User login
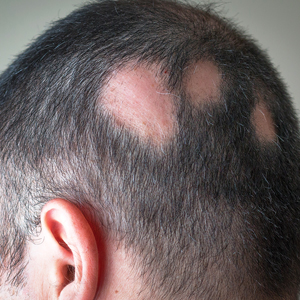
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
Culprits of Medication-Induced Telogen Effluvium, Part 2
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s) such as heparin and its derivatives.
- Infection, illness, or hospitalization around the time of initiation of the suspected culprit medication may complicate identification of the inciting cause and may contribute to TE.
- Angiotensin-converting enzyme inhibitors and β-blockers are unlikely culprits of medication-induced TE, and the benefits of discontinuing a suspected culprit medication should be weighed carefully against the risks of medication cessation.
Androgenetic Alopecia: What Works?
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.
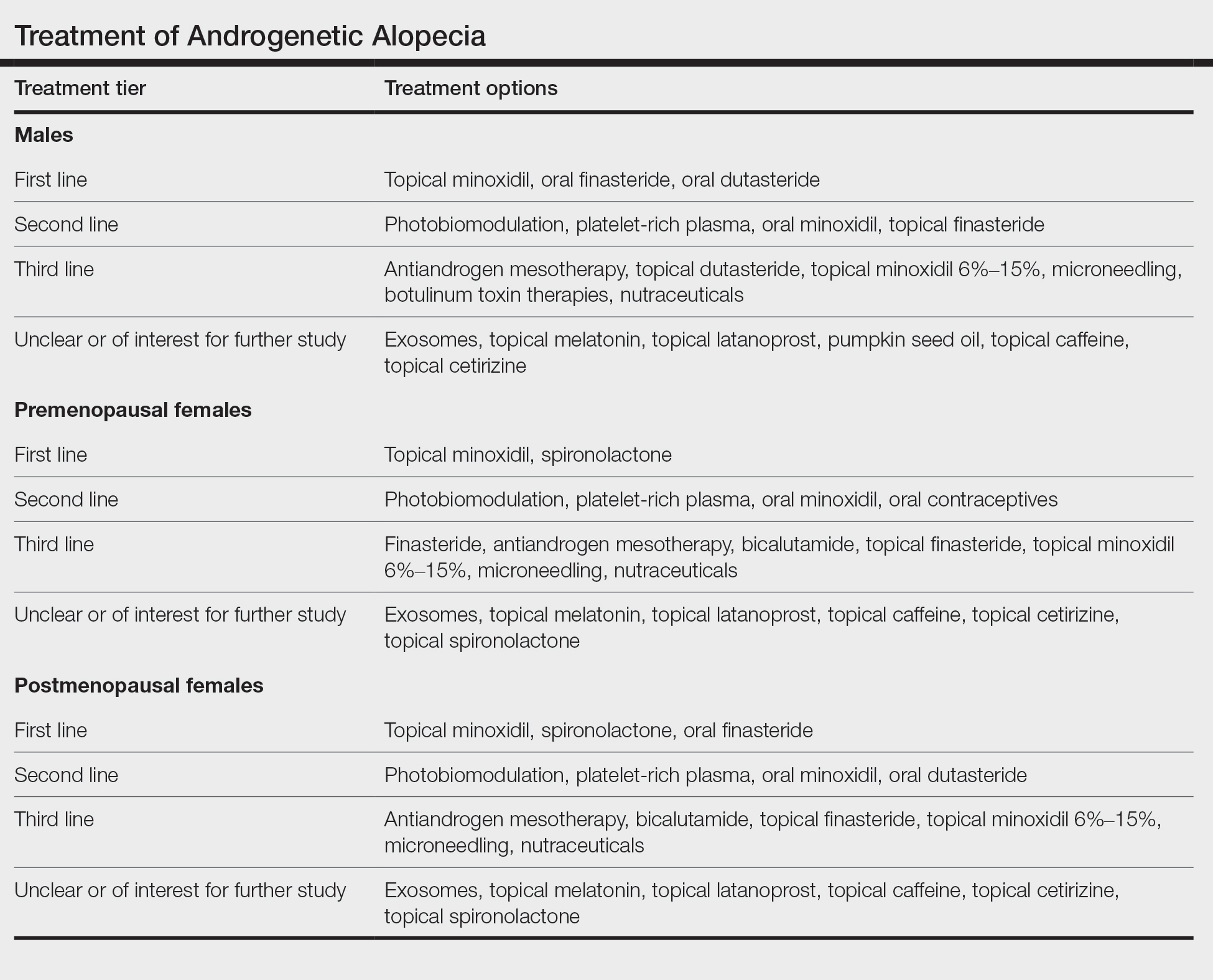
When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
US Dermatologic Drug Approvals Rose Between 2012 and 2022
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
Navigating Hair Loss in Medical School: Experiences of 2 Young Black Women
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
Practice Points
- Hair loss is a common dermatologic concern among Black women and can represent a diagnostic challenge to dermatologists who may not be familiar with textured hair.
