User login
Second Treatment for Prurigo Nodularis Approved by FDA
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
Storybooks Can Help Children Deal with Skin Conditions
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Experts Highlight Challenges That Remain for AI Devices in Triaging Skin Cancer
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Who’s Behind Cosmetic Procedures at MedSpas?
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
A 7-Month-Old Female Presented With Nail Changes
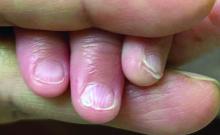
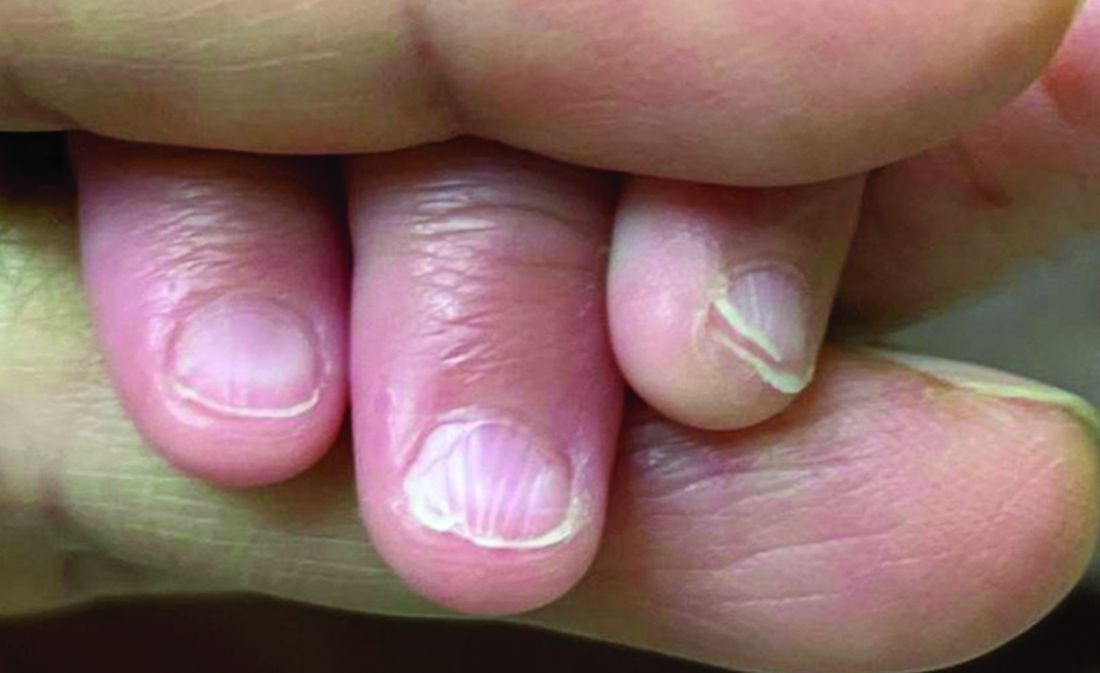
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
Given the clinical presentation and the absence of other systemic or dermatological findings, the diagnosis of chevron nails was made.
Discussion
The condition is characterized by transverse ridges on the nails that converge towards the center, forming a V or chevron shape. This condition was first described by Perry et al. and later by Shuster et al., who explained that the condition might result from axial growth of the nail with synchronous growth occurring from a chevron-shaped growing edge of the nail root. Alternatively, Shuster suggested that sequential growth, with localized variation in the nail production rate, could propagate a wave from the center of the nail to the edge.
The etiology of chevron nails is not well understood, but it is believed to result from temporary disruptions in the nail matrix, possibly related to minor illness or physiological stress during infancy.
In the case of our 7-month-old patient, the history of mild upper respiratory infections might have contributed to the development of chevron nails. However, the lack of other significant illness, skin involvement, or systemic findings supports the benign and self-limiting nature of this condition. Parents were reassured that chevron nails typically resolve on their own as the child grows and that no specific treatment is necessary.
Differential Diagnosis
The differential diagnosis of transverse nail changes in children includes other conditions such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita.
Trachyonychia, also known as “sandpaper nails,” trachyonychia is characterized by the roughening of the nail surface, giving it a dull and ridged appearance. The condition may affect all 20 nails and is often associated with underlying dermatological conditions such as lichen planus or alopecia areata. Unlike chevron nails, trachyonychia presents with more diffuse nail changes and does not typically feature the distinct V-shaped ridging seen in this patient.
Lichen planus is an inflammatory condition that can affect the skin, mucous membranes, and nails. Nail involvement in lichen planus can lead to longitudinal ridging, thinning, and sometimes even complete nail loss. The absence of other characteristic features of lichen planus, such as violaceous papules on the skin or white lacy patterns on mucous membranes (Wickham striae), makes this diagnosis less likely in our patient.
Darier disease, also known as keratosis follicularis, is a genetic disorder characterized by greasy, warty papules primarily on seborrheic areas of the skin, nail abnormalities, and sometimes mucosal involvement. Nail changes in Darier disease include longitudinal red and white streaks, V-shaped notching at the free edge of the nails, and subungual hyperkeratosis. These nail changes are more severe and distinct than the simple transverse ridging seen in chevron nails. The absence of other clinical signs of Darier disease, such as skin papules or characteristic nail notching, makes this diagnosis unlikely in our patient.
