User login
MD-IQ only
The ovarian remnant syndrome
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
Consider cutaneous endometriosis in women with umbilical lesions
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Claims data suggest endometriosis ups risk of chronic opioid use
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
NASHVILLE, TENN. – Women with endometriosis are at increased risk of chronic opioid use, compared with those without endometriosis, based on an analysis of claims data.
The 2-year rate of chronic opioid use was 4.4% among 36,373 women with endometriosis, compared with 1.1% among 2,172,936 women without endometriosis (odds ratio, 3.94) – a finding with important implications for physician prescribing considerations, Stephanie E. Chiuve, ScD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The OR was 3.76 after adjusting for age, race, and geographic region, said Dr. Chiuve of AbbVie, North Chicago.
Notably, the prevalence of other pain conditions, depression, anxiety, abuse of substances other than opioids, immunologic disorders, and use of opioids and other medications at baseline was higher in women with endometriosis versus those without. In any year, women with endometriosis were twice as likely to fill at least one opioid prescription, and were 3.5-4 times more likely to be a chronic opioid user than were women without endometriosis, she and her colleagues wrote in a poster presented at the meeting.
“Up to 60% of women with endometriosis experience significant chronic pain, including dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia,” they explained, adding that opioids may be prescribed for chronic pain management or for acute pain in the context of surgical procedures for endometriosis.
“This was due in part to various comorbidities that are also risk factors for chronic opioid use,” Dr. Chiuve said.
Women included in the study were aged 18-50 years (mean, 35 years), and were identified from a U.S. commercial insurance claims database and followed for 2 years after enrolling between January 2006 and December 2017. Chronic opioid use was defined as at least 120 days covered by an opioid dispensing or at least 10 fills of an opioid over a 1-year period during the 2-year follow-up study.
“With a less restrictive definition of chronic opioid use [of at least 6 fills] in any given year, the OR for chronic use comparing women with endometriosis to [the referent group] was similar [OR, 3.77],” the investigators wrote. “The OR for chronic use was attenuated to 2.88 after further adjustment for comorbidities and other medication use.”
Women with endometriosis in this study also experienced higher rates of opioid-associated clinical sequelae, they noted. For example, the adjusted ORs were 17.71 for an opioid dependence diagnosis, 12.52 for opioid overdose, and 10.39 for opioid use disorder treatment in chronic versus nonchronic users of opioids.
Additionally, chronic users were more likely to be prescribed high dose opioids (aOR, 6.45) and to be coprescribed benzodiazepines and sedatives (aORs, 5.87 and 3.78, respectively).
In fact, the findings of this study – though limited by factors such as the use of prescription fills rather than intake to measure exposure, and possible misclassification of endometriosis because of a lack of billing claims or undiagnosed disease – raise concerns about harmful opioid-related outcomes and dangerous prescribing patterns, they said.
In a separate poster presentation at the meeting, the researchers reported that independent risk factors for chronic opioid use in this study population were younger age (ORs, 0.90 and 0.72 for those aged 25-35 and 35-40 years, respectively, vs. those under age 25 years); concomitant chronic pain conditions, including fibromyalgia (OR, 1.49), chronic back pain (OR, 1.55), headaches/migraines (OR, 1.49), irritable bowel syndrome (OR, 1.61), and rheumatoid arthritis (OR, 2.52); the use of antipsychiatric drugs, including antidepressants (OR, 2.0), antipsychotics (OR, 1.66), and benzodiazepines (OR, 1.87); and baseline opioid use (OR, 3.95).
Hispanic ethnicity and Asian race predicted lower risk of chronic opioid use (ORs, 0.56 and 0.39, respectively), they found.
“These data contribute to the knowledge of potential risks of opioid use and may inform benefit-risk decision making of opioid use among women with endometriosis for management of endometriosis and its associated pain,” they concluded.
This study was funded by AbbVie. Dr. Chiuve is an employee of AbbVie, and she reported receiving stock/stock options.
REPORTING FROM ACOG 2019
Survey: Patient-provider communication regarding dyspareunia disappoints
NASHVILLE, TENN. – Many women with endometriosis experience dyspareunia, but they are largely unsatisfied when it comes to discussions with health care providers about their symptoms, the results of an online survey suggest.
