User login
MD-IQ only
Challenges in managing chronic pelvic pain in women
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
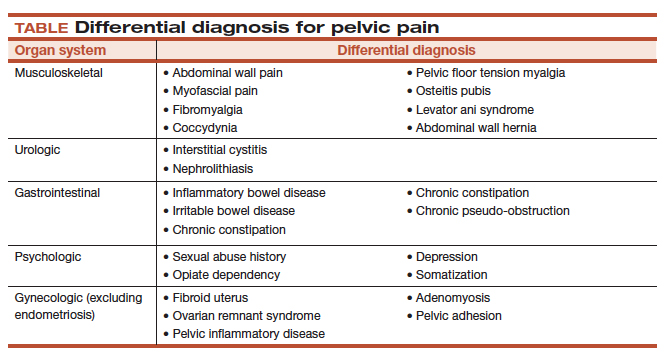
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
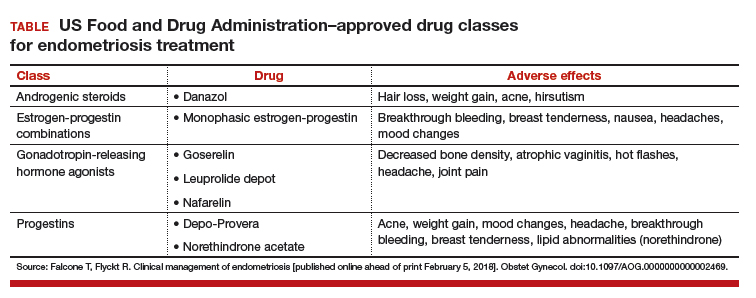
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
Medical science’s broad knowledge of endometriosis notwithstanding, “many questions remain unanswered” about the management of a condition that is often refractory to established therapies, observed Sawsan As-Sanie, MD, MPH, at the 2018 Pelvic Anatomy and Gynecologic Surgery Symposium meeting in Las Vegas, Nevada. Dr. As-Sanie is Associate Professor and Director, Minimally Invasive Gynecologic Surgery Fellowship, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor. How, then, should clinicians approach the challenge of caring for women with this enigmatic disease in the larger context of chronic pelvic pain, in which, as Dr. As-Sanie said, “one size never fits all”?
Complex correlation between endometriosis and CPP
Despite high prevalence and negative impact on the health and quality of life of women who suffer from endometriosis, Dr. As-Sanie emphasized, it remains unclear why only some women with endometriosis develop chronic pelvic pain (CPP) and why there is little, if any, correlation between disease severity and the intensity of pain.
The clinical approach to endometriosis and CPP can be frustrating for several reasons: there is minimal relationship between extent or location of disease with pain symptoms; there is no consistent relationship among inflammatory markers, nerve-fiber density, and pain symptoms; and pain can recur after medical and surgical therapy—often without evidence of recurrent endometriosis. Furthermore, the differential diagnosis of CPP is broad, and also includes adenomyosis, adhesions, chronic pelvic inflammatory disease, uterine fibroids, pelvic congestion, ovarian remnant, and residual ovarian syndrome. Chronic overlapping pain conditions are prevalent, too, including interstitial cystitis, irritable bowel syndrome, and vulvodynia, to name a few.1
_
CPP is not just a pain disorder
Dr. As-Sanie said that understanding of CPP must extend to include fatigue, memory difficulties, poor sleep, and heightened sensitivity to multiple sensory stimuli (e.g., sound and light).2 So what, she asked, do we know about endometriosis, chronic pelvic pain, and the brain? We know that CPP, with and without endometriosis, is associated with increased pain sensitivity and altered central nervous system structure and function.3-5 Central amplification of pain can lead to chronic pain independent of nociceptive signals, including multifocal, widespread pain; higher lifetime history of pain throughout the body; and pain triggered or exacerbated by stressors. And CPP brings with it other, potentially debilitating problems, including elevated distress, decreased activity, isolation, poor sleep, and maladaptive illness behaviors.
Finding, then addressing, the culprit
Identifying the underlying cause(s) of CPP in the individual woman should guide clinical care. This includes the decision to proceed with, or avoid, surgery. Remember: Patients with centralized pain respond differently to therapy; surgery is less likely to help relieve the pain.
Dr. As-Sanie offered several fundamental guidelines for managing CPP:
- Treat early, to prevent transition from acute to chronic pain; treatment delay increases connectivity between pain regulatory regions.
- Hysterectomy is not definitive therapy for all women with endometriosis or CPP.6
- Take a multisystem approach, comprising medical, behavioral, and interventional strategies.
- If an organ- or disease-based diagnostic and treatment approach does not work, reconsider the diagnosis; re-evaluate comorbid psychosocial variables; and consider treating centralized pain.
