstrated no difference between PRP and corticosteroid (CS) injection treatments in the short term for symptomatic lateral elbow epicondylitis. At 15 days, 1 month, and 6 months postinjection, no significant difference was found between PRP and CS injections in dynamometer strength measurements nor patient outcome scores (VAS, DASH, OES, and Mayo Clinic Performance Index for Elbow [MMCPIE]).40,41 In fact, multiple randomized controlled trials have demonstrated PRP to be less effective at 1 and 3 months compared to CS injections, as assessed by the Patient Rated Tennis-Elbow Evaluation (PRTEE) questionnaire, VAS, MMCPIE, and Nirschl scores.42,43 One mid-term, multi-center randomized controlled trial published by Mishra and colleagues44 compared PRP injections to an active control group, demonstrating a significant improvement in VAS pain scores at 24 weeks, but no difference in the PRTEE outcome. The available evidence indicates PRP injection therapy remains limited in utility for treatment of lateral epicondylitis, particularly in the short term when compared to CS injections. In the midterm to long term, PRP therapy may provide some benefit, but ultimately, well-designed prospective randomized controlled trials are needed to delineate the effects of PRP versus the natural course of tendon healing and symptom resolution.
Hamstring Injuries
Acute hamstring injuries are common across all levels and types of sport, particularly those in which sprinting or running is involved. While there is no consensus within the literature on how RTP after hamstring injury should be managed or defined, most injuries seem to resolve around 3 to 6 weeks.45 The proximal myotendinous junction of the long head of the biceps femoris and semitendinosus are commonly associated with significant pain and edema after acute hamstring injury.46 The amount of edema resulting from grade 1 and 2 hamstring injuries has been found to correlate (minimally) with time to RTP in elite athletes.47 PRP injection near the proximal myotendinous hamstring origin has been theorized to help speed the recovery process after acute hamstring injury. To date, the literature demonstrates mixed and limited benefit of PRP injection therapy for acute hamstring injury.
Few studies have shown improvements of PRP therapy over typical nonoperative management (rest, physical therapy, nonsteroidal anti-inflammatory drugs) in acute hamstring injury, but the results must be interpreted carefully.48,49 Wetzel and colleagues48 retrospectively reviewed 17 patients with acute hamstring injury, 12 of whom failed typical management and received PRP injection at the hamstring origin. This group demonstrated significant improvements in their VAS and Nirschl scores at follow-up, whereas the 5 patients who did not receive the injection did not. However, this study exhibited significant limitations inherent to a retrospective review with a small sample size. Hamid and colleagues49 conducted a randomized controlled trial of 24 athletes with diagnosed grade 2a acute hamstring injuries, comparing autologous PRP therapy combined with a rehabilitation program versus rehabilitation program alone. RTP, changes in pain severity (Brief Pain Injury-Short Form [BPI-SF] questions 2-6), and pain interference (BPI-SF questions 9A-9G) scores over time were examined. Athletes in the PRP group exhibited no difference in outcomes scores, but returned to play sooner than controls (26.7 vs 42.5 days).
Mejia and Bradley50 have reported their experience in treating 12 National Football League (NFL) players with acute MRI grade 1 or 2 hamstring injuries with a series of PRP injections at the site of injury. They found a 1-game difference in earlier RTP when compared to the predicted RTP based on MRI grading. Similarly, Hamid and colleagues49 performed a randomized control trial published in 2014, reporting an earlier RTP (26.7 vs 42.5 days) when comparing single PRP injection vs rehabilitation alone in 28 patients diagnosed with acute ultrasound grade 2 hamstring injuries. On the contrary, a small case-control study of NFL players and a retrospective cohort study of athletes with severe hamstring injuries demonstrated no difference in RTP when PRP injected patients were compared with controls.51,52 Larger randomized controlled trials have demonstrated comparable results, including a study of 90 professional athletes in whom a single PRP injection did not decrease RTP or lessen the risk of re-injury at 2 and 6 months.53 In another large multicenter randomized controlled trial examining 80 competitive and recreational athletes, PRP did not accelerate RTP, lessen the risk of 2-month or 1-year re-injury rate, or improve secondary measures of MRI parameters, subjective patient satisfaction, or the hamstring outcome score.54 Although further study is warranted, available evidence suggests limited utility of PRP injection in the treatment of acute hamstring injuries.
Achilles Tendinopathy
Noninsertional Achilles tendinopathy is a common source of pain for both recreational and competitive athletes. Typically thought of as an overuse syndrome, Achilles tendinopathy may result in significant pain and swelling, often at the site of its tenuous blood supply, approximately 2 to 7 cm proximal to its insertion.55 Conservative management frequently begins with rest, activity/shoe modification, physical therapy, and eccentric loading exercises.56 For those whom conservative management has failed to reduce symptoms after 6 months, more invasive treatment options may be considered. Peritendinous PRP injection has become an alternative approach in treating Achilles tendinopathy refractory to conservative treatment.
In the few randomized controlled trials published, the data demonstrates no significant improvements in clinical outcomes from PRP injection for Achilles tendinopathy. Kearney and colleagues57 conducted a pilot study of 20 patients randomized into PRP injection or eccentric loading program for mid-substance Achilles tendinopathy, in which Victorian Institute of Sports Assessment (VISA-A), EuroQol 5 dimensions questionnaire (EQ-5D), and complications associated with the injection were recorded at 6 weeks, 3 months, and 6 months. Although this was a pilot study with a small sample size, no significant difference was found between groups across these time periods. Similarly, de Vos and colleagues58,59 conducted a double-blind randomized controlled trial of 54 patients with chronic mid-substance Achilles tendinopathy and randomized them into eccentric exercise therapy with either a PRP injection or a saline injected placebo groups. VISA-A scores were recorded and imaging parameters assessing tendon structure by ultrasonographic tissue characterization and color Doppler ultrasonography were taken with follow-up at 6, 12, and 24 weeks. VISA-A scores improved significantly in both groups after 24 weeks, but the difference was not statistically significant between groups. In addition, tendon structure and neovascularization (exhibited by color Doppler ultrasonography) improved in both groups, with no significant difference between groups. The current literature does not support the use of PRP in treatment of Achilles tendinopathy, as it has failed to reveal additional benefits over conventional treatment alone. Future prospective, well-designed randomized controlled trials with large sample sizes will need to be conducted to ultimately conclude whether or not PRP deserves a role in the treatment of Achilles tendinopathy.
Summary
In theory, the use of PRP within orthopedic surgery makes a great deal of sense to accelerate and augment the healing process of the aforementioned musculoskeletal injuries. However, the vast majority of published literature is Level III and IV evidence. Future research may provide the missing critical information of optimal growth factor, platelet, and leukocyte concentrations necessary for the desired effect, as well as the appropriate delivery method and timing of PRP application in different target tissues. Evidence-based guidelines to direct the use of PRP will benefit from more homogenous, repeatable, and randomized controlled trials.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
1. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
2. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
3. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
4. Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy. 2001;17(9):905-912.
5. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: A long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234-238.
6. Huang R, Wang S, Wang Y, Qin X, Sun Y. Systematic review of all-arthroscopic versus mini-open repair of rotator cuff tears: a meta-analysis. Sci Rep. 2016;6:22857.
7. Watson EM, Sonnabend DH. Outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):201-211.
8. Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303-329.
9. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219-224.
10. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
11. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
14. Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. J Bone Joint Surg Am. 2012;94(15):1345-1352.
15. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
16. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
17. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125-135.
18. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376-387.
19. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
20. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-1078.
23. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-2221.
24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 2016;32(3):495-505.
25. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-891.
26. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
27. Herquelot E, Gueguen A, Roquelaure Y, et al. Work-related risk factors for incidence of lateral epicondylitis in a large working population. Scand J Work Environ Health. 2013;39(6):578-588.
28. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159-164.
29. Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979;7(4):234-238.
30. Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071.
31. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
32. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384-393.
33. Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9(4):419-446.
34. Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc. 2014;22(3):e1-e6.
35. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):952-956.
36. Ahmad Z, Brooks R, Kang SN, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy. 2013;29(11):1851-1862.
37. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101-112.
38. Chaudhury S, de La Lama M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
39. Brkljac M, Kumar S, Kalloo D, Hirehal K. The effect of platelet-rich plasma injection on lateral epicondylitis following failed conservative management. J Orthop. 2015;12(Suppl 2):S166-S170.
40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05-RC07.
41. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
42. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
43. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6-10.
44. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463-471.
45. van der Horst N, van de Hoef S, Reurink G, Huisstede B, Backx F. Return to play after hamstring injuries: a qualitative systematic review of definitions and criteria. Sports Med. 2016;46(6):899-912.
46. Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317-322.
47. Ekstrand J, Lee JC, Healy JC. MRI findings and return to play in football: a prospective analysis of 255 hamstring injuries in the UEFA Elite Club Injury Study. Br J Sports Med. 2016;50(12):738-743.
48. Wetzel RJ, Patel RM, Terry MA. Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics. 2013;36(1):e64-e70.
49. Hamid A, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418.
50. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Operative Techniques in Sports Medicine. 2011;19(3):149-153.
51. Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284-288.
52. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: Clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
53. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943-950.