Of 638 women with self-reported endometriosis who responded to the survey, 81% said they always or usually experience pain during intercourse, 51% described their pain as severe, and 49% said they experience pain lasting more than 24 hours, Roberta Renzelli-Cain, DO, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The results of our survey suggest that said Dr. Renzelli-Cain, director of the West Virginia National Center of Excellence in Women’s Health and an ob.gyn. at West Virginia University, Morgantown.
In fact, survey responses suggested that dyspareunia has a marked impact on quality of life; 69% of respondents said they find sexual intercourse unpleasant, 31% said they always or usually avoid intercourse, 44% strongly agreed that dyspareunia has affected their relationship with their spouse or partner, 63% said they worry that their spouse or partner will leave, and 63% said they feel depressed because of their dyspareunia, she and her colleagues found.
Most respondents (88%) discussed their symptoms with health care providers (HCPs), and 85% did so with their ob.gyn. Among the other HCPs who respondents spoke with about their dyspareunia were primary care physicians, nurse practitioners, emergency department doctors, fertility specialists, and pain specialists.
Among the reasons given for avoiding discussions with HCPs about painful intercourse were embarrassment (34% of respondents), thinking nothing would help (26%), the physician was a man (5%), and a feeling that the provider was not understanding (3%).
Overall, 18% of respondents said they received no advice from their HCPs regarding how to deal with their dyspareunia, and 39% found nothing that their HCPs suggested to be effective.
Advice given by HCPs included surgery, lubricant use, over-the-counter pain medication, and trying different sexual positions. The percentages of respondents receiving this advice, and the percentages who considered the advice effective, respectively, were 46%, 25% for surgery; 32%, 21% for lubricant use; 36%, 18% for OTC medication; and 21%, 14% for trying different sexual positions, the investigators said.
Importantly, 42% of respondent said they felt it would be easier to discuss dyspareunia if their HCP initiated the subject.
The findings are notable given that 6%-10% of women of childbearing age are affected by endometriosis, and about 30% of those women have related dyspareunia – a “challenging symptom associated with lower sexual functioning, as well as lower self-esteem, and body image,” the investigators wrote.
The 24-question English-language survey was conducted online among women aged 19 years or older who reported having endometriosis and dyspareunia. Participants were recruited via a social network for women with endometriosis (MyEndometriosisTeam.com) and invited by e-mail to participate.
Of the 32,865 invited participants, 361 U.S.-based women and 277 women from outside the United States completed the survey. Most (83%) were aged 19-29 years.
In this online survey, the majority of women reported suboptimal communication with HCPs when seeking help for dyspareunia, the investigators said, concluding that “these results were similar between the U.S.- and non-U.S.–based women, highlighting the need for better medical communication between patients and HCPs, and better advice for patients regarding dyspareunia.”
Dr. Renzelli-Cain reported having no relevant financial disclosures.
NASHVILLE, TENN. – Many women with endometriosis experience dyspareunia, but they are largely unsatisfied when it comes to discussions with health care providers about their symptoms, the results of an online survey suggest.
Of 638 women with self-reported endometriosis who responded to the survey, 81% said they always or usually experience pain during intercourse, 51% described their pain as severe, and 49% said they experience pain lasting more than 24 hours, Roberta Renzelli-Cain, DO, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The results of our survey suggest that said Dr. Renzelli-Cain, director of the West Virginia National Center of Excellence in Women’s Health and an ob.gyn. at West Virginia University, Morgantown.
In fact, survey responses suggested that dyspareunia has a marked impact on quality of life; 69% of respondents said they find sexual intercourse unpleasant, 31% said they always or usually avoid intercourse, 44% strongly agreed that dyspareunia has affected their relationship with their spouse or partner, 63% said they worry that their spouse or partner will leave, and 63% said they feel depressed because of their dyspareunia, she and her colleagues found.
Most respondents (88%) discussed their symptoms with health care providers (HCPs), and 85% did so with their ob.gyn. Among the other HCPs who respondents spoke with about their dyspareunia were primary care physicians, nurse practitioners, emergency department doctors, fertility specialists, and pain specialists.
Among the reasons given for avoiding discussions with HCPs about painful intercourse were embarrassment (34% of respondents), thinking nothing would help (26%), the physician was a man (5%), and a feeling that the provider was not understanding (3%).