- Dermatologists should practice cultural sensitivity and provide relevant recommendations to Black patients dealing with hair loss.
Pilot study educates barbers about pseudofolliculitis barbae
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
FROM JAMA DERMATOLOGY
Treatment and Current Policies on Pseudofolliculitis Barbae in the US Military
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.
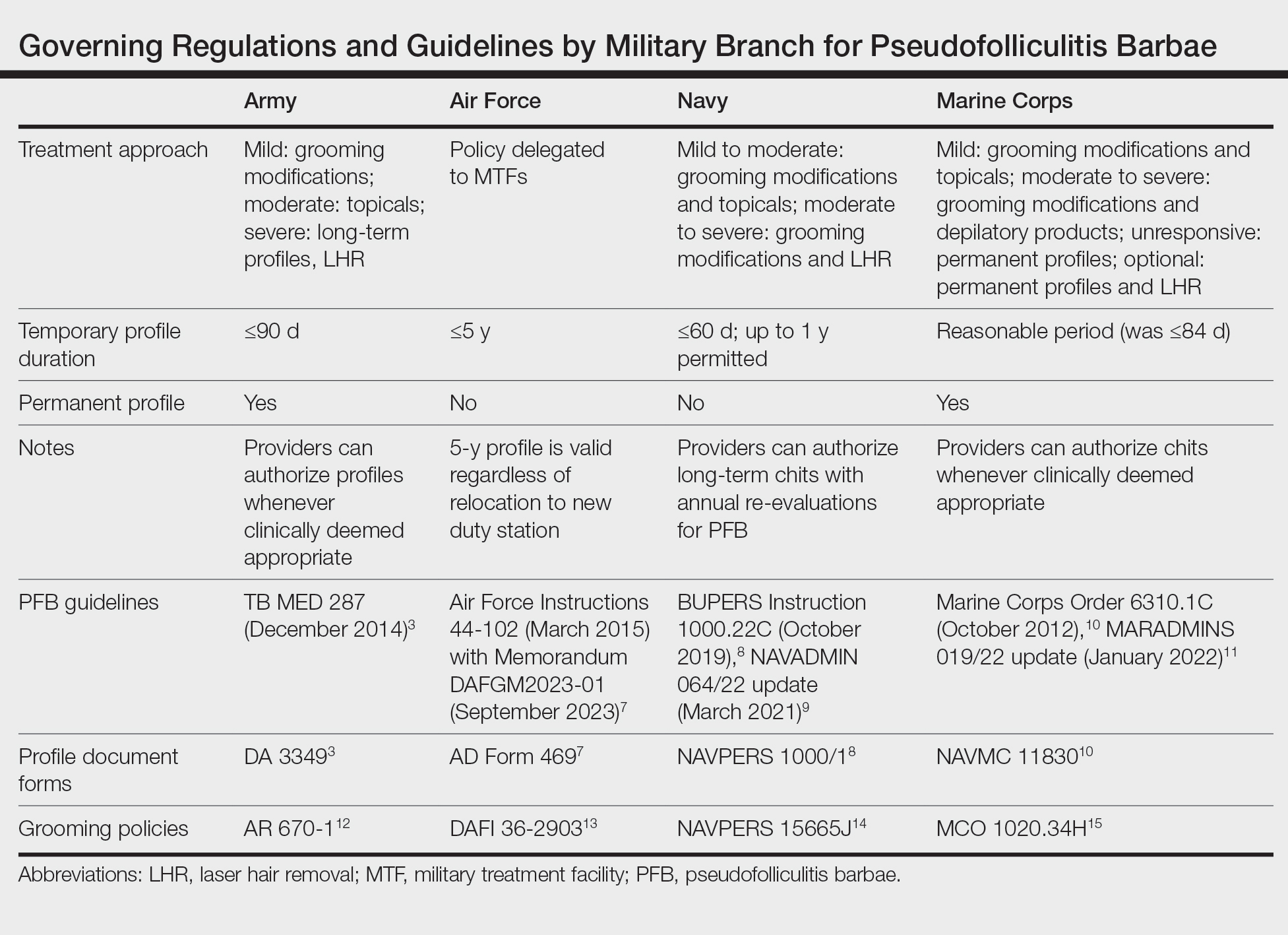
Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
Pseudofolliculitis barbae (PFB)(also referred to as razor bumps) is a skin disease of the face and neck caused by shaving and remains prevalent in the US Military. As the sharpened ends of curly hair strands penetrate back into the epidermis, they can trigger inflammatory reactions, leading to papules and pustules as well as hyperpigmentation and scarring.1 Although anyone with thick curly hair can develop PFB, Black individuals are disproportionately affected, with 45% to 83% reporting PFB symptoms compared with 18% of White individuals.2 In this article, we review the treatments and current policies on PFB in the military.