54. Reurink G, Goudswaard GJ, Moen MH, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206-1212.
55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135.
56. Alfredson H. Clinical commentary of the evolution of the treatment for chronic painful mid-portion Achilles tendinopathy. Braz J Phys Ther. 2015;19(5):429-432.
57. Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232.
58. de Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387-392.
59. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
Stem Cells in Orthopedics: A Comprehensive Guide for the General Orthopedist
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
Biologic use in orthopedics is a continuously evolving field that complements technical, anatomic, and biomechanical advancements in orthopedics. Biologic agents are receiving increasing attention for their use in augmenting healing of muscles, tendons, ligaments, and osseous structures. As biologic augmentation strategies become increasingly utilized in bony and soft-tissue injuries, research on stem cell use in orthopedics continues to increase. Stem cell-based therapies for the repair or regeneration of muscle and tendon represent a promising technology going forward for numerous diseases.1
Stem cells by definition are undifferentiated cells that have 4 main characteristics: (1) mobilization during angiogenesis, (2) differentiation into specialized cell types, (3) proliferation and regeneration, and (4) release of immune regulators and growth factors.2 Mesenchymal stem cells (MSCs) have garnered the most attention in the field of surgery due to their ability to differentiate into the tissues of interest for the surgeon.3 This includes both bone marrow-derived mesenchymal stem cells (bm-MSCs) and adipose-derived mesenchymal stem cells (a-MSCs). These multipotent stem cells in adults originate from mesenchymal tissues, including bone marrow, tendon, adipose, and muscle tissue.4 They are attractive for clinical use because of their multipotent potential and relative ease of growth in culture.5 They also exert a paracrine effect to modulate and control inflammation, stimulate endogenous cell repair and proliferation, inhibit apoptosis, and improve blood flow through secretion of chemokines, cytokines, and growth factors.6,7
Questions exist regarding the best way to administer stem cells, whether systematic administration is possible for these cells to localize to the tissue in need, or more likely if direct application to the pathologic area is necessary.8,9 A number of sources, purification process, and modes of delivery are available, but the most effective means of preparation and administration are still under investigation. The goal of this review is to illustrate the current state of knowledge surrounding stem cell therapy in orthopedics with a focus on osteoarthritis, tendinopathy, articular cartilage, and enhancement of surgical procedures.
Important Considerations
Common stem cell isolates include embryonic, induced pluripotent, and mesenchymal formulations (Table 1). MSCs can be obtained from multiple sites, including but not limited to the adult bone marrow, adipose, muscular, or tendinous tissues, and their use has been highlighted in the study of numerous orthopedic and nonorthopedic pathologies over the course of the last decade. Research on the use of embryonic stem cells in medical therapy with human implications has received substantial attention, with many ethical concerns by those opposed, and the existence of a potential risk of malignant alterations.8,10 Amniotic-derived stem cells can be isolated from amniotic fluid, umbilical cord blood, or the placenta and thus do not harbor the same social constraints as the aforementioned embryonic cells; however, they do not harbor the same magnitude of multi-differentiation potential, either.4
Adult MSCs are more locally available and easy to obtain for treatment when compared with embryonic and fetal stem cells, and the former has a lower immunogenicity, which allows allogeneic use.11 Safety has been preliminarily demonstrated in use thus far; Centeno and colleagues12 found no neoplastic tissue generation at the site of stem cell injection after 3 years postinjection for a cohort of patients who were treated with autologous bm-MSCs for various pathologies. Self-limited pain and swelling are the most commonly reported adverse events after use.13 However, long-term data are lacking in many instances to definitively suggest the absence of possible complications.
Basic Science
Stem cell research encompasses a wide range of rapidly developing treatment strategies that are applicable to virtually every field of medicine. In general, stem cells can be classified as embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells, or adult-derived MSCs. ESCs are embryonic cells derived typically from fetal tissue, whereas iPS cells are dedifferentiated from adult tissue, thus avoiding many of the ethical and legal challenges imposed by research with ESCs. However, oncogenic and lingering politico-legal concerns with introducing dedifferentiated ESCs or iPS cells into healthy tissue necessitate the development, isolation, and expansion of multi- but not pluripotent stem cell lines.14 To date, the most advantageous and widely utilized from any perspective are MSCs, which can further differentiate into cartilage, tendon, muscle, and bony tissue.7,15,16
MSCs are defined by their ability to demonstrate in vitro differentiation into osteoblasts, adipocytes, or chondroblasts, adhere to plastic, express CD105, CD73, and CD90, and not express CD43, CD23, CD14 or CD11b, CD79 or CD19, or HLA-DR.17 Porada and Almeida-Porada18 have outlined 6 reasons highlighting the advantages of MSCs: 1) ease of isolation, 2) high differentiation capabilities, 3) strong colony expansion without differentiation loss, 4) immunosuppression following transplantation, 5) powerful anti-inflammatory properties, and 6) their ability to localize to damaged tissue. The anti-inflammatory properties of MSCs are particularly important as they promote allo- and xenotransplantation from donor tissues.19,20 MSCs can be isolated from numerous sources, including but not limited to bone marrow, periosteum, adipocyte, and muscle.21-23 Interestingly, the source tissue used to isolate MSCs can affect differentiation capabilities, colony size, and growth rate (Table 2).24 Advantages of a-MSCs include high prevalence and ease of harvest; however, several animal studies have shown inferior results when compared to bm-MSCs.25-27 More research is needed to determine the ideal source material for MSCs, which will likely depend in part on the procedure for which they are employed.27
Following harvesting, isolation, and expansion, MSC delivery methods for treatments typically consist of either cell-based or tissue engineering approaches. Cell-based techniques involve the injection of MSCs into damaged tissues. Purely cell-based therapy has shown success in limited clinical trials involving knee osteoarthritis, cartilage repair, and meniscal repair.28-30 However, additional studies with longer follow-up are required to validate these preliminary findings. Tissue engineering approaches involve the construction of a 3-dimensional scaffold seeded with MSCs that is later surgically implanted. While promising in theory, limited and often conflicting data exist regarding the efficacy of tissue-engineered MSC implantation.31-32 Suboptimal scaffold vascularity is a major limitation to scaffold design, which may be alleviated in part with the advent of 3-dimensional printing and the ability to more precisely alter scaffold architecture.14,33 Additional limitations include ensuring MSC purity and differentiation potential following harvesting and expansion. At present, the use of tissue engineering with MSCs is promising but it remains a nascent technology with additional preclinical studies required to confirm implant efficacy and safety.
Clinical Entities
Osteoarthritis
MSC therapies have emerged as promising treatment strategies in the setting of early osteoarthritis (OA). In addition to their regenerative potential, MSCs demonstrate potent anti-inflammatory properties, increasing their attractiveness as biologic agents in the setting of OA.34 Over the past decade, multiple human trials have been published demonstrating the efficacy of MSC injections into patients with OA.35,36 In a study evaluating a-MSC injection into elderly patients (age >65 years) with knee OA, Koh and colleagues29 found that 88% demonstrated improved cartilage status at 2-year follow-up, while no patient underwent a total knee arthroplasty during this time period. In another study investigating patients with unicompartmental knee OA with varus alignment undergoing high tibial osteotomy and microfracture, Wong and colleagues37 reported improved clinical, patient-reported, and magnetic resonance imaging (MRI)-based outcomes in a group receiving a preoperative MSC injection compared to a control group. Further, in a recent randomized control trial of patients with knee osteoarthritis, Vega and colleagues38 reported improved cartilage and quality of life outcomes at 1 year following MSC injection compared to a control group receiving a hyaluronic acid injection. In addition to knee OA, studies have also reported improvement in ankle OA following MSC injection.39 While promising, many of the preliminary clinical studies evaluating the efficacy of MSC therapies in the treatment of OA are hindered by small patient populations and short-term follow-up. Additional large-scale, randomized studies are required and many are ongoing presently in hopes of validating these preliminary findings.36
Tendinopathy
The quality of repaired tissue in primary tendon-to-tendon and tendon-to-bone healing has long been a topic of great interest.40 The healing potential of tendons is inferior to that of other bony and connective tissues,41 with tendon healing typically resulting in a biomechanically and histologically inferior structure to the native tissue.42 As such, this has been a particularly salient opportunity for stem cell use with hopes of recapitulating a more normal tendon or tendon enthesis following injury. In addition to the acute injury, there is great interest in the application of stem cells to chronic states of injury such as tendinopathy.