Pachyonychia congenita is a rare genetic disorder characterized by thickened nails (pachyonychia), painful plantar keratoderma, and sometimes oral leukokeratosis. The condition typically presents with significant nail thickening and other systemic findings, which were absent in our patient. The distinct pattern of V-shaped ridging observed in chevron nails does not align with the typical presentation of pachyonychia congenita.
Next Steps
No specific treatment is required for chevron nails. The condition is typically self-resolving, and the nails usually return to a normal appearance as the child continues to grow. Parents were advised to monitor the nails for any changes or new symptoms and were reassured about the benign nature of the findings. Follow-up was scheduled to ensure the resolution of the condition as the child develops.
Conclusion
Chevron nails are an important consideration in the differential diagnosis of transverse nail ridging in infants and young children. While the condition is benign and self-limiting, it is crucial to differentiate it from other nail dystrophies, such as trachyonychia, lichen planus, Darier disease, and pachyonychia congenita, which may require further investigation or intervention. Awareness of chevron nails can help prevent unnecessary worry and provide reassurance to parents and caregivers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Delano S, Belazarian L. Chevron nails: A normal variant in the pediatric population. Pediatr Dermatol. 2014 Jan-Feb;31(1):e24-5. doi: 10.1111/pde.12193.
John JM et al. Chevron nail — An under-recognised normal variant of nail development. Arch Dis Child. 2024 Jul 18;109(8):648. doi: 10.1136/archdischild-2024-326975.
Shuster S. The significance of chevron nails. Br J Dermatol. 1996;135:151–152. doi: 10.1046/j.1365-2133.1996.d01-961.x.
Starace M et al. Nail disorders in children. Skin Appendage Disord. 2018 Oct;4(4):217-229. doi: 10.1159/000486020.
There was no family history of similar nail findings and no relatives had a history of chronic skin conditions or congenital nail disorders.
On physical examination, several of the child’s fingernails exhibited distinct longitudinal ridges, with a characteristic pattern where the ridges converged at the center of the nail, forming a V-shape. There were no other concerning dermatologic findings, such as rashes, plaques, or erosions, and the skin and hair appeared otherwise normal. The rest of the physical exam was unremarkable.
Chronic Back Pain in Patients With Psoriasis, Uveitis, or Colitis: How Often Is It Axial Spondyloarthritis?
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with psoriasis, uveitis, or colitis who present with undiagnosed chronic back pain should be referred to a rheumatologist for the assessment of axial spondyloarthritis (axSpA), with MRI being a more accurate diagnostic method than clinical features.
METHODOLOGY:
- Researchers assessed the prevalence of axSpA according to the extra-articular presentation and human leukocyte antigen B27 (HLA-B27) status in two Canadian cohorts (SASPIC 1 and 2).
- Overall, 363 adult patients aged ≤ 45 years with psoriasis, uveitis, or colitis who presented with chronic undiagnosed back and/or buttock pain lasting 3 months or more were included.
- Participants were referred to rheumatologists with expertise in axSpA for structured diagnostic evaluations, including history, physical exam, levels of C-reactive protein, HLA-B27 status, and imaging studies.
- An MRI of the sacroiliac joints was conducted in all patients in the SASPIC-2 cohort and in 62.3% of those in the SASPIC-1 cohort.
- The primary outcome was the proportion of patients diagnosed with axSpA after final global evaluation, and the secondary outcome was the impact of MRI on diagnosis and classification.
TAKEAWAY:
- AxSpA diagnoses were made in 46.7% with psoriasis, 61.6% with uveitis, and 46.8% with colitis in the SASPIC-1 cohort and in 23.5%, 57.9%, and 23.3%, respectively, in the SASPIC-2 cohort.
- Being positive for HLA-B27 was linked to the presence of axSpA in 56%-88% of those in both the cohorts.
- Musculoskeletal clinical features were not helpful in differentiating between patients with and without axSpA.
- In both the cohorts, the MRI of the sacroiliac joints was indicative of axSpA in a significantly greater number of patients with psoriasis, uveitis, or colitis who were diagnosed with axSpA than in those not diagnosed with axSpA (P < .05 for all).
IN PRACTICE:
“Our data supports the benefit of recent referral recommendations that advocate referral to a rheumatologist of patients with chronic back pain and extra-articular features related to axSpA,” the authors wrote.
SOURCE:
The study was led by Walter P. Maksymowych, MB ChB, University of Alberta, Edmonton, Alberta, Canada. It was published online in Arthritis & Rheumatology.
LIMITATIONS:
MRI readers had to rely on their own expertise to decide if an MRI was indeed positive and thus indicative of axSpA. This study included only patients with undiagnosed back pain, and a longer follow-up duration could have led to a higher number of patients being diagnosed with axial inflammation. In SASPIC-1, local rheumatologists conducted MRI evaluations of the spinal lesions only when necessary, while in SASPIC-2, MRI of only the sacroiliac joints was required.
DISCLOSURES:
SASPIC-1 was supported by AbbVie Canada and Janssen Canada, and SASPIC-2 was supported by AbbVie Canada. The authors disclosed receiving grants, consulting fees, speaking fees, and/or honoraria and having other ties with AbbVie and several other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Federal Health Care Data Trends 2024
US Experience With Infliximab Biosimilars Suggests Need for More Development Incentives
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nonmelanoma Skin Cancer: Encouraging Data on Laser Treatment
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
Nemolizumab Benefits Seen in Adults, Teens With Atopic Dermatitis
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.