Treatment Options
Shaving Guidelines—Daily shaving remains the grooming standard for US service members who are encouraged to follow prescribed grooming techniques to prevent mild cases of PFB, defined as having “few, scattered papules with scant hair growth of the beard area,” according to the technical bulletin of the US Army, which provides the most detailed guidelines among the branches.3 The bulletin recommends hydrating the face with warm water, followed by a preshave lotion and shaving with a single pass superiorly to inferiorly. Following shaving, postrazor hydration lotion is recommended. Single-bladed razors are preferred, as there is less trauma to existing PFB and less potential for hair retraction under the epidermis, though multibladed razors can be used with adequate preshave and postrazor hydration.4 Shaving can be undertaken in the evening to ensure adequate time for preshave preparation and postshave hydration. Waterless shaving uses waterless soaps or lotions containing α-hydroxy acid just prior to shaving in lieu of preshaving and postshaving procedures.4
Topical Medications—For PFB cases that are recalcitrant to management by changes in shaving, topical retinoids are commonly prescribed, as they reduce follicular hyperkeratosis that may lead to PFB.5 The Army medical bulletin recommends a pea-sized amount of tretinoin cream or gel 0.025%, 0.05%, or 0.1% for moderate cases, defined as “heavier beard growth, more scattered papules, no evidence of pustules or denudation.”3 Adapalene cream 0.1% may be used instead of tretinoin for sensitive skin. Oral doxycycline or topical benzoyl peroxide–clindamycin may be added for secondary bacterial skin infections. Clinical trials have demonstrated that combination benzoyl peroxide–clindamycin significantly reduces papules and pustules in up to 63% of patients with PFB (P<.029).6 Azelaic acid can be prescribed for prominent postinflammatory hyperpigmentation. The bulletin also suggests depilatories such as barium sulfide to obtund the hair ends and make them less likely to re-enter the skin surface, though it notes low compliance rates due to strong sulfur odor, messy application, and irritation and reactions to ingredients in the preparations.4
Shaving Waivers and Laser Hair Removal—The definitive treatment of PFB is to not shave, and a shaving waiver or laser hair removal (LHR) are the best options for severe PFB or PFB refractory to other treatments. A shaving waiver (or shaving profile) allows for growth of up to 0.25 inches of facial hair with maintenance of the length using clippers. The shaving profile typically is issued by the referring primary care manager (PCM) but also can be recommended by a dermatologist. Each military branch implements different regulations on shaving profiles, which complicates care delivery at joint-service military treatment facilities (MTFs). The Table provides guidelines that govern the management of PFB by the US Army, Air Force, Navy, and Marine Corps. The issuance and duration of shaving waivers vary by service.

Laser hair removal therapy uses high-wavelength lasers that largely bypass the melanocyte-containing basal layer and selectively target hair follicles located deeper in the skin, which results in precise hair reduction with relative sparing of the epidermis.16 Clinical trials at military clinics have demonstrated that treatments with the 1064-nm long-pulse Nd:YAG laser generally are safe and effective in impeding hair growth in Fitzpatrick skin types IV, V, and VI.17 This laser, along with the Alexandrite 755-nm long-pulse laser for Fitzpatrick skin types I to III, is widely available and used for LHR at MTFs that house dermatologists. Eflornithine cream 13.9%, which is approved by the US Food and Drug Administration to treat hirsutism, can be used as monotherapy for treatment of PFB and has a synergistic depilatory effect in PFB patients when used in conjunction with LHR.18,19 Laser hair removal treatments can induce a permanent change in facial hair density and pattern of growth. Side effects and complications of LHR include discomfort during treatment and, in rare instances, blistering and dyspigmentation of the skin as well as paradoxical hair growth.17
TRICARE, the uniformed health care program, covers LHR in the civilian sector if the following criteria are met: candidates must work in an environment that may require breathing protection, and they must have failed conservative therapy; an MTF dermatologist must evaluate each case and attempt LHR at an MTF to limit outside referrals; and the MTF dermatologist must process each outside referral claim to completion and ensure that the LHR is rendered by a civilian dermatologist and is consistent with branch-specific policies.20
Service Policies on PFB
Army—
The technical bulletin also allows a permanent shaving profile for soldiers who demonstrate a severe adverse reaction to treatment or progression of the disease despite a trial of all these methods.3 The regulation stipulates that 0.125 to 0.25 inches of beard growth usually is sufficient to prevent PFB. Patients on profiles must be re-evaluated by a PCM or a dermatologist at least once a year.3