In equine models, the effect of autologous bm-MSCs treatment on tendinopathy of the superficial digital flexor tendon has been studied. Godwin and colleagues43 evaluated 141 race horses with spontaneous superficial digital flexor tendinopathy treated in this manner, and reported a reinjury percentage in these treated horses of just 27.4%, which compared favorably to historical controls and alternative therapeutics. Machova Urdzikova and colleagues44 injected MSCs at Achilles tendinopathy locations to augment nonoperative healing in 40 rats, and identified more native histological organization and improved vascularization in comparison to control rat specimens. Oshita and colleagues45 reported histologic improvement of tendinopathy findings in 8 rats receiving a-MSCs at the location of induced Achilles tendinopathy that was significantly superior to a control cohort. Bm-MSCs were used by Yuksel and colleagues46 in comparison with platelet-rich plasma (PRP) for treatment of Achilles tendon ruptures created surgically in rat models. They demonstrated successful effects with its use in terms of recovery for the tendon’s histopathologic, immunohistochemical, and biomechanical properties, related to significantly greater levels of anti-inflammatory cytokines. However, these aforementioned findings have not been uniform across the literature—other authors have reported findings that MSC transplantation alone did not repair Achilles tendon injury with such high levels of success.47
Human treatment of tendinopathies with stem cells has been scarcely studied to date. Pascual-Garrido and colleagues48 evaluated 8 patients with refractory patellar tendinopathy treated with injection of autologous bm-MSCs and reported successful results at 2- to 5-year follow-up, with significant improvements in patient-reported outcome measures for 100% of patients. Seven of 8 (87.5%) noted that they would undergo the procedure again.
Articular Cartilage Injury
Chondral injury is a particularly important subject given the limited potential of chondrocytes to replicate or migrate to the site of pathology.49 Stem cell use in this setting assists with programmed growth factor release and alteration of the anatomic microenvironment to facilitate regeneration and repair of the chondral surface. Autologous stem cell use through microfracture provides a perforation into the bone marrow and a subsequent fibrin clot formation containing platelets, growth factors, vascular elements, and MSCs.50 A similar concept to PRP is currently being explored with bm-MSCs. Isolated bm-MSCs are commonly referred to as bone marrow aspirate or bone marrow aspirate concentrate (BMAC). Commercially available systems are now available to aid in the harvesting and implementation of BMAC. One of the more promising avenues for BMAC implementation is in articular cartilage repair or regeneration due to chondrogenic potential of BMAC when used in isolation or when combined with microfracture, chondrocyte transfer, or collagen scaffolds.19,51 Synovial-derived stem cells as an additional source for stem cell use has demonstrated excellent chondrogenic potential in animal studies with full-thickness lesion healing and native-appearing cartilage histologically.52 Incorporation of a-MSCs into scaffolds for surgical implantation has demonstrated success in repairing full-thickness chondral defects with continuous joint surface and extracellular proteins, surface markers, and gene products similar to the native cartilage in animal models.53,54 In light of the promising basic science and animal studies, clinical studies have begun to emerge.55-57
Fortier and colleagues58 found MRI and histologic evidence of full-thickness chondral repair and increased integration with neighboring cartilage when BMAC was concurrently used at the time of microfracture in an equine model. Fortier and colleagues58 also demonstrated greater healing in equine models with acute full-thickness cartilage defects treated by microfracture with MSCs than without delivery of MSCs. Kim and colleagues59,60 similarly reported superiority in clinical outcomes for patients with osteochondral lesions of the talus treated with marrow stimulation and MSC injection than by the former in isolation.
In humans, stem cell use for chondral repair has additionally proven promising. A systematic review of the literature suggested good to excellent overall outcomes for the treatment of moderate focal chondral defects with BMAC with or without scaffolds and microfracture with inclusion of 8 total publications.61 This review included Gobbi and colleagues,62 who prospectively treated 15 patients with a mean focal chondral defect size of 9.2 cm2 about the knee. Use of BMAC covered with a collagen I/III matrix produced significant improvements in patient-reported outcome scores and MRI demonstrated complete hyaline-like cartilage coverage in 80%, with second-look arthroscopy demonstrating normal to nearly normal tissue. Gobbi and colleagues55 also found evidence for superiority of chondral defects treated with BMAC compared to matrix-induced autologous chondrocyte implantation (MACI) for patellofemoral lesions in 37 patients (MRI showed complete filling of defects in 81% of BMAC-treated patients vs 76% of MACI-treated patients).
Meniscal Repair
Clinical application of MSCs in the treatment of meniscal pathology is evolving as well. ASCs have been added to modify the biomechanical environment of avascular zone meniscal tears at the time of suture repair in a rabbit, and have demonstrated increased healing rates in small and larger lesions, although the effect lessens with delay in repair.63 Angele and colleagues64 treated meniscal defects in a rabbit model with scaffolds with bm-MSCs compared with empty scaffolds or control cohorts and found a higher proportion of menisci with healed meniscus-like fibrocartilage when MSCs were utilized.
In humans, Vangsness and colleagues30 treated knees with partial medial meniscectomy with allogeneic stem cells and reported an increase in meniscal volume and decrease in pain in those patients when compared to a cohort of knees treated with hyaluronic acid. Despite promising early results, additional clinical studies are necessary to determine the external validity and broad applicability of stem cell use in meniscal repair.
Rotator Cuff Repair
The number of local resident stem cells at the site of rotator cuff tear has been shown to decrease with tear size, chronicity, and degree of fatty infiltration, suggesting that those with the greatest need for a good reparative environment are those least equipped to heal.65 The need for improvement in this domain is related to the still relatively high re-tear rate after rotator cuff repair despite improvements in instrumentation and surgical technique.66 The native fibrocartilaginous transition zone between the humerus and the rotator cuff becomes a fibrovascular scar tissue after rupture and repair with poorer material properties than the native tissue.67 Thus, a-MSCs have been evaluated in this setting to determine if the biomechanical and histological properties of the repair may improve.68
In rat models, Valencia Mora and colleagues68 reported on the application of a-MSCs in a rat rotator cuff repair model compared to an untreated group. They found no differences between those treated rats and those without a-MSCs use in terms of biomechanical properties of the tendon-to-bone healing, but those with stem cell use had less inflammation shown histologically (diminished presence of edema and neutrophils) at 2- and 4-week time points, which the authors suggested may lead to a more elastic repair and less scar at the bone-tendon healing site. Oh and colleagues1 evaluated the use of a-MSCs in a rabbit subscapularis tear model, and reported significantly reduced fatty infiltration at the site of chronic rotator cuff tear after repair with its application at the repair site; while the load-to-failure was higher in those rabbits with ASCs administration, it was short of reaching statistical significance. Yokoya and colleagues69 demonstrated regeneration of rotator cuff tendon-to-bone insertional site anatomy and in the belly of the cuff tendon in a rabbit model with MSCs applied at the operative site. However, Gulotta and colleagues70 did not see the same improvement in their similar study in the rat model; these authors failed to see improvement in structure, strength, or composition of the tendinous attachment site despite addition of MSCs.
Clinical studies on augmented rotator cuff repair have also found mixed results. MSCs for this purpose have been cultivated from arthroscopic bone marrow aspiration of the proximal humerus71 and subacromial bursa72 with successful and reproducibly high concentrations of stem cells. Hernigou and colleagues73 found a significant improvement in rate of healing (87% intact cuffs vs 44% in the control group) and repair surface tendon integrity (via ultrasound and MRI) for patients at a minimum of 10 years after rotator cuff repair with MSC injection at the time of surgery. The authors found a direct correlation in these outcomes with the number of MSCs injected at the time of repair. Ellera Gomes and colleagues74 injected bm-MSCs obtained from the iliac crest into the tendinous repair site in 14 consecutive patients with full-thickness rotator cuff tears treated by transosseous sutures via a mini-open approach. MRI demonstrated integrity of the repair site in all patients at more than 1-year follow-up.
Achilles Tendon Repair
The goal with stem cell use in Achilles repair is to accelerate the healing and rehabilitation. Several animal studies have demonstrated improved mechanical properties and collagen composition of tendon repairs augmented with stem cells, including Achilles tendon repair in a rat model. Adams and colleagues75 compared suture alone (36 tendons) to suture plus stem cell concentrate injection (36 tendons) and stem cell loaded suture (36 tendons) in Achilles tendon repair with rat models. The suture-alone cohort had lower ultimate failure loads at 14 days after surgery, indicating biomechanical superiority with stem cell augmentation means. Transplantation of hypoxic MSCs at the time of Achilles tendon repair may be a promising option for superior biomechanical failure loads and histologic findings as per recent rat model findings by Huang and colleagues.76 Yao and colleagues77 demonstrated increased strength of suture repair for Achilles repair in rat models at early time points when using MSC-coated suture in comparison to standard suture, and suggested that the addition of stem cells may improve early mechanical properties during the tendon repair process. A-MSC addition to PRP has provided significantly increased tensile strength to rabbit models with Achilles tendon repair as well.78
In evaluation of stem cell use for this purpose with humans, Stein and colleagues79 reviewed 28 sports-related Achilles tendon ruptures in 27 patients treated with open repair and BMAC injection. At a mean follow-up of 29.7 months, the authors reported no re-ruptures, with 92% return to sport at 5.9 months, and excellent clinical outcomes. This small cohort study found no adverse outcomes related to the BMAC addition, and thus proposed further study of the efficacy of stem cell treatment for Achilles tendon repair.
