User login
Have you asked your patients: What is your ideal outpatient gynecology experience?
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.
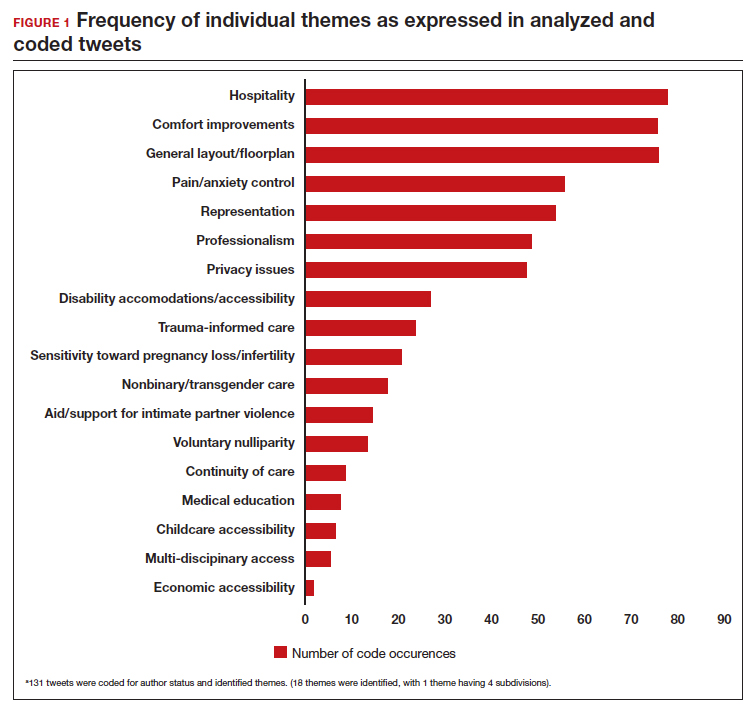
Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).
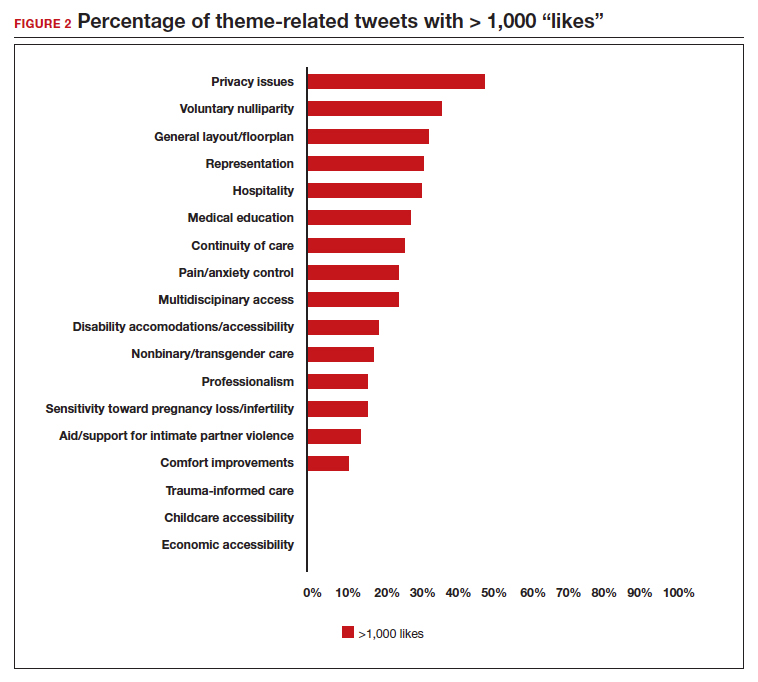
Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).