Air Force—Air Force Instruction 44-102 delegates PFB treatment and management strategies to each individual MTF, which allows for decentralized management of PFB, resulting in treatment protocols that can differ from one MTF to another.7 Since 2020, waivers have been valid for 5 years regardless of deployment or permanent change of station location. Previously, shaving profiles required annual renewals.7 Special duties, such as Honor Guard, Thunderbirds, Special Warfare Mission Support, recruiters, and the Air Force Band, often follow the professional appearance standards more strictly. Until recently, the Honor Guard used to reassign those with long-term medical shaving waivers but now allows airmen with shaving profiles to serve with exceptions (eg, shaving before ceremonies).21
Navy—BUPERS (Bureau of Naval Personnel) Instruction 1000.22C divides PFB severity into 2 categories.8 For mild to moderate PFB cases, topical tretinoin and adapalene are recommended, along with improved shaving hygiene practices. As an alternative to topical steroids, topical eflornithine monotherapy can be used twice daily for 60 days. For moderate to severe PFB cases, continued grooming modifications and LHR at military clinics with dermatologic services are expected.8
Naval administrative memorandum NAVADMIN 064/22 (released in 2022) no longer requires sailors with a shaving “chit,” or shaving waiver, to fully grow out their beards.9 Sailors may now outline or edge their beards as long as doing so does not trigger a skin irritation or outbreak. Furthermore, sailors are no longer required to carry a physical copy of their shaving chit at all times. Laser hair removal for sailors with PFB is now considered optional, whereas sailors with severe PFB were previously expected to receive LHR.9
Marine Corps—The Marine Corps endorses a 4-phase treatment algorithm (Table). As of January 2022, permanent shaving chits are authorized. Marines no longer need to carry physical copies of their chits at all times and cannot be separated from service because of PFB.10 New updates explicitly state that medical officers, not the commanding officers, now have final authority for granting shaving chits.11
Final Thoughts
The Army provides the most detailed bulletin, which defines the clinical features and treatments expected for each stage of PFB. All 4 service branches permit temporary profiles, albeit for different lengths of time. However, only the Army and the Marine Corps currently authorize permanent shaving waivers if all treatments mentioned in their respective bulletins have failed.
The Air Force has adopted the most decentralized approach, in which each MTF is responsible for implementing its own treatment protocols and definitions. Air Force regulations now authorize a 5-year shaving profile for medical reasons, including PFB. The Air Force also has spearheaded efforts to create more inclusive policies. A study of 10,000 active-duty male Air Force members conducted by Air Force physicians found that shaving waivers were associated with longer times to promotion. Although self-identified race was not independently linked to longer promotion times, more Black service members were affected because of a higher prevalence of PFB and shaving profiles.22
The Navy has outlined the most specific timeline for therapy for PFB. The regulations allow a 60-day temporary shaving chit that expires on the day of the appointment with the dermatologist or PCM. Although sailors were previously mandated to fully grow out their beards without modifications during the 60-day shaving chit period, Navy leadership recently overturned these requirements. However, permanent shaving chits are still not authorized in the Navy.
Service members are trying to destigmatize shaving profiles and facial hair in our military. A Facebook group called DoD Beard Action Initiative has more than 17,000 members and was created in 2021 to compile testimonies and data regarding the effects of PFB on airmen.23 Soldiers also have petitioned for growing beards in the garrison environment with more than 100,000 signatures, citing that North Atlantic Treaty Organization allied nations permit beard growth in their respective ranks.24 A Sikh marine captain recently won a lawsuit against the US Department of the Navy to maintain a beard with a turban in uniform on religious grounds.25
The clean-shaven look remains standard across the military, not only for uniformity of appearance but also for safety concerns. The Naval Safety Center’s ALSAFE report concluded that any facial hair impedes a tight fit of gas masks, which can be lethal in chemical warfare. However, the report did not explore how different hair lengths would affect the seal of gas masks.26 It remains unknown how 0.25 inch of facial hair, the maximum hair length authorized for most PFB patients, affects the seal. Department of Defense occupational health researchers currently are assessing how each specific facial hair length diminishes the effectiveness of gas masks.27
Furthermore, the COVID-19 pandemic has led to frequent N95 respirator wear in the military. It is likely that growing a long beard disrupts the fitting of N95 respirators and could endanger service members, especially in clinical settings. However, one study confirmed that 0.125 inch of facial hair still results in 98% effectiveness in filtering particles for the respirator wearers.28 Although unverified, it is surmisable that 0.25 inch of facial hair will likely not render all respirators useless. However, current Occupational Safety and Health Administration guidelines require fit tests to be conducted only on clean-shaven faces.29 Effectively, service members with facial hair cannot be fit-tested for N95 respirators.