Anterior Cruciate Ligament Reconstruction
Bm-MSCs genetically modified with bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have shown great promise in improvement of the formation of mechanically sound tendon-bone interface in anterior cruciate ligament (ACL) reconstruction.80 Similar to the other surgical procedures mentioned in this review, animal studies have successfully evaluated the augmentation of osteointegration of tendon to bone in the setting of ACL reconstruction. Jang and colleagues3 investigated the use of nonautologous transplantation of human umbilical cord blood-derived MSCs in a rabbit ACL reconstruction model. The authors demonstrated a lack of immune rejection, and enhanced tendon-bone healing with broad fibrocartilage formation at the transition zone (similar to the native ACL) and decreased femoral and tibial tunnel widening as compared to a control cohort at 12-weeks after surgery. In a rat model, Kanaya and colleagues81 reported improved histological scores and slight improvements in biomechanical integrity of partially transected rat ACLs treated with intra-articular MSC injection. Stem cell use in the form of suture-supporting scaffolds seeded with MSCs has been evaluated in a total ACL transection rabbit model; the authors of this report demonstrated total ACL regeneration in one-third of samples treated with this augmentation option, in comparison to complete failure in all suture and scaffold alone groups.82
The use of autologous MSCs in ACL healing remains limited to preclinical research and small case series of patients. One human trial by Silva and colleagues83 evaluated the graft-to-bone site of healing in ACL reconstruction for 20 patients who received an intraoperative infiltration of their graft with adult bm-MSCs. MRI and histologic analysis showed no difference in comparison to control groups, but the authors’ conclusion proposed that the number of stem cells injected might have been too minimal to show a clinical effect.
Other Applications
Although outside the scope of this article, stem cells have demonstrated efficacy in the treatment of a number of osseous clinical entities. This includes the treatment of fracture nonunion, augmentation of spinal fusion, and assistance in the treatment of osteonecrosis.84
Summary
As a scientific community, our understanding of the use of stem cells, their nuances, and their indications has expanded dramatically over the last several years. Stem cell treatment has particularly infiltrated the world of operative and nonoperative sports medicine, given in part the active patient population seeking greater levels of improvement.85 Stem cell therapy offers a potentially effective therapy for a multitude of pathologies because of these cells’ anti-inflammatory, immunoregulatory, angiogenic, and paracrine effects.86 It thus remains a very dynamic option in the study of musculoskeletal tissue regeneration. While the potential exists for stem cell use in daily surgery practices, it is still premature to predict whether this can be expected.
The ideal stem cell sources (including allogeneic or autologous), preparation, cell number, timing, and means of application continue to be evaluated, as well as those advantageous pathologies that can benefit from the technology. In order to better answer these pertinent questions, we need to make sure we have a safe, economic, and ethically acceptable means for stem cell translational research efforts. More high-level studies with standardized protocols need to be performed. It is necessary to improve national and international collaboration in research, as well as collaboration with governing bodies, to attempt to further scientific advancement in this field of research.49 Further study on embryonic stem cell use may be valuable as well, pending governmental approval. Finally, more dedicated research efforts must be placed on the utility of adjuncts with stem cell use, including PRP and scaffolds, which may increase protection, nutritional support, and mechanical stimulation of the administered stem cells.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
1. Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg. 2014;23(4):445-455.
2. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795-1803.
3. Jang KM, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539.
4. Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2(3):187-192.
5. Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39(12):2363-2372.
6. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181-192.
7. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198-1211.
8. Hirzinger C, Tauber M, Korntner S, et al. ACL injuries and stem cell therapy. Arch Orthop Trauma Surg. 2014;134(11):1573-1578.
9. Becerra P, Valdés Vázquez MA, Dudhia J, et al. Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J Orthop Res. 2013;31(7):1096-1102.
10. Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
11. García-Gómez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453-1468.
12. Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6(4):368-378.
13. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016 Mar 30. [Epub ahead of print]
14. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:394962.
15. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45.
16. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
17. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
18. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156-1566.
19. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717-1729.
20. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286.
21. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.
22. De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209-1221.
23. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279-4295.
24. Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248.
25. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675-1680.
26. Vidal MA, Robinson SO, Lopez MJ, et al. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37(8):713-724.
27. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449-462.
28. Hogan MV, Walker GN, Cui LR, Fu FH, Huard J. The role of stem cells and tissue engineering in orthopaedic sports medicine: current evidence and future directions. Arthroscopy. 2015;31(5):1017-1021.
29. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.
30. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98.
31. Goodrich LR, Chen AC, Werpy NM, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am. 2016;98(1):23-34.
32. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176-185.
33. Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237-247.
34. Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027-2035.
35. Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016 Mar 10. [Epub ahead of print]
36. Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117-124.
37. Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020-2028.
38. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681-1690.
39. Kim YS, Lee M, Koh YG. Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exp Orthop. 2016;3(1):12.
40. Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17(1):148.
41. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832-839.
42. Müller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2097-3105.
43. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44(1):25-32.
44. Machova Urdzikova L, Sedlacek R, Suchy T, et al. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13:42.
45. Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 2016 Apr 11. [Epub ahead of print]
46. Yuksel S, Guleç MA, Gultekin MZ, et al. Comparison of the early-period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on achilles tendon ruptures in rats. Connect Tissue Res. 2016 May 18. [Epub ahead of print]
47. Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153-2168.
48. Pascual-Garrido C, Rolón A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510.
49. Zlotnicki JP, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4(4):2325967116642433.
50. McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013). 2014;72(1):89-96.
51. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19(10):1449-1470.
52. Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327-338.
53. Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615-1621.
54. Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006 79(1):25-34.
55. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-657.
56. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505-1511.
57. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91-98.
58. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-1937.
59. Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090-1099.
60. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434.
61. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481.
62. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299.
63. Ruiz-Ibán MÁ, Díaz-Heredia J, García-Gómez I, Gonzalez-Lizán F, Elías-Martín E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27(12):1688-1696.
64. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445-455.
65. Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219-1225.
66. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550-554.
67. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622-633.
68. Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45 Suppl 4:S22-S27.
69. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40(6):1259-1268.
70. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133.
71. Beitzel K, McCarthy MB, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29(2):301-308.
72. Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41(3):657-668.
73. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
74. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373-377.
75. Adams SB Jr, Thorpe MA, Parks BG, Aghazarian G, Allen E, Schon LC. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293-299.
76. Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 2013;41(5):1117-1125.
77. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012;37(8):1639-1645.
78. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712-1719.
79. Stein BE, Stroh DA, Schon LC. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop. 2015;39(5):901-905.
80. Chen B, Li B, Qi YJ, et al. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci Rep. 2016;6:25940.
81. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617.
82. Figueroa D, Espinosa M, Calvo R, et al. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196-1202.
83. Silva A, Sampaio R, Fernandes R, Pinto E. Is there a role for adult non-cultivated bone marrow stem cells in ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2014;22(1):66-71.
84. Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
85. Kopka M, Bradley JP. The use of biologic agents in athletes with knee injuries. J Knee Surg. 2016 May 20. [Epub ahead of print]
86. Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, Barco Laakso R, Cuéllar R, García Arranz M. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells. 2015;7(4):691-699.
87. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-272.
88. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528-1530.
89. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
90. Fukuda K. Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto). 2002;42(1):1-9.
91. Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9(6):444-450.
92. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.
93. Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587-591.
94. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537-544.
95. Funk JF, Matziolis G, Krocker D, Perka C. [Promotion of bone healing through clinical application of autologous periosteum derived stem cells in a case of atrophic non-union]. Z Orthop Unfall. 2007;145(6):790-794.
Current and Future Stem Cell Regulation: A Call to Action
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
The 2 cardinal properties of stem cells are the ability to self-renew and the ability to differentiate into distinctive end-stage cell types. The work of Caplan1 captured our early attention, with cells cultured from bone marrow differentiating into a number of different cell types of orthopedic interest. Our latest attention has been captured by the additional abilities of these cells to mobilize, monitor, and interact with their surrounding environment.2-4 In response to their environment, stem cells are able to release a broad spectrum of macromolecules with trophic, chemotactic, and immunomodulatory potential, which allows them to participate in injury response, tissue healing, and tissue regeneration.4 These cells are innate to the body’s monitoring, maintenance, repair, and stress response systems.2,4-11 Basic science and animal studies have illustrated the potential of cells with stem potential regardless of their environment/source of harvest.
Where Can We Get Stem Cells?