Overall, 18% of respondents said they received no advice from their HCPs regarding how to deal with their dyspareunia, and 39% found nothing that their HCPs suggested to be effective.
Advice given by HCPs included surgery, lubricant use, over-the-counter pain medication, and trying different sexual positions. The percentages of respondents receiving this advice, and the percentages who considered the advice effective, respectively, were 46%, 25% for surgery; 32%, 21% for lubricant use; 36%, 18% for OTC medication; and 21%, 14% for trying different sexual positions, the investigators said.
Importantly, 42% of respondent said they felt it would be easier to discuss dyspareunia if their HCP initiated the subject.
The findings are notable given that 6%-10% of women of childbearing age are affected by endometriosis, and about 30% of those women have related dyspareunia – a “challenging symptom associated with lower sexual functioning, as well as lower self-esteem, and body image,” the investigators wrote.
The 24-question English-language survey was conducted online among women aged 19 years or older who reported having endometriosis and dyspareunia. Participants were recruited via a social network for women with endometriosis (MyEndometriosisTeam.com) and invited by e-mail to participate.
Of the 32,865 invited participants, 361 U.S.-based women and 277 women from outside the United States completed the survey. Most (83%) were aged 19-29 years.
In this online survey, the majority of women reported suboptimal communication with HCPs when seeking help for dyspareunia, the investigators said, concluding that “these results were similar between the U.S.- and non-U.S.–based women, highlighting the need for better medical communication between patients and HCPs, and better advice for patients regarding dyspareunia.”
Dr. Renzelli-Cain reported having no relevant financial disclosures.
NASHVILLE, TENN. – Many women with endometriosis experience dyspareunia, but they are largely unsatisfied when it comes to discussions with health care providers about their symptoms, the results of an online survey suggest.
Of 638 women with self-reported endometriosis who responded to the survey, 81% said they always or usually experience pain during intercourse, 51% described their pain as severe, and 49% said they experience pain lasting more than 24 hours, Roberta Renzelli-Cain, DO, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The results of our survey suggest that said Dr. Renzelli-Cain, director of the West Virginia National Center of Excellence in Women’s Health and an ob.gyn. at West Virginia University, Morgantown.
In fact, survey responses suggested that dyspareunia has a marked impact on quality of life; 69% of respondents said they find sexual intercourse unpleasant, 31% said they always or usually avoid intercourse, 44% strongly agreed that dyspareunia has affected their relationship with their spouse or partner, 63% said they worry that their spouse or partner will leave, and 63% said they feel depressed because of their dyspareunia, she and her colleagues found.
Most respondents (88%) discussed their symptoms with health care providers (HCPs), and 85% did so with their ob.gyn. Among the other HCPs who respondents spoke with about their dyspareunia were primary care physicians, nurse practitioners, emergency department doctors, fertility specialists, and pain specialists.
Among the reasons given for avoiding discussions with HCPs about painful intercourse were embarrassment (34% of respondents), thinking nothing would help (26%), the physician was a man (5%), and a feeling that the provider was not understanding (3%).
Overall, 18% of respondents said they received no advice from their HCPs regarding how to deal with their dyspareunia, and 39% found nothing that their HCPs suggested to be effective.
Advice given by HCPs included surgery, lubricant use, over-the-counter pain medication, and trying different sexual positions. The percentages of respondents receiving this advice, and the percentages who considered the advice effective, respectively, were 46%, 25% for surgery; 32%, 21% for lubricant use; 36%, 18% for OTC medication; and 21%, 14% for trying different sexual positions, the investigators said.
Importantly, 42% of respondent said they felt it would be easier to discuss dyspareunia if their HCP initiated the subject.
The findings are notable given that 6%-10% of women of childbearing age are affected by endometriosis, and about 30% of those women have related dyspareunia – a “challenging symptom associated with lower sexual functioning, as well as lower self-esteem, and body image,” the investigators wrote.
The 24-question English-language survey was conducted online among women aged 19 years or older who reported having endometriosis and dyspareunia. Participants were recruited via a social network for women with endometriosis (MyEndometriosisTeam.com) and invited by e-mail to participate.
Of the 32,865 invited participants, 361 U.S.-based women and 277 women from outside the United States completed the survey. Most (83%) were aged 19-29 years.