More research is needed to optimize treatment protocols and regulations for PFB in our military. As long as the current grooming standards remain in place, treatment of PFB will be a controversial topic. Guidelines will need to be continuously updated to balance the needs of our service members and to minimize risk to unit safety and mission success. Department of Defense Instruction 6130.03, Volume 1, revised in late 2022, now no longer designates PFB as a condition that disqualifies a candidate from entering service in any military branch.30 The Department of Defense is demonstrating active research and adoption of policies regarding PFB that will benefit our service members.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27.
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. Published December 10, 2014. Accessed November 16, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/tbmed287.pdf
- Tshudy M, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:52-57.
- Kligman AM, Mills OH. Pseudofolliculitis of the beard and topically applied tretinoin. J Am Acad Dermatol. 1973;107:551-552.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- US Department of the Air Force. Air Force Instruction 44-102. Medical Care Management. March 17, 2015. Updated July 13, 2022. Accessed October 1, 2022. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-102/afi44-102.pdf
- Chief of Naval Personnel, Department of the Navy. BUPERS Instruction 1000.22C. Management of Navy Uniformed Personnel Diagnosed With Pseudofolliculitis Barbae. October 8, 2019. Accessed November 16, 2023. https://www.mynavyhr.navy.mil/Portals/55/Reference/Instructions/BUPERS/BUPERSINST%201000.22C%20Signed.pdf?ver=iby4-mqcxYCTM1t3AOsqxA%3D%3D
- Chief of Naval Operations, Department of the Navy. NAVADMIN 064/22. BUPERSINST 1000,22C Management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 9, 2022. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064.txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Commandant of the Marine Corps, Department of the Navy. Marine Corps Order 6310.1C. Pseudofolliculitis Barbae. October 9, 2012. Accessed November 16, 2023. https://www.marines.mil/Portals/1/Publications/MCO%206310.1C.pdf
- US Marine Corps. Advance Notification of Change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). January 21, 2022. Accessed November 16, 2023. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900
- Department of the Army. Army Regulation 670-1. Uniform and Insignia. Wear and Appearance of Army Uniforms and Insignia. January 26, 2021. Accessed November 19, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Department of the Air Force. Department of the Air Force Guidance Memorandum to DAFI 36-2903, Dress and Personal Appearance of United States Air Force and United States Space Force Personnel. Published March 31, 2023. Accessed November 20, 2023. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR website. Accessed November 19, 2023. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- US Marine Corps. Marine Corps Uniform Regulations. Published May 1, 2018. Accessed November 20, 2023. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Xia Y, Cho SC, Howard RS, et al. Topical eflornithine hydrochloride improves effectiveness of standard laser hair removal for treating pseudofolliculitis barbae: a randomized, double-blinded, placebo-controlled trial. J Am Acad Dermatol. 2012;67:694-699.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525. doi:10.1111/jocd.14027
- TRICARE operations manual 6010.59-M. Supplemental Health Care Program (SHCP)—chapter 17. Contractor responsibilities. Military Health System and Defense Health Agency website. Revised November 5, 2021. Accessed November 16, 2023. https://manuals.health.mil/pages/DisplayManualHtmlFile/2022-08-31/AsOf/TO15/C17S3.html
- Air Force Honor Guard: Recruiting. Accessed November 16, 2023. https://www.honorguard.af.mil/About-Us/Recruiting/
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- DoD Beard Action Initiative Facebook group. Accessed November 5, 2023. https://www.facebook.com/groups/326068578791063/
- Geske R. Petition gets 95K signatures in push for facial hair for soldiers. KWTX. February 4, 2021. Accessed November 16, 2023. https://www.kwtx.com/2021/02/04/petition-gets-95k-signatures-in-push-for-facial-hair-for-soldiers/
- Athey P. A Sikh marine is now allowed to wear a turban in uniform. Marine Corps Times. October 5, 2021. Accessed November 16, 2023. https://www.marinecorpstimes.com/news/your-marine-corps/2021/10/05/a-sikh-marine-is-now-allowed-to-wear-a-turban-in-uniform
- US Department of the Navy. Face Seal Guidance update (ALSAFE 18-008). Naval Safety Center. Published November 18, 2018. Accessed October 22, 2022. https://navalsafetycommand.navy.mil/Portals/29/ALSAFE18-008.pdf
- Garland C. Navy and Marine Corps to study facial hair’s effect on gas masks, lawsuit reveals. Stars and Stripes. January 25, 2022. Accessed November 16, 2023. https://www.stripes.com/branches/navy/2022-01-25/court-oversee-navy-marine-gas-mask-facial-hair-study-4410015.html
- Floyd EL, Henry JB, Johnson DL. Influence of facial hair length, coarseness, and areal density on seal leakage of a tight-fitting half-face respirator. J Occup Environ Hyg. 2018;15:334-340.