Cells with stem properties are present in many environmental niches, including the bone marrow, peripheral circulatory system, adipose tissue, synovial tissue, muscle tissue, and tendon tissue.12-15 A number of cell types with stem properties populate the bone marrow niche, including hematopoietic stem/progenitor cells (HSPC), perivascular stromal cells (PSC), endothelial stem cells (ESC), and immature cells with qualities like embryonal stem cells termed very small embryonal-like stem cells (VESL).12,15-19 All of these cells have stem properties and have been shown to differentiate to tissues of orthopedic interest.The interplay, interaction, and potential of these cell types is complex and incompletely understood.12,15-19 When bone marrow is aspirated for culturing purposes, it is unclear which cell line produces the plastic-adherent multipotent cells grown in culture, which are often referred to as mesenchymal stem cells (MSCs). Researches propose that HSPC and/or VESL circulate peripherally in small numbers but leave the bone marrow in certain mobilization instances and are important for the monitoring and maintenance of the majority of tissues in our bodies.5,16 Current clinical utilization of these cell types by the orthopedic community primarily utilizes point-of-care bone marrow aspiration and concentration, while the hematology oncology community mobilizes cells from the bone marrow to the blood stream with pharmaceutical agents and harvests cells via apheresis. Bone marrow aspiration produces variable numbers of stem cells, with studies ranging from 1 stem cell per mL of tissue collected to 300,000 stem cells per mL of tissue collected.20Mobilization and apheresis can produce large volumes of peripheral blood-derived cells with 600,000 HSPC per mL and 2.32 million PSC per mL of tissue collected.21
In adipose tissue, cells adherent to the abluminal side of blood vessels known as pericytes also carry stem qualities. Aspiration and processing of adipose tissue can access these stem cells, producing a product often referred to as stromal vascular fraction (SVF). Processing of lipoaspirate to create stromal vascular fraction requires mechanical or enzymatic processing. This also produces variable numbers of stem cells, with quantitative studies ranging from 5000 to 1.5 million stem cells per mL of tissue collected.20 Similar to adipose-derived stem cells, synovial-derived and muscle-derived stem cells also require mechanical or enzymatic processing. For applications where it is believed that a large number of cells is necessary, investigators often utilize culturing techniques for all sources with the exception of mobilization and apheresis harvest. As clinicians, 3 challenges have proven more important than which cell type to utilize: 1) patient-care logistics regarding collection and application; 2) the undefined dose-response curve regarding stem cell treatments; and 3) evolving government/community regulation.
Regulation of Stem Cell Therapies
The regulation of stem cell technologies is a double-edged sword for development. While loose regulation encourages clinical application and experimentation, patient safety and efficacy concerns are raised, and a technology’s worth is not proven before clinical application. Tight regulation temporarily hampers progress, yet ensures the proof of safety and efficacy prior to widespread implementation. Within the United States, the Food and Drug Administration (FDA) has tightened regulation, established precedent, and intervened in the ability of clinicians to utilize stem cell therapies in humans, through “warning letters,” “untitled letters,” and industry guidance documents.22-30
The FDA categorizes stem cell therapies as human cells, tissues, and cellular- and tissue-based products (HCT/Ps). Section 361 of the Public Health Safety (PHS) Act established and outlined the authority of the FDA to regulate low-risk HCT/Ps in order to prevent the introduction, transmission, and spread of communicable disease. Section 361 provided standards for safety without requiring preclinical development. The FDA established 4 principles to determine the risk of HCT/Ps: the extent of manipulation involved in manufacture, the metabolic activity/autologous nature of the product, whether the product represents a tissue combined with another product, and whether the product is utilized in a fashion homologous with its original function (Figure 1). If a product/therapy meets requirements around all 4 of these principles, then it is deemed a low-risk product and regulated under Section 361 alone. If a product/therapy does not meet requirements around all 4 of these principles, then the FDA regulates the product/therapy under additional codes including Section 351 of the PHS Act. Section 351 outlines a developmental process including preclinical animal trials, phased clinical study, and premarket review by the FDA prior to offering the product/treatment in clinical practice. The developmental process requires investigators and/or industry developers to initiate an Investigational New Drug (IND) program whose end goal is to present data from all developmental study and obtain a Biologic License Application (BLA) approval to market the product.22-23 To establish safety and efficacy, the traditional IND program involves a preclinical animal study, a small pilot human study (Phase I), a small initial randomized controlled trial (Phase II), followed by a large multicenter randomized controlled trial (Phase III) (Figure 2). The FDA has recognized little to no stem cell treatments as products regulated by Section 361 alone. Additionally, the FDA has established precedent regarding allograft stem cells, cells obtained from fat harvest, amniotic/placental products, and cultured cells, suggesting that these products are not low risk and require an IND pathway outlined in Section 351.24-30
Bone Marrow Aspiration
Surprisingly, the FDA has not moved to regulate the point-of-care use of bone marrow aspirate or platelet-rich plasma and has labeled these as “not HCTPs.” The stem cell concentration of bone marrow aspirate is technique-dependent, declines with age, and has been found to be an important factor for clinical benefit.31 While it is possible to aspirate from multiple sites, posterior iliac crest harvest produces the highest stem cell yield.32-34 Hernigou and colleagues35-36 have outlined safe zones for trocar placement and illustrated that strong aspiration with small-volume syringes, 10-mL syringes, optimizes stem cell harvest. Additionally, studies by Hernigou and colleagues31,37-38 involving tibial nonunion, avascular necrosis of the femur, and augmentation of rotator cuff repair are guideposts to clinicians utilizing bone marrow aspirate.
Amniotic Stem Cell Technologies and Adipose-Derived Stem Cells
While some argue that there is regulatory confusion around amniotic/placental-derived tissues and adipose-derived products, the FDA has clearly established precedent establishing these as products requiring Section 351 development.26-29 Companies are marketing products derived from perinatal byproducts, yet there are multiple FDA letters suggesting that these are not products regulated solely under PHS Act 361 because they do not meet the criteria of homologous use and are not autologous.28-29 Use of these products places risk upon the clinician and the patient. Some argue that adipose-derived stem cell products are 361 products. While the FDA has approved devices for the mechanical processing of lipoaspirate, they have established precedent suggesting that they consider orthopedic applications nonhomologous and any processing that “alters the original relevant characteristics of adipose tissue relating to the tissue’s utility for reconstruction, repair, or replacement” as more than minimal manipulation.26,27 The FDA originally planned an open forum for discussion with clinicians and industry for April 2016. This open forum was delayed due to the volume of interest, and a workshop has been planned for Fall 2016.
Future Regulation of Stem Cell Technologies
While many countries have mirrored the FDA with tight regulatory mechanisms, a few countries have established modern regulatory mechanisms aimed at the promotion of conscientious development, including South Korea, Japan, and England. For example, in 2014 Japan labeled stem cell technologies as “regenerative medicine products,” setting them apart from pharmaceuticals, and implemented a new approval system allowing early observed commercialization with reimbursement after less stringent safety and efficacy milestones.22The observed commercialization lowers time and financial hurdles for development while still requiring the proof of the technology’s worth. Countries that have effected change have positioned themselves to be pioneers in this emerging field.
In March 2016, the Reliable and Effective Growth for Regenerative Health Options that Improve Wellness (REGROW) Act of 2016 (S. 2689 / H.R. 4762) was introduced into the United States Congress. This bipartisan, bicameral legislation was introduced, read twice, and referred to subcommittee. Its goal is to reduce barriers and accelerate development of biologic therapies while keeping the frame work set forth under Sections 351 and 361 of the PHS Act.39 Similar to the pathway in Japan, the REGROW Act would establish a conditional approval pathway that would ensure products are safe and effective while also evolving the regulatory pathway towards progress (Figure 3). Development would still require an IND application after preclinical animal study. However, after safety was established with human Phase I data and preliminary evidence of efficacy with Phase II data, patients could be treated with the investigational therapies and reimbursement collected for a limited period of time (5 years) prior to a large Phase III human clinical trial. Patients treated with the new therapy would be monitored closely. All results would be reported to the FDA in a BLA. This change in legislation would lower but not remove regulatory hurdles necessary for development.
Conclusion
The future of stem cell treatments hinges upon the creation of new favorable regulatory mechanisms that will promote clinical application while ensuring that safety and efficacy milestones are reached. Clinical researchers require freedom to develop these technologies while protecting patients and ensuring the validity of treatments. The coordination of research and regulatory affairs on a global level is necessary focusing on the harmonization of guidelines, regulations, and mechanisms for simultaneous adoption in different countries. The global orthopedic community has made strides regarding the science of stem cell technologies; it is time for us to initiate progressive change regarding regulation so that we can determine what is effective clinically.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.
1. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650.
2. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936.
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
4. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
5. Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.” Blood Cells Mol Dis. 2013;51(1):3-8.
6. Cesselli D, Beltrami AP, Rigo S, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104(10):1225-1234.
7. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008.
8. Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92(6):768-774.
9. Mansilla E, Marín GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967-969.
10. Rankin SM. Impact of bone marrow on respiratory disease. Curr Opin Pharmacol. 2008;8(3):236-241.
11. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202-2208.
12. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535-2547.
13. Harvanová D, Tóthová T, Sarišský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 2011;57(3):119-124.
14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.
15. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316.
16. Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
17. Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz K, Zbucka-Kretowska M, Moniuszko M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Adv Med Sci. 2014;59(2):273-280.
18. Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044-1050.
19. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.
20. Vangsness CT Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31(9):1836-1843.
21. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506.
22. Board on Health Sciences Policy; Board on Life Sciences; Division on Earth and Life Studies; Institute of Medicine; National Academy of Sciences. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop. Washington, DC: National Academies Press (US); 2014.
23. US Food and Drug Administration. Minimal manipulation of human cells, tissues, and cellular and tissue-based products: draft guidance for industry and food and drug administration staff. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm427692.htm. Updated February 3, 2015. Accessed June 10, 2016.
24. US Food and Drug Administration. PureGen™ osteoprogenitor cell allograft, parcell laboratories, LLC - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm264011.htm. Published June 23, 2011. Accessed June 10, 2016.
25. US Food and Drug Administration. Map3 chips allograft-untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm418126.htm. Updated December 30, 2014. Accessed June 10, 2016.
26. US Food and Drug Administration. Irvine stem cell treatment center 12/30/15: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm479837.htm. Published December 30, 2015. Accessed June 10, 2016.