In this online survey, the majority of women reported suboptimal communication with HCPs when seeking help for dyspareunia, the investigators said, concluding that “these results were similar between the U.S.- and non-U.S.–based women, highlighting the need for better medical communication between patients and HCPs, and better advice for patients regarding dyspareunia.”
Dr. Renzelli-Cain reported having no relevant financial disclosures.
REPORTING FROM ACOG 2019
Modern surgical techniques for gastrointestinal endometriosis
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
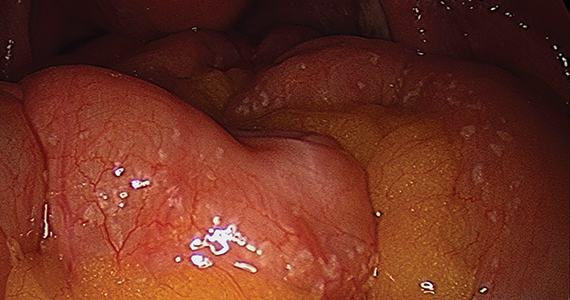
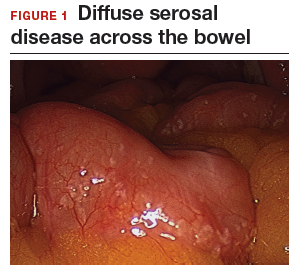
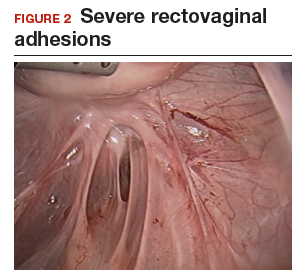
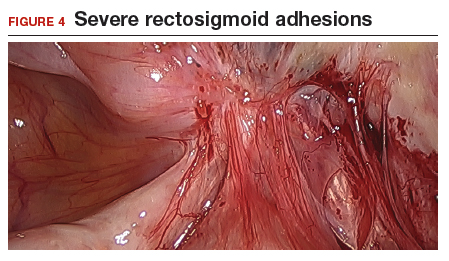
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.


Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
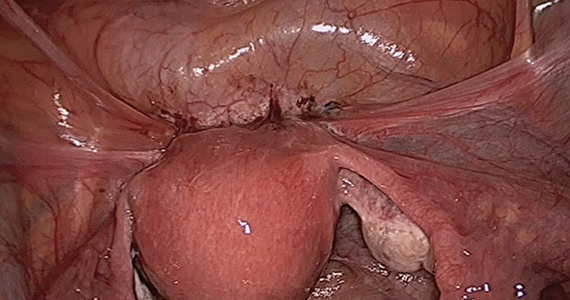
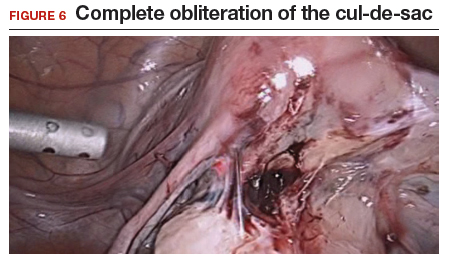
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.
Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
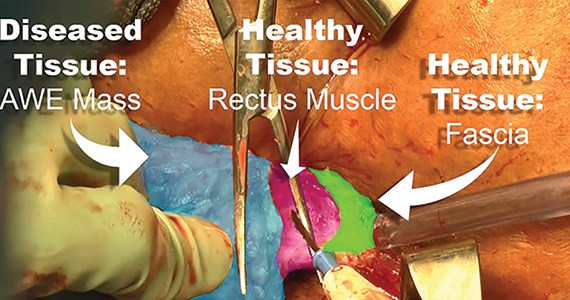
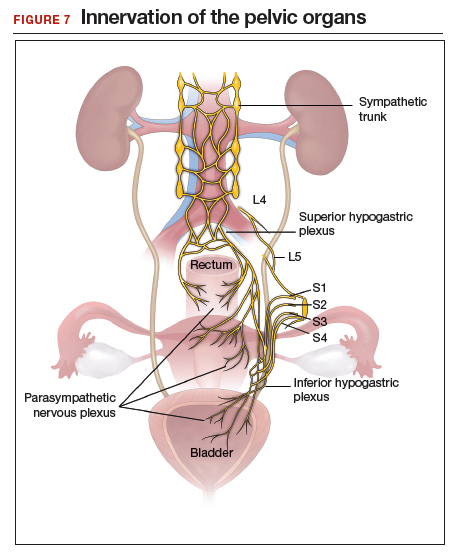
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2
Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
Excision of abdominal wall endometriosis
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

Endometriosis, defined by the ectopic growth of functioning endometrial glands and stroma,1,2 usually affects the peritoneal cavity. However, endometriosis has been identified in the pneumothorax, brain, and within the extraperitoneum, such as the abdominal wall.1-3 Incidence of abdominal wall endometriosis can be up to 12%.3-5 If patients report symptoms, they can include abdominal pain, a palpable mass, pelvic pain consistent with endometriosis, and bleeding from involvement of the overlying skin. Abdominal wall endometriosis can be surgically resected, with complete resolution and a low rate of recurrence.