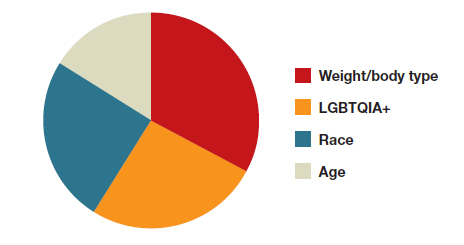
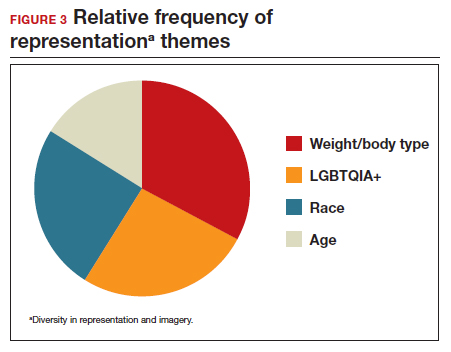
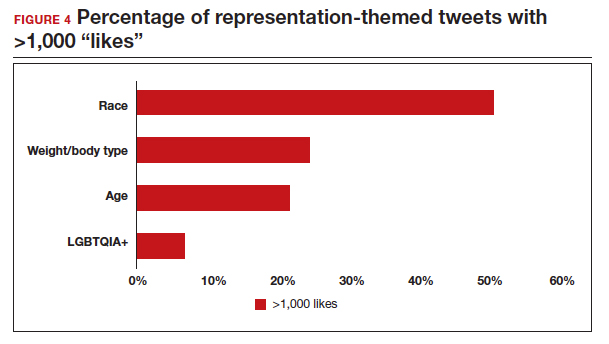
A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).
Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
The HPV vaccine: Time for ObGyn physicians to up our game
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
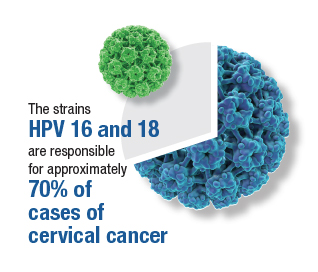
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.
Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
‘Vaginal dryness’ can be fatal. No, really.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
Young women rate top sources for STI self-testing
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
FROM SEXUALLY TRANSMITTED DISEASES
Pelvic yoga, physical conditioning both improve urinary incontinence
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
PHILADELPHIA – Both a pelvic yoga program and a general physical conditioning program for incontinence led to improvements in women’s incontinence, according to a study presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“As clinicians, we’re usually focused on treatments that we ourselves can prescribe, perform, or administer. We’re not as good as recommending or supporting treatment or management strategies that don’t rely on costly or intensive visits with clinical specialists,” lead author Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco, said in an interview.
“But our findings suggest that women who try pelvic yoga as a complementary management strategy for genitourinary conditions like urinary incontinence that often emerge in midlife are likely to experience substantial improvement in their genitourinary symptoms and function,” Dr. Huang said. “Some of these improvements may be shared with other forms of low-impact physical movement or exercise.”
The 240 participants from communities around three Northern California sites ranged in age from 45 to 90 years old, with an average age of 62, and all had at least daily urgency, stress, or mixed-type urinary incontinence. While most were White women, 40% identified as racial/ethnic minorities, including 14% Hispanic, 6% Black, 16% Asian American, and 4% multiracial.
Participants needed to be able to walk two blocks on level ground and get from a supine to a standing position on their own, but they should not have recently participated in any organized yoga or physical conditioning exercise classes. They also needed to forgo behavioral, invasive, or pharmacologic treatments for urinary incontinence for at least 3 months. The trial ran from 2019 to 2022, with most women completing the 3-month program virtually once the pandemic began.
The 121 women randomly assigned to the pelvic yoga program had twice-weekly group instruction by trained yoga instructors and once-weekly individual practice. The practice focused on 16 standard Hatha yoga poses in standing, seated, supine, and prone positions with an emphasis on precise alignment of their postures during each pose. Yoga props, such as blocks, straps, or bolsters, were available to minimize risk of injury and to accommodate women with less flexibility.
The 119 women randomly assigned to the physical conditioning group spent the same amount of group and individual class time on skeletal muscle stretching and strengthening exercises. These exercises focused on strengthening and stretching exercises for the upper and lower extremities in standing, sitting, or supine positions. The only props needed were exercise straps and handles and an exercise mat, and the program was designed to be safe and feasible for women across all ages.