- Occupational Safety and Health Administration. Occupational Safety and Health Standards 1910.134 App A. Fit Testing Procedures—General Requirements. US Department of Labor. April 23, 1998. Updated August 4, 2004. Accessed November 16, 2023. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- US Department of Defense. DoD Instruction 6130.03, Volume 1. Medical Standards for Military Service: Appointment, Enlistment, or Induction. November 16, 2022. Accessed November 16, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D
Practice Points
- Pseudofolliculitis barbae (PFB) is common among US service members due to grooming standards in the military.
- Each military branch follows separate yet related guidelines to treat PFB.
- The best treatment for severe or refractory cases of PFB is a long-term shaving restriction or laser hair removal.
Culprits of Medication-Induced Telogen Effluvium, Part 1
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s).
- A careful history of new medications and dose adjustments 1 to 6 months prior to notable hair loss may identify the most likely inciting cause.
- Medication-induced TE often improves with cessation or dose reduction of the culprit medication.
Autoimmune Skin Diseases Linked To Risk Of Adverse Pregnancy Outcomes
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
FROM ACR 2023
Laser epilation may reduce pilonidal disease recurrences when added to standard care
according to the results of a randomized trial.
The study, recently published in JAMA Surgery, enrolled 302 patients ages 11-21 with pilonidal disease. Half of the participants were assigned to receive LE (laser hair removal) plus standard treatment (improved hygiene plus mechanical or chemical hair removal), and half were assigned to receive standard care alone.
At 1 year, 10.4% of the patients who had received LE plus standard treatment had experienced a recurrence of pilonidal disease, compared with 33.6% of patients in the standard treatment group (P < .001). Rates were based on the data available on 96 patients in the LE group and 134 patients in the standard care group.
“These results provide further evidence that laser epilation is safe, well-tolerated, and should be available as an initial treatment option or adjunct treatment modality for all eligible patients,” first author Peter C. Minneci, MD, chair of surgery at Nemours Children’s Health, Delaware Valley, Wilmington, Del, said in a press release reporting the results. “There have been few comparative studies that have investigated recurrence rates after LE versus other treatment modalities,” he and his coauthors wrote in the study, noting that the study “was the first, to our knowledge, to compare LE as an adjunct to standard care versus standard care alone and demonstrate a decrease in recurrence rates.”
Pilonidal disease, a common condition, results when cysts form between the buttocks and is most common in adolescents and young adults. It is thought to recur about 33% of the time, with most cases recurring within 1 year of treatment.
In practice, there are large variations in management strategies for pilonidal disease because evidence for an ideal treatment approach is lacking, Dr. Minneci and coauthors wrote. Although lifestyle modifications and nonepilation hair removal strategies have been linked to a reduced need for surgery, compliance with these strategies is low. Additionally, recurrence contributes to “a high degree of psychosocial stress in patients, who often miss school or sports and may avoid social activities,” Dr. Minneci said in the press release. Therefore, some practitioners have begun using LE – which uses selective thermolysis to remove the hair shaft, follicle, and bulb – as an adjunct to standard treatments in the hopes of avoiding surgery.
A few studies have shown LE is effective in reducing pilonidal disease recurrence, but these studies had small sample sizes, according to the authors.
Study methods
The randomized, nonblinded clinical trial was conducted between 2017 and 2022 at Nationwide Children’s Hospital, Columbus, and enrolled patients aged 11-21 years with a history of pilonidal disease, who did not have active disease.
Those in the control group (151 patients) had an in-person clinic visit where they received education and training about hair removal in the gluteal cleft, and were provided with supplies for hair removal (chemical epilation or shaving) for 6 months (standard of care). Those in the LE group (151 patients) received standard of care therapy, and also received one LE treatment every 4-6 weeks for a total of five treatments. They were encouraged to perform hair removal using chemical or mechanical depilation between visits.
At the 1-year follow-up, data were available in 96 patients in the LE group and 134 patients in the standard care group. At that time, the proportion of those who had a recurrence within 1 year was significantly lower in the LE group than in the standard care group (mean difference, –23.2%; 95% CI, –33.2% to –13.1%; P < .001).
In addition, over the course of a year, those in the LE-treated group had significantly higher Child Attitude Toward Illness scores, indicating that they felt more positively about their illness at 6 months than participants in the standard care group. There were no differences between the groups in terms of patient or caregiver disability days, patient- or caregiver-reported health-related quality of life, health care satisfaction, or perceived stigma. In the LE group, no burns were reported, and no inability to tolerate treatment because of pain.