- Choice of treatment should include consideration of cost and adverse-effect profile.
- If one modality is ineffective, try another.
Continue to: What are the levels of evidence for centralized pain treatment?
What are the levels of evidence for centralized pain treatment?
Available pharmacotherapeutic agents have modest benefit, possibly because the population of pain patients is heterogeneous, with various underlying mechanisms of pain. And, Dr. As-Sanie pointed out, clinical tools do not currently exist to pre-emptively select the right medicine for individual patients.
Evidence is strong, Dr. As-Sanie noted, for dual reuptake-inhibitor antidepressants, such as tricyclic compounds (amitriptyline, cyclobenzaprine) and serotonin–norepinephrine reuptake inhibitors, and for anticonvulsants with analgesic properties (pregabalin, gabapentin). Evidence is “modest,” Dr. As-Sanie said, for tramadol, gamma hydroxybutyrate, and low-dose naltrexone, and “weak” for cannabinoids, human growth hormone, 5-hydroxytryptamine, tropisetron, and S-adenosyl-L-methionine. There is no evidence for using opioids, corticosteroids, nonsteroidal anti-inflammatory drugs, benzodiazepine and non–benzodiazepine hypnotics, or guaifenesin.7
When surgery or pharmacotherapy alone fail to yield the necessary outcome, consider adjunctive nonpharmacotherapy.8 For example, there is strong evidence for patient education, aerobic exercise, and cognitive behavior therapy; modest evidence for acupressure, acupuncture, strength training, hypnotherapy, biofeedback, trigger-point injection, and neuromodulation; but only weak evidence for chiropractic, manual and massage therapy, electrotherapy, and ultrasound. 7
_
With CPP, “one size never fits all”
Dr. As-Sanie concluded with a reminder that CPP can be the product of any of a range of underlying contributory causes. Pathology might stand foremost as you search for the source of pain and an effective treatment, but keep in mind that genetics, environment, co-existing pain conditions, the patient’s ability to cope, and her resilience and social support might play a role.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
- Veasley C, Clare D, Clauw DJ, et al; Chronic Pain Research Alliance. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment. 2015 analysis and policy recommendations. May 2015. http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf. Accessed December 10, 2018.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555.
- As-Sanie S, Harris RE, Harte SE, et al. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047-1055.
- As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006-1014.
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1-13.
- Brandsborg B. Pain following hysterectomy: epidemiological and clinical aspects. Dan Med J. 2012;59:B4374.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388-2395.
- Till SR, Wahl HN, As-Sanie S. The role of nonpharmacologic therapies in management of chronic pelvic pain: what to do when surgery fails. Curr Opin Obstet Gynecol. 2017;29:231-239.
Meaningful endometriosis treatment requires a holistic approach and an understanding of chronic pain
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
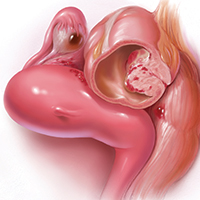
Laparoscopic hysterectomy with obliterated cul-de-sac needs specialist care
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
LAS VEGAS – When stage IV endometriosis with obliterated posterior cul-de-sac is discovered during laparoscopic hysterectomy, or suspected beforehand, women should be referred to a minimally invasive gynecologic surgery specialist because the procedure will be much more difficult, investigators said at the meeting sponsored by AAGL.
They reviewed 333 laparoscopic hysterectomies where endometriosis was discovered in the operating room. The disease is known to increase the complexity of hysterectomy; the investigators wanted to quantify the risk by endometriosis severity. Among their subjects, 237 women (71%) had stage I, II, or III endometriosis; 96 (29%) had stage IV disease, including 55 women (57%) with obliterated posterior cul-de-sacs.
Surgery was longer among stage IV cases (137 vs. 116 minutes), and there was greater blood loss; 66% of stage IV women required laparoscopic-modified radical hysterectomy versus about a quarter of women with stage I-III endometriosis.
A total of 93% required modified radical hysterectomies versus 29% of stage IV women with intact cul-de-sacs. Additional procedures were far more likely in this population, including salpingectomy, ureterolysis, enterolysis, cystoscopy, ureteral stenting, proctoscopy, bowel oversew, and anterior resection anastomosis. The differences all were statistically significant.
Among stage IV cases, mean operating time was longer in obliterated cul-de-sac cases (159 vs. 108 minutes), with higher blood loss, 100 mL versus 50 mL.
“Patients with obliterated cul-de-sacs identified intraoperatively should be referred to minimally invasive gynecologic surgeons because of the ... extra training required to safely perform [laparoscopic hysterectomy] with limited morbidity,” said lead investigator Alexandra Melnyk, MD, a University of Pittsburgh ob.gyn resident.
There was no industry funding and the investigators reported no disclosures.