In the following video, we review the diagnosis of abdominal wall endometriosis, including our imaging of choice, and treatment options. In addition, we illustrate a surgical technique for the excision of abdominal wall endometriosis in a 38-year-old patient with symptomatic disease. We conclude with a review of key surgical steps.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211:363.e1-e5.
- Horton JD, Dezee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207-212.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41-44.
- Chang Y, Tsai EM, Long CY, et al. Abdominal wall endometriosis. J Reproductive Med. 2009;54:155-159.
Diagnosing endometriosis: Is laparoscopy the gold standard?
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
This CME supplement to OBG Management provides readers with an understanding of the following topics:
- The relationship between chronic pelvic pain and endometriosis
- The limitations of laparocopy in the diagnosis of endometriosis
- The emerging role of imaging in the diagnosis of endometriosis
Click Here to read the full supplement and access a posttest and evaluation for CME credit.
Genitourinary endometriosis: Diagnosis and management
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18
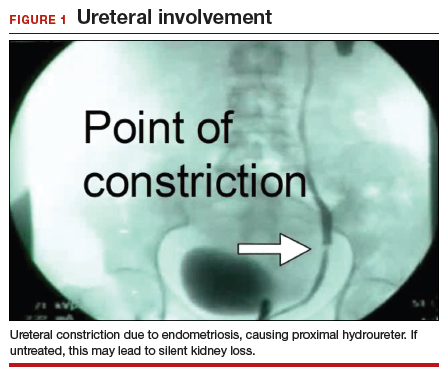
Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36
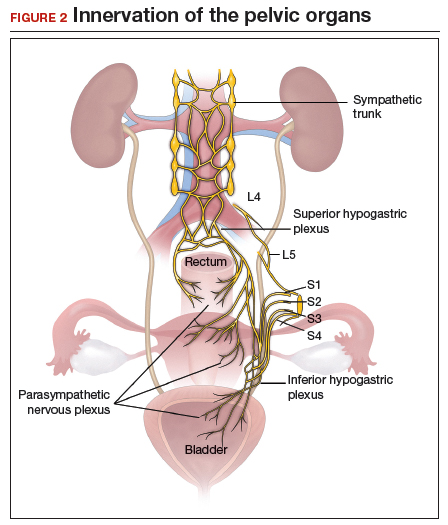
Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43
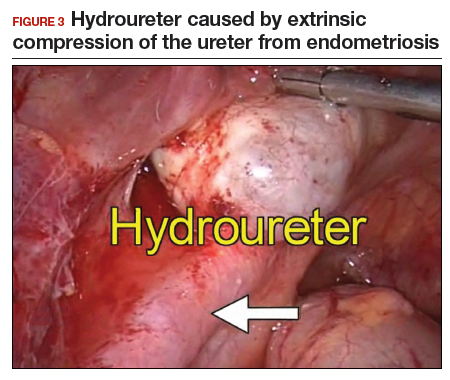
Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
For pelvic pain, think outside the lower body
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
EXPERT ANALYSIS FROM PAGS
Endometriosis surgery: Women can expect years-long benefits
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: Endometriosis excision improves quality of life for at least 7 years, even when women have conservative, fertility-sparing surgery.
Major finding: The overall score on the Endometriosis Health Profile-30 fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at the 7-year follow-up.
Study details: A review of 61 cases
Disclosures: The investigators didn’t report any disclosures.
Source: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.