The study had several limitations, including the potential for participation bias, and because of a loss to follow-up, primary and secondary outcomes were missing data points, which was higher in the LE group. Loss to follow-up in the LE arm increased after 6 months, when laser treatments ended, with many of those patients not completing surveys at 9 and 12 months. The hospital’s pilonidal clinic shut down for 3 months during the COVID-19 pandemic, and when the clinic reopened, 15 patients in the LE arm withdrew from the study.
|In the press release, Dr. Minneci said that confirmation of the effectiveness of LE could help justify insurance coverage for pilonidal disease, noting that LE is usually not covered with insurance, and a course of treatment could cost $800-$1,500.
Dr. Minneci and four of the other six coauthors reported receiving grants from Patient-Centered Outcomes Research Institute during the conduct of the study. One author reported receiving grants from the National Institute on Minority Health and Health Disparities outside the submitted work. The research was funded by a grant from the Patient-Centered Outcomes Research Institute.
according to the results of a randomized trial.
The study, recently published in JAMA Surgery, enrolled 302 patients ages 11-21 with pilonidal disease. Half of the participants were assigned to receive LE (laser hair removal) plus standard treatment (improved hygiene plus mechanical or chemical hair removal), and half were assigned to receive standard care alone.
At 1 year, 10.4% of the patients who had received LE plus standard treatment had experienced a recurrence of pilonidal disease, compared with 33.6% of patients in the standard treatment group (P < .001). Rates were based on the data available on 96 patients in the LE group and 134 patients in the standard care group.
“These results provide further evidence that laser epilation is safe, well-tolerated, and should be available as an initial treatment option or adjunct treatment modality for all eligible patients,” first author Peter C. Minneci, MD, chair of surgery at Nemours Children’s Health, Delaware Valley, Wilmington, Del, said in a press release reporting the results. “There have been few comparative studies that have investigated recurrence rates after LE versus other treatment modalities,” he and his coauthors wrote in the study, noting that the study “was the first, to our knowledge, to compare LE as an adjunct to standard care versus standard care alone and demonstrate a decrease in recurrence rates.”
Pilonidal disease, a common condition, results when cysts form between the buttocks and is most common in adolescents and young adults. It is thought to recur about 33% of the time, with most cases recurring within 1 year of treatment.
In practice, there are large variations in management strategies for pilonidal disease because evidence for an ideal treatment approach is lacking, Dr. Minneci and coauthors wrote. Although lifestyle modifications and nonepilation hair removal strategies have been linked to a reduced need for surgery, compliance with these strategies is low. Additionally, recurrence contributes to “a high degree of psychosocial stress in patients, who often miss school or sports and may avoid social activities,” Dr. Minneci said in the press release. Therefore, some practitioners have begun using LE – which uses selective thermolysis to remove the hair shaft, follicle, and bulb – as an adjunct to standard treatments in the hopes of avoiding surgery.
A few studies have shown LE is effective in reducing pilonidal disease recurrence, but these studies had small sample sizes, according to the authors.
Study methods
The randomized, nonblinded clinical trial was conducted between 2017 and 2022 at Nationwide Children’s Hospital, Columbus, and enrolled patients aged 11-21 years with a history of pilonidal disease, who did not have active disease.
Those in the control group (151 patients) had an in-person clinic visit where they received education and training about hair removal in the gluteal cleft, and were provided with supplies for hair removal (chemical epilation or shaving) for 6 months (standard of care). Those in the LE group (151 patients) received standard of care therapy, and also received one LE treatment every 4-6 weeks for a total of five treatments. They were encouraged to perform hair removal using chemical or mechanical depilation between visits.
At the 1-year follow-up, data were available in 96 patients in the LE group and 134 patients in the standard care group. At that time, the proportion of those who had a recurrence within 1 year was significantly lower in the LE group than in the standard care group (mean difference, –23.2%; 95% CI, –33.2% to –13.1%; P < .001).
In addition, over the course of a year, those in the LE-treated group had significantly higher Child Attitude Toward Illness scores, indicating that they felt more positively about their illness at 6 months than participants in the standard care group. There were no differences between the groups in terms of patient or caregiver disability days, patient- or caregiver-reported health-related quality of life, health care satisfaction, or perceived stigma. In the LE group, no burns were reported, and no inability to tolerate treatment because of pain.
The study had several limitations, including the potential for participation bias, and because of a loss to follow-up, primary and secondary outcomes were missing data points, which was higher in the LE group. Loss to follow-up in the LE arm increased after 6 months, when laser treatments ended, with many of those patients not completing surveys at 9 and 12 months. The hospital’s pilonidal clinic shut down for 3 months during the COVID-19 pandemic, and when the clinic reopened, 15 patients in the LE arm withdrew from the study.
|In the press release, Dr. Minneci said that confirmation of the effectiveness of LE could help justify insurance coverage for pilonidal disease, noting that LE is usually not covered with insurance, and a course of treatment could cost $800-$1,500.