SOURCE: Melnyk A et al. 2018 AAGL Global Congress, Abstract 81.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Elagolix: A new treatment for pelvic pain caused by endometriosis
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3
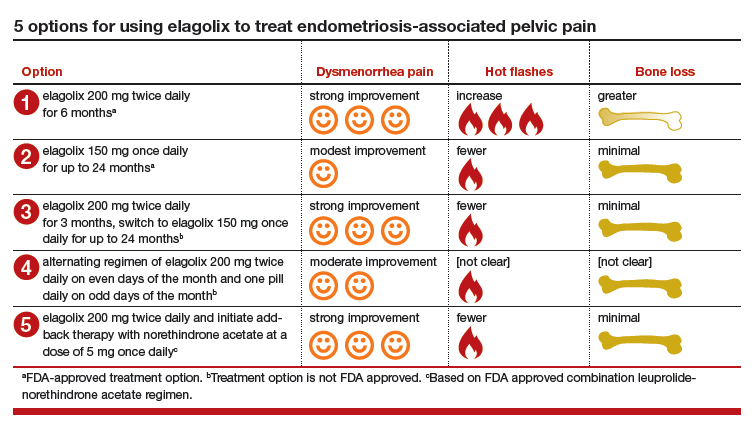
Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Diagnosis is an ongoing concern in endometriosis
according to a new survey by Health Union, a family of online health communities.
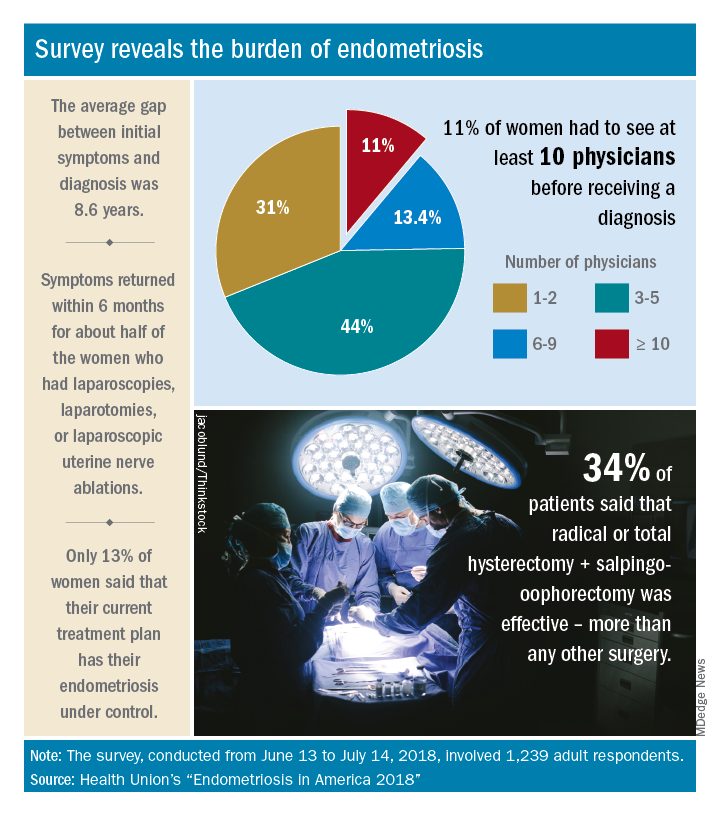
Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
Optimize the medical treatment of endometriosis—Use all available medications
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Deep infiltrating endometriosis: Evaluation and management
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Take-home points
- Specific MRI or TVUS protocols are highly accurate in making a nonsurgical diagnosis of deep infiltrating endometriosis (DIE).
- The combination of compelling clinical signs and symptoms and absence of imaging findings for DIE can be used to make a presumptive nonsurgical diagnosis of endometriosis.
- Empiric medical therapy may provide pain relief.
- Conservative treatment, including observation alone, may be considered in asymptomatic patients with DIE and in those with minimal pain.
- Before surgery, it is imperative to know lesion size, depth, circumferential bowel involvement, and location (or distance from the anal verge in cases of rectosigmoid lesion) to optimize surgical outcomes.
Endometriosis: Expert perspectives on medical and surgical management
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Take-home points
- Endometriosis management involves fluidity of care. Treatment approaches will change throughout a patient's reproductive life, depending on the patient's presenting symptoms and reproductive goals.
- Inform the patient of the disease process and how it may affect her menstrual pain symptoms and family planning.
- Educate patients so they may effectively participate in the management discussion. Hear the voice of the patient to make a tailored plan of care for each individual.
- Endometriosis can be a complex medical problem. Use a comprehensive multidisciplinary approach when appropriate.
Watch: Video roundtable–Endometriosis: Expert perspectives on medical and surgical management
Video roundtable–Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management
Endometriosis surgery on a young woman: $483,351 award
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.