27. US Food and Drug Administration. IntelliCell Biosciences, Inc. 3/13/12: warning letter. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm297245.htm. Published March 13, 2012. Accessed June 10, 2016.
28. US Food and Drug Administration. Osiris Therapeutics, Inc. - untitled letter. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm371540.htm. Updated October 21, 2013. Accessed June 10, 2016.
29. US Food and Drug Administration. BioD- untitled letter. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/UCM452862.pdf. Published June 22, 2015. Accessed June 10, 2016.
30. US Food and Drug Administration. Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm. Published July 25, 2008. Accessed June 10, 2016.
31. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
32. Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47.
33. Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95(14):1312-1316.
34. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101-1107.
35. Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377-2384.
36. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287.
37. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
38. Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40-45.
39. 114th Congress (2015-2016). S.2689 - REGROW Act. https://www.congress.gov/bill/114th-congress/senate-bill/2689/text. Accessed June 10, 2016.
“I Want What Kobe Had”: A Comprehensive Guide to Giving Your Patients the Biologic Solutions They Crave
The sun has finally set on Kobe Bryant’s magnificent career. After all the tributes and tearful goodbyes, he has finally played his last game and become a part of basketball history. Ever since his field trip to Germany for interleukin-1 receptor antagonist protein (IRAP) treatments to his knee, and his subsequent return to high-level play, I’ve been under siege in the office by patients who “want what Kobe had.” I’ve had to explain, time and time again, that IRAP treatment is not available in the United States and that platelet-rich plasma (PRP) is the closest alternative treatment, convince them that PRP may be even better, and then let them know that it’s considered experimental and not covered by insurance.
In the last issue, we discussed the future of orthopedics, which in my opinion will rely heavily on the biologic therapies now considered experimental. In this issue, we will look into our crystal balls and imagine what that future might look like. To do so, we should first consider what we hope to accomplish through the incorporation of biologic therapies.
The regeneration of articular cartilage, acceleration of fracture and tissue healing, and faster incorporation of tendon grafts to bone have long been considered the Holy Grail of Orthopedics. In his best seller, The Da Vinci Code, Dan Brown makes a compelling argument that the Holy Grail, the chalice thought to have held the blood of Christ, was in fact a mistranslated reference to his living descendants. Whenever I have a visitor or student in the operating room, I focus the scope on the synovial capillaries so they can see the individual red blood cells passing single-file through the vessels on their way to supply cells with the nutrients they need.
Perhaps, like in The Da Vinci Code, the solution to our greatest biologic challenges lies in the blood, already there, just waiting to be unlocked.
PRP has been utilized for everything from tendinopathy to arthropathy, with varied results in the literature. The lack of standardization of PRP preparations, which vary in inclusion of white cells and absolute platelet count, confounds these results even further. In this issue, we review its use in sports medicine and knee arthritis, taking a closer look at partial ulnar collateral ligament tears in baseball players.
In “Tips of the Trade,” we present a technique for “superior capsular reconstruction” that provides a novel solution for patients with pseudoparalysis from massive rotator cuff tears with little other options beside reverse total shoulder arthroplasty.
The one absolute statement I can make regarding biologics is that we currently have more questions than answers, and every hypothesis we prove simply begets more questions. More randomized controlled studies are needed in virtually every aspect of biologics, and we should all consider taking part. While the solutions our patients crave may not arrive during our careers, or even our lifetimes, the groundwork we do now will set the stage for future generations to enjoy biologically enhanced outcomes.
The sun has finally set on Kobe Bryant’s magnificent career. After all the tributes and tearful goodbyes, he has finally played his last game and become a part of basketball history. Ever since his field trip to Germany for interleukin-1 receptor antagonist protein (IRAP) treatments to his knee, and his subsequent return to high-level play, I’ve been under siege in the office by patients who “want what Kobe had.” I’ve had to explain, time and time again, that IRAP treatment is not available in the United States and that platelet-rich plasma (PRP) is the closest alternative treatment, convince them that PRP may be even better, and then let them know that it’s considered experimental and not covered by insurance.
In the last issue, we discussed the future of orthopedics, which in my opinion will rely heavily on the biologic therapies now considered experimental. In this issue, we will look into our crystal balls and imagine what that future might look like. To do so, we should first consider what we hope to accomplish through the incorporation of biologic therapies.
The regeneration of articular cartilage, acceleration of fracture and tissue healing, and faster incorporation of tendon grafts to bone have long been considered the Holy Grail of Orthopedics. In his best seller, The Da Vinci Code, Dan Brown makes a compelling argument that the Holy Grail, the chalice thought to have held the blood of Christ, was in fact a mistranslated reference to his living descendants. Whenever I have a visitor or student in the operating room, I focus the scope on the synovial capillaries so they can see the individual red blood cells passing single-file through the vessels on their way to supply cells with the nutrients they need.
Perhaps, like in The Da Vinci Code, the solution to our greatest biologic challenges lies in the blood, already there, just waiting to be unlocked.
PRP has been utilized for everything from tendinopathy to arthropathy, with varied results in the literature. The lack of standardization of PRP preparations, which vary in inclusion of white cells and absolute platelet count, confounds these results even further. In this issue, we review its use in sports medicine and knee arthritis, taking a closer look at partial ulnar collateral ligament tears in baseball players.
In “Tips of the Trade,” we present a technique for “superior capsular reconstruction” that provides a novel solution for patients with pseudoparalysis from massive rotator cuff tears with little other options beside reverse total shoulder arthroplasty.
The one absolute statement I can make regarding biologics is that we currently have more questions than answers, and every hypothesis we prove simply begets more questions. More randomized controlled studies are needed in virtually every aspect of biologics, and we should all consider taking part. While the solutions our patients crave may not arrive during our careers, or even our lifetimes, the groundwork we do now will set the stage for future generations to enjoy biologically enhanced outcomes.
The sun has finally set on Kobe Bryant’s magnificent career. After all the tributes and tearful goodbyes, he has finally played his last game and become a part of basketball history. Ever since his field trip to Germany for interleukin-1 receptor antagonist protein (IRAP) treatments to his knee, and his subsequent return to high-level play, I’ve been under siege in the office by patients who “want what Kobe had.” I’ve had to explain, time and time again, that IRAP treatment is not available in the United States and that platelet-rich plasma (PRP) is the closest alternative treatment, convince them that PRP may be even better, and then let them know that it’s considered experimental and not covered by insurance.
In the last issue, we discussed the future of orthopedics, which in my opinion will rely heavily on the biologic therapies now considered experimental. In this issue, we will look into our crystal balls and imagine what that future might look like. To do so, we should first consider what we hope to accomplish through the incorporation of biologic therapies.
The regeneration of articular cartilage, acceleration of fracture and tissue healing, and faster incorporation of tendon grafts to bone have long been considered the Holy Grail of Orthopedics. In his best seller, The Da Vinci Code, Dan Brown makes a compelling argument that the Holy Grail, the chalice thought to have held the blood of Christ, was in fact a mistranslated reference to his living descendants. Whenever I have a visitor or student in the operating room, I focus the scope on the synovial capillaries so they can see the individual red blood cells passing single-file through the vessels on their way to supply cells with the nutrients they need.
Perhaps, like in The Da Vinci Code, the solution to our greatest biologic challenges lies in the blood, already there, just waiting to be unlocked.
PRP has been utilized for everything from tendinopathy to arthropathy, with varied results in the literature. The lack of standardization of PRP preparations, which vary in inclusion of white cells and absolute platelet count, confounds these results even further. In this issue, we review its use in sports medicine and knee arthritis, taking a closer look at partial ulnar collateral ligament tears in baseball players.
In “Tips of the Trade,” we present a technique for “superior capsular reconstruction” that provides a novel solution for patients with pseudoparalysis from massive rotator cuff tears with little other options beside reverse total shoulder arthroplasty.
The one absolute statement I can make regarding biologics is that we currently have more questions than answers, and every hypothesis we prove simply begets more questions. More randomized controlled studies are needed in virtually every aspect of biologics, and we should all consider taking part. While the solutions our patients crave may not arrive during our careers, or even our lifetimes, the groundwork we do now will set the stage for future generations to enjoy biologically enhanced outcomes.
Biomechanical Evaluation of All-Polyethylene Pegged Bony Ingrowth Glenoid Fixation Techniques on Implant Micromotion
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Progressive Cardiomyopathy in a Patient With Elevated Cobalt Ion Levels and Bilateral Metal-on-Metal Hip Arthroplasties
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Knee Extensor Mechanism Reconstruction With Complete Extensor Allograft After Failure of Patellar Tendon Repair
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
Rationale for Strategic Graft Placement in Anterior Cruciate Ligament Reconstruction: I.D.E.A.L. Femoral Tunnel Position
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
In the United States, surgeons perform an estimated 200,000 anterior cruciate ligament reconstructions (ACLRs) each year. Over the past decade, there has been a surge in interest in defining anterior cruciate ligament (ACL) anatomy to guide ACLR. With this renewed interest in the anatomical features of the ACL, particularly the insertion site, many authors have advocated an approach for complete or near-complete “footprint restoration” for anatomical ACLR.1,2 Some have recommended a double-bundle (DB) technique that completely “fills” the footprint, but it is seldom used. Others have proposed centralizing the femoral tunnel position within the ACL footprint in the hope of capturing the function of both the anteromedial (AM) and posterolateral (PL) bundles.1,3,4 Indeed, a primary surgical goal of most anatomical ACLR techniques is creation of a femoral tunnel based off the anatomical centrum (center point) of the ACL femoral footprint.3,5 With a single-bundle technique, the femoral socket is localized in the center of the entire footprint; with a DB technique, sockets are created in the centrums of both the AM and PL bundles.