Dr. Minneci and four of the other six coauthors reported receiving grants from Patient-Centered Outcomes Research Institute during the conduct of the study. One author reported receiving grants from the National Institute on Minority Health and Health Disparities outside the submitted work. The research was funded by a grant from the Patient-Centered Outcomes Research Institute.
according to the results of a randomized trial.
The study, recently published in JAMA Surgery, enrolled 302 patients ages 11-21 with pilonidal disease. Half of the participants were assigned to receive LE (laser hair removal) plus standard treatment (improved hygiene plus mechanical or chemical hair removal), and half were assigned to receive standard care alone.
At 1 year, 10.4% of the patients who had received LE plus standard treatment had experienced a recurrence of pilonidal disease, compared with 33.6% of patients in the standard treatment group (P < .001). Rates were based on the data available on 96 patients in the LE group and 134 patients in the standard care group.
“These results provide further evidence that laser epilation is safe, well-tolerated, and should be available as an initial treatment option or adjunct treatment modality for all eligible patients,” first author Peter C. Minneci, MD, chair of surgery at Nemours Children’s Health, Delaware Valley, Wilmington, Del, said in a press release reporting the results. “There have been few comparative studies that have investigated recurrence rates after LE versus other treatment modalities,” he and his coauthors wrote in the study, noting that the study “was the first, to our knowledge, to compare LE as an adjunct to standard care versus standard care alone and demonstrate a decrease in recurrence rates.”
Pilonidal disease, a common condition, results when cysts form between the buttocks and is most common in adolescents and young adults. It is thought to recur about 33% of the time, with most cases recurring within 1 year of treatment.
In practice, there are large variations in management strategies for pilonidal disease because evidence for an ideal treatment approach is lacking, Dr. Minneci and coauthors wrote. Although lifestyle modifications and nonepilation hair removal strategies have been linked to a reduced need for surgery, compliance with these strategies is low. Additionally, recurrence contributes to “a high degree of psychosocial stress in patients, who often miss school or sports and may avoid social activities,” Dr. Minneci said in the press release. Therefore, some practitioners have begun using LE – which uses selective thermolysis to remove the hair shaft, follicle, and bulb – as an adjunct to standard treatments in the hopes of avoiding surgery.
A few studies have shown LE is effective in reducing pilonidal disease recurrence, but these studies had small sample sizes, according to the authors.
Study methods
The randomized, nonblinded clinical trial was conducted between 2017 and 2022 at Nationwide Children’s Hospital, Columbus, and enrolled patients aged 11-21 years with a history of pilonidal disease, who did not have active disease.
Those in the control group (151 patients) had an in-person clinic visit where they received education and training about hair removal in the gluteal cleft, and were provided with supplies for hair removal (chemical epilation or shaving) for 6 months (standard of care). Those in the LE group (151 patients) received standard of care therapy, and also received one LE treatment every 4-6 weeks for a total of five treatments. They were encouraged to perform hair removal using chemical or mechanical depilation between visits.
At the 1-year follow-up, data were available in 96 patients in the LE group and 134 patients in the standard care group. At that time, the proportion of those who had a recurrence within 1 year was significantly lower in the LE group than in the standard care group (mean difference, –23.2%; 95% CI, –33.2% to –13.1%; P < .001).
In addition, over the course of a year, those in the LE-treated group had significantly higher Child Attitude Toward Illness scores, indicating that they felt more positively about their illness at 6 months than participants in the standard care group. There were no differences between the groups in terms of patient or caregiver disability days, patient- or caregiver-reported health-related quality of life, health care satisfaction, or perceived stigma. In the LE group, no burns were reported, and no inability to tolerate treatment because of pain.
The study had several limitations, including the potential for participation bias, and because of a loss to follow-up, primary and secondary outcomes were missing data points, which was higher in the LE group. Loss to follow-up in the LE arm increased after 6 months, when laser treatments ended, with many of those patients not completing surveys at 9 and 12 months. The hospital’s pilonidal clinic shut down for 3 months during the COVID-19 pandemic, and when the clinic reopened, 15 patients in the LE arm withdrew from the study.
|In the press release, Dr. Minneci said that confirmation of the effectiveness of LE could help justify insurance coverage for pilonidal disease, noting that LE is usually not covered with insurance, and a course of treatment could cost $800-$1,500.
Dr. Minneci and four of the other six coauthors reported receiving grants from Patient-Centered Outcomes Research Institute during the conduct of the study. One author reported receiving grants from the National Institute on Minority Health and Health Disparities outside the submitted work. The research was funded by a grant from the Patient-Centered Outcomes Research Institute.
FROM JAMA SURGERY