Both groups received standard self-management pamphlets describing pelvic floor muscle exercises and recommendations on timed urination and urging suppression. After early dropouts from both arms, 107 women remained for analysis in the pelvic yoga group, and 113 women remained for analysis in the physical conditioning group.
Researchers assessed participants’ genitourinary quality of life at baseline and after 3 months using the Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire (IIQ), and Patient Perception of Bladder Condition (PPBC). At baseline, the women’s average scores were 38.8 on the UDI-6, 101 on the IIQ, and 3.4 on the PPBC.
About one-third of the women in both groups attended all 24 group classes, and 57% of women in both groups attended 20-23 classes. In addition, 65% of the women in the pelvic yoga group and 73% of the women in the physical conditioning group completed all of the recommended additional hours of individual practice. Only 15% of pelvic yoga participants and 9% of physical conditioning participants completed less than 80% of the recommended individual practice hours. No differences in participation between the groups were statistically significant.
“Over 3 months, scores on all genitourinary quality of life measures improved by more than the minimum important difference thresholds in the pelvic yoga group,” the researchers reported, but only the UDI-6 score improved significantly – albeit still modestly – in the pelvic yoga group, compared with the physical conditioning group. Average scores improved 18.9 points in the pelvic yoga group and 13.1 points in the physical conditioning group (5.8-point difference; P = .02).
The scores on the IIQ improved an average 38.5 points in the pelvic yoga group and 31.4 points in the physical conditioning group (P = .48). PPBC scores improved 0.7 points in both groups.
“While yoga may offer benefits for genitourinary quality of life, it may not offer superior benefits compared to equivalent-time practice of other activities that improve general physical function,” Dr. Huang told attendees.
“The bottom line is that physical activity toward incontinence is a helpful technique,” Stephanie Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health and medical director for the Menopause Society, said in an interview regarding the findings. Urinary incontinence is under-recognized, Dr. Faubion said, “because women are embarrassed, so they don’t bring it up, so it doesn’t get managed.” But it’s a common problem, so clinicians need to ask patients about it, she said.
“We should realize that, in midlife and older age, genitourinary health is often connected to overall health,” Dr. Huang said in an interview. “We shouldn’t focus exclusively on treatments that are directed solely at the genital or lower urinary tract organs or tissues. We should consider the ways in which women’s urinary and sexual function are influenced by other aspects of their physical and cognitive health.”
The research was funded by the National Institutes of Health. Dr. Huang and Dr. Faubion had no disclosures.
AT NAMS 2023
False-positive Pap smear may indicate genitourinary syndrome
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY ANNUAL MEETING
This symptom signals UTI in 83% of cases
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study: Unexpected vaginal bleeding rises after COVID vaccination
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
2023 Update on abnormal uterine bleeding
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
The safety of vaginal estrogen in breast cancer survivors
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
However, concomitant use of vaginal estrogen and aromatase inhibitors was associated with a higher risk for breast cancer recurrence than was use of vaginal estrogen alone.
Although this important study’s findings have the limitations characteristic of observational studies, its large size and careful analyses suggest that
Dr. Kaunitz is associate chairman, department of obstetrics and gynecology, University of Florida College of Medicine, Jacksonville. This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
However, concomitant use of vaginal estrogen and aromatase inhibitors was associated with a higher risk for breast cancer recurrence than was use of vaginal estrogen alone.
Although this important study’s findings have the limitations characteristic of observational studies, its large size and careful analyses suggest that
Dr. Kaunitz is associate chairman, department of obstetrics and gynecology, University of Florida College of Medicine, Jacksonville. This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
However, concomitant use of vaginal estrogen and aromatase inhibitors was associated with a higher risk for breast cancer recurrence than was use of vaginal estrogen alone.
Although this important study’s findings have the limitations characteristic of observational studies, its large size and careful analyses suggest that
Dr. Kaunitz is associate chairman, department of obstetrics and gynecology, University of Florida College of Medicine, Jacksonville. This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.