Because of the complex shape of the native ACL, however, the strategy of restoring the femoral footprint with use of either a central tunnel or a DB approach has been challenged. The femoral footprint is 3.5 times larger than the midsubstance of the ACL.6 Detailed anatomical dissections have recently demonstrated that the femoral origin of the ACL has a stout anterior band of fibers with a fanlike extension posteriorly.7 As the ACL fibers extend off the bony footprint, they form a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick.2,8 Within this structure, there is no clear separation of the AM and PL bundles. The presence of this structure makes sense given the anatomical constraints inherent in the notch. Indeed, the space for the native ACL is narrow, as the posterior cruciate ligament (PCL) occupies that largest portion of the notch with the knee in full extension, leaving only a thin, 5-mm slot through which the ACL must pass.9 Therefore, filling the femoral footprint with a tubular ACL graft probably does not reproduce the dynamic 3-dimensional morphology of the ACL.
In light of the discrepancy between the sizes of the femoral footprint and the midsubstance of the native ACL, it seems reasonable that optimizing the position of the ACL femoral tunnel may be more complex than simply centralizing the tunnel within the footprint or attempting to maximize footprint coverage. In this article, we amalgamate the lessons of 4 decades of ACL research into 5 points for strategic femoral tunnel positioning, based on anatomical, histologic, isometric, biomechanical, and clinical data. These points are summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension.
1. Anatomy Considerations
In response to study results demonstrating that some transtibial ACLRs were associated with nonanatomical placement of the femoral tunnel—resulting in vertical graft placement, PCL impingement, and recurrent rotational instability10-16—investigators have reexamined both the anatomy of the femoral origin of the native ACL and the ACL graft. Specifically, a large body of research has been devoted to characterizing the osseous landmarks of the femoral origin of the ACL17 and the dimensions of the femoral footprint.3 In addition, authors have supported the concept that the ACL contains 2 functional bundles, AM and PL.5,17 Several osseous landmarks have been identified as defining the boundaries of the femoral footprint. The lateral intercondylar ridge is the most anterior aspect of the femoral footprint and was first defined by Clancy.18 More recently, the lateral bifurcate ridge, which separates the AM and PL bundle insertion sites, was described19 (Figure 1A).
These osseous ridges delineate the location of the femoral footprint. Studies have shown that ACL fibers attach from the lateral intercondylar ridge on the anterior border of the femoral footprint and extend posteriorly to the cartilage of the lateral femoral condyle (Figure 1B).
ACL fibers from this oblong footprint are organized such that the midsubstance of the ACL is narrower than the femoral footprint. Anatomical dissections have demonstrated that, though the femoral footprint is oval, the native ACL forms a flat, ribbonlike structure 9 to 16 mm wide and only 2 to 4 mm thick as it takes off from the bone.8,20 There is a resulting discrepancy between the femoral footprint size and shape and the morphology of the native ACL, and placing a tunnel in the center of the footprint or “filling the footprint” with ACL graft may not reproduce the morphology or function of the native ACL. Given this size mismatch, strategic decisions need to be made to place the femoral tunnel in a specific region of the femoral footprint to optimize its function.
2. Histologic Findings
Histologic analysis has further clarified the relationship between the femoral footprint and functional aspects of the native ACL. The femoral origin of the ACL has distinct direct and indirect insertions, as demonstrated by histology and 3-dimensional volume-rendered computed tomography.21 The direct insertion consists of dense collagen fibers anterior in the footprint that is attached to a bony depression immediately posterior to the lateral intercondylar ridge.19 Sasaki and colleagues22 found that these direct fibers extended a mean (SD) of 5.3 (1.1) mm posteriorly but did not continue to the posterior femoral articular cartilage. The indirect insertion consists of more posterior collagen fibers that extend to and blend into the articular cartilage of the posterior aspect of the lateral femoral condyle. Mean (SD) width of this membrane-like tissue, located between the direct insertion and the posterior femoral articular cartilage, was found by Sasaki and colleagues22 to be 4.4 (0.5) mm anteroposteriorly(Figure 2). This anterior band of ACL tissue with the direct insertion histologically corresponds to the fibers in the anterior, more isometric region of the femoral footprint. Conversely, the more posterior band of fibers with its indirect insertion histologically corresponds to the more anisometric region and is seen macroscopically as a fanlike projection extending to the posterior articular cartilage.7
The dense collagen fibers of the direct insertion and the more membrane-like indirect insertion regions of the femoral footprint of the native ACL suggest that these regions have different load-sharing characteristics. The direct fibers of the insertion form a firm, fixed attachment that allows for gradual load distribution into the subchondral bone. From a biomechanical point of view, this attachment is extremely important, a key ligament–bone link transmitting mechanical load to the joint.23 A recent kinematic analysis revealed that the indirect fibers in the posterior region of the footprint, adjacent to the posterior articular cartilage, contribute minimally to restraint of tibial translation and rotations during stability examination.24 This suggests it may be strategically wise to place a tunnel in the direct insertion region of the footprint—eccentrically anterior (high) in the footprint rather than in the centrum.
3. Isometric Considerations
Forty years ago, Artmann and Wirth25 reported that a nearly isometric region existed in the femur such that there is minimal elongation of the native ACL during knee motion. The biomechanical rationale for choosing an isometric region of an ACL graft is that it will maintain function throughout the range of flexion and extension. A nonisometric graft would be expected to slacken during a large portion of the flexion cycle and not restrain anterior translation of the tibia, or, if fixed at the wrong flexion angle, it could capture the knee and cause graft failure by excessive tension. These 2 theoretical undesirable effects from nonisometric graft placement are supported by many experimental and clinical studies demonstrating that nonisometric femoral tunnel placement at time of surgery can cause recurrent anterior laxity of the knee.26-28 Multiple studies have further clarified that the isometric characteristics of an ACL graft are largely determined by femoral positioning. The most isometric region of the femoral footprint is consistently shown to be localized eccentrically within the footprint, in a relatively narrow bandlike region that is proximal (deep) and anterior (along the lateral intercondylar ridge within the footprint)19,29,30 (Figure 3).
A large body of literature has demonstrated that a tunnel placed in the center of the femoral footprint is less isometric than a tunnel in the more anterior region.25,29,31,32 Indeed, the anterior position (high in the footprint) identified by Hefzy and colleagues29 demonstrated minimal anisometry with 1 to 4 mm of length change through the range of motion. In contrast, a central tunnel would be expected to demonstrate 5 to 7 mm of length change, whereas a lower graft (in the PL region of the footprint) would demonstrate about 1 cm of length change through the range of motion.31,32 As such, central grafts, or grafts placed in the PL portion of the femoral footprint, would be expected to see high tension or graft forces as the knee is flexed, or to lose tension completely if the graft is fixed at full extension.32
Importantly, Markolf and colleagues33 reported that the native ACL does not behave exactly in a so-called isometric fashion during the last 30° of extension. They showed that about 3 mm of retraction of a trial wire into the joint during the last 30° of extension (as measured with an isometer) is reasonable to achieve graft length changes approximating those of the intact ACL. Given this important caveat, a primary goal for ACLR is placement of the femoral tunnel within this isometric region so that the length change in the ACL graft is minimized to 3 mm from 30° to full flexion. In addition, results of a time-zero biomechanical study suggested better rotational control with anatomical femoral tunnel position than with an isometric femoral tunnel34 placed outside the femoral footprint. Therefore, maximizing isometry alone is not the goal; placing the graft in the most isometric region within the anatomical femoral footprint is desired. This isometric region in the footprint is in the histologic region that corresponds to the direct fibers. Again, this region is eccentrically located in the anterior (high) and proximal (deep) portion of the footprint.
4. Biomechanical Considerations
Multiple cadaveric studies have investigated the relationship between femoral tunnel positioning and time-zero stability. These studies often demonstrated superior time-zero control of knee stability, particularly in pivot type maneuvers, with a femoral tunnel placed more centrally in the femoral footprint than with a tunnel placed outside the footprint.34-37 However, an emerging body of literature is finding no significant difference in time-zero stability between an anteriorly placed femoral tunnel within the anatomical footprint (eccentrically located in the footprint) and a centrally placed graft.38,39 Returning to the more isometric tunnel position, still within the femoral footprint, would be expected to confer the benefits of an anatomically based graft position with the advantageous profile of improved isometry, as compared with a centrally placed or PL graft. Biomechanical studies40 have documented that ACL graft fibers placed posteriorly (low) in the footprint cause high graft forces in extension and, in some cases, graft rupture (Figure 4). Accordingly, the importance of reconstructing the posterior region of the footprint to better control time-zero stability is questioned.41
In addition to time-zero control of the stability examination, restoring the low tension-flexion pattern in the ACL graft to replicate the tension-flexion behavior of the native ACL is a fundamental biomechanical principle of ACLR.15,33,42,43 These studies have demonstrated that a femoral tunnel localized anterior (high) and proximal (deep) within the footprint better replicates the tension-flexion behavior of the native ACL, as compared with strategies that attempt to anatomically “fill the footprint.”40 Together, these studies have demonstrated that an eccentric position in the footprint, in the anterior (high) and proximal (deep) region, not only maximizes isometry and restores the direct fibers, but provides favorable time-zero stability and a low tension-flexion pattern biomechanically, particularly as compared with a tunnel in the more central or posterior region of the footprint.
5. Clinical Data
Clinical studies of the traditional transtibial ACLR have shown good results.44,45 However, when the tibial tunnel in the coronal plane was drilled vertical with respect to the medial joint line of the tibia, the transtibially placed femoral tunnel migrated anterior to the anatomical femoral footprint, often on the roof of the notch.10,14 This nonanatomical, vertical placement of the femoral tunnel led to failed normalization of knee kinematics.46-50 Indeed, a higher tension-flexion pattern was found in this nonanatomical “roof” position for the femoral tunnel as compared with the native ACL—a pattern that can result in either loss of flexion or recurrent instability.13,15,51
Clinical results of techniques used to create an anatomical ACLR centrally within the footprint have been mixed. Registry data showed that the revision rate at 4 years was higher with the AM portal technique (5.16%) than with transtibial drilling (3.20%).52 This higher rate may be associated with the more central placement of the femoral tunnel with the AM portal technique than with the transtibial technique, as shown in vivo with high-resolution magnetic resonance imaging.12 Recent reports have documented a higher rate of failure with DB or central ACLR approaches than with traditional transtibial techniques.53 As mentioned, in contrast to a more isometric position, a central femoral tunnel position would be expected to demonstrate 5 to 7 mm of length change, whereas moving the graft more posterior in the footprint (closer to the articular cartilage) would result in more than 1 cm of length change through the range of motion.31,32 As such, these more central grafts, or grafts placed even lower (more posterior) in the footprint, would be expected to see high tension in extension (if fixed in flexion), or to lose tension completely during flexion (if the graft is fixed at full extension).32 This may be a mechanistic cause of the high failure rate in the more posterior bundles of the DB approach.54
Together, these clinical data suggest that the femoral tunnel should be placed within the anatomical footprint of the ACL. However, within the footprint, a more eccentric femoral tunnel position capturing the isometric and direct region of the insertion may be preferable to a more central or posterior (low region) position.
Summary
Anatomical, histologic, isometric, biomechanical, and clinical data from more than 4 decades collectively point to an optimal position for the femoral tunnel within the femoral footprint. This position can be summarized by the acronym I.D.E.A.L., which refers to placing a femoral tunnel in a position that reproduces the Isometry of the native ACL, that covers the fibers of the Direct insertion histologically, that is Eccentrically located in the anterior (high) and proximal (deep) region of the footprint, that is Anatomical (within the footprint), and that replicates the Low tension-flexion pattern of the native ACL throughout the range of flexion and extension (Figure 5).
In vivo and in vitro studies as well as surgical experience suggest a need to avoid both (a) the nonanatomical vertical (roof) femoral tunnel placement that causes PCL impingement, high tension in the ACL graft in flexion, and ultimately graft stretch-out with instability and (b) the femoral tunnel placement in the posterior (lowest) region of the footprint that causes high tension in extension and can result in graft stretch-out with instability.13,15,39,40 The transtibial and AM portal techniques can both be effective in properly placing the femoral tunnel and restoring motion, stability, and function to the knee. Their effectiveness, however, depends on correct placement of the femoral tunnel. We think coming studies will focus on single-bundle ACLR and will be designed to improve the reliability of the transtibial and AM portal techniques for placing a femoral tunnel in keeping with the principles summarized by the I.D.E.A.L. acronym.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.
1. Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):699-706.
2. Siebold R, Schuhmacher P. Restoration of the tibial ACL footprint area and geometry using the modified insertion site table. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1845-1849.
3. Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy. 2012;28(6):872-881.
4. Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS. Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech. 2013;2(2):e99-e104.
5. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984-992.
6. Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15(7):741-749.
7. Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):336-344.
8. Siebold R, Schuhmacher P, Fernandez F, et al. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site [published correction appears in Knee Surg Sports Traumatol Arthrosc. 2014 Aug 23. Epub ahead of print]. Knee Surg Sports Traumatol Arthrosc. 2014 May 20. [Epub ahead of print]
9. Triantafyllidi E, Paschos NK, Goussia A, et al. The shape and the thickness of the anterior cruciate ligament along its length in relation to the posterior cruciate ligament: a cadaveric study. Arthroscopy. 2013;29(12):1963-1973.
10. Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194-199.
11. Ayerza MA, Múscolo DL, Costa-Paz M, Makino A, Rondón L. Comparison of sagittal obliquity of the reconstructed anterior cruciate ligament with native anterior cruciate ligament using magnetic resonance imaging. Arthroscopy. 2003;19(3):257-261.
12. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27(11):1511-1522.
13. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(5):567-574.
14. Kopf S, Forsythe B, Wong AK, et al. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427-1431.
15. Simmons R, Howell SM, Hull ML. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am. 2003;85(6):1018-1029.
16. Stanford FC, Kendoff D, Warren RF, Pearle AD. Native anterior cruciate ligament obliquity versus anterior cruciate ligament graft obliquity: an observational study using navigated measurements. Am J Sports Med. 2009;37(1):114-119.
17. Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218-1225.
18. Hutchinson MR, Ash SA. Resident’s ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy. 2003;19(9):931-935.
19. Fu FH, Jordan SS. The lateral intercondylar ridge—a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103-2104.
20. Smigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2014 Jun 28. [Epub ahead of print]
21. Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 suppl):S13-S20.
22. Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28(8):1135-1146.
23. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50(10):3306-3313.
24. Pathare NP, Nicholas SJ, Colbrunn R, McHugh MP. Kinematic analysis of the indirect femoral insertion of the anterior cruciate ligament: implications for anatomic femoral tunnel placement. Arthroscopy. 2014;30(11):1430-1438.
25. Artmann M, Wirth CJ. Investigation of the appropriate functional replacement of the anterior cruciate ligament (author’s transl) [in German]. Z Orthop Ihre Grenzgeb. 1974;112(1):160-165.
26. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;(6 suppl 1):S2-S12.
27. Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29(2):161-166.
28. O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop. 1992;(277):201-209.
29. Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17(2):208-216.
30. Zavras TD, Race A, Bull AM, Amis AA. A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):28-33.
31. Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534-1541.
32. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195.
33. Markolf KL, Burchfield DM, Shapiro MM, Davis BR, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg Am. 1996;78(11):1720-1727.
34. Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712-718.
35. Bedi A, Musahl V, Steuber V, et al. Transtibial versus anteromedial portal reaming in anterior cruciate ligament reconstruction: an anatomic and biomechanical evaluation of surgical technique. Arthroscopy. 2011;27(3):380-390.
36. Lim HC, Yoon YC, Wang JH, Bae JH. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: a cadaveric study of comparison of knee stability. Clin Orthop Surg. 2012;4(4):249-255.
37. Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19(3):297-304.
38. Cross MB, Musahl V, Bedi A, et al. Anteromedial versus central single-bundle graft position: which anatomic graft position to choose? Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1276-1281.
39. Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament–reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38(5):912-917.
40. Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107-118.
41. Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805-809.
42. Markolf KL, Burchfield DM, Shapiro MM, Cha CW, Finerman GA, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1996;78(11):1728-1734.
43. Wallace MP, Howell SM, Hull ML. In vivo tensile behavior of a four-bundle hamstring graft as a replacement for the anterior cruciate ligament. J Orthop Res. 1997;15(4):539-545.
44. Harner CD, Marks PH, Fu FH, Irrgang JJ, Silby MB, Mengato R. Anterior cruciate ligament reconstruction: endoscopic versus two-incision technique. Arthroscopy. 1994;10(5):502-512.
45. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision technique for reconstructing a torn anterior cruciate ligament using hamstring tendons. J Arthroscopy. 1999;15(6):594-606.
46. Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon–bone autografts: an in vivo study comparing tibial internal–external rotation. Am J Sports Med. 2007;35(2):189-196.
47. Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984-992.
48. Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006-2012.
49. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975-983.
50. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;(454):66-73.
51. Wallace MP, Hull ML, Howell SM. Can an isometer predict the tensile behavior of a double-looped hamstring graft during anterior cruciate ligament reconstruction? J Orthop Res. 1998;16(3):386-393.
52. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind MC. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy. 2013;29(1):98-105.
53. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40(4):800-807.
54. Ahn JH, Choi SH, Wang JH, Yoo JC, Yim HS, Chang MJ. Outcomes and second-look arthroscopic evaluation after double-bundle anterior cruciate ligament reconstruction with use of a single tibial tunnel. J Bone Joint Surg Am. 2011;93(20):1865-1872.