User login
‘Game changer’ semaglutide halves diabetes risk from obesity
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Continuous cuffless monitoring may fuel lifestyle change to lower BP
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
FROM AHA HYPERTENSION 2022
Ultrasonic renal denervation passes 2-month test in uncontrolled HTN: RADIANCE II
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.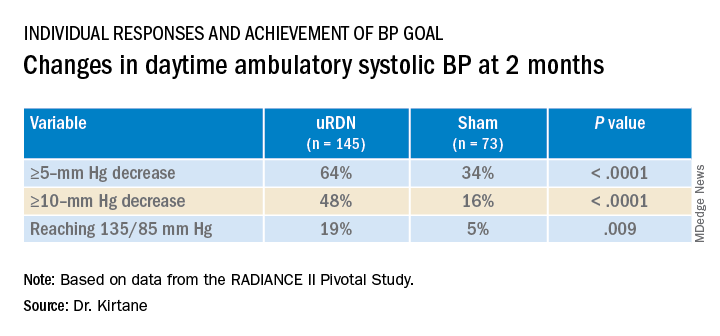
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).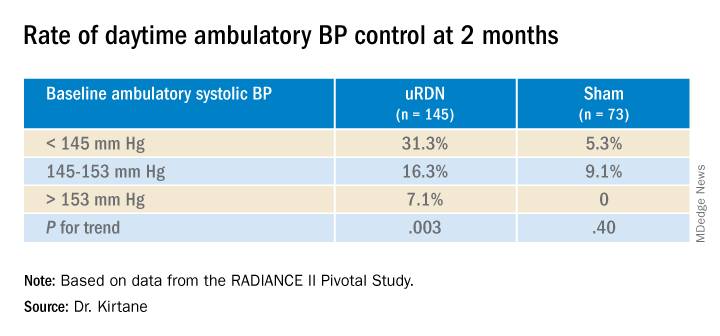
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
Systolic blood pressure went down safely and consistently 2 months after renal denervation achieved by ultrasound ablation in patients with uncontrolled, mild to moderate hypertension (HTN) in a key sham-controlled test of the balloon-equipped catheter.
The BP reductions were significant almost regardless of how they were measured – at home, in the office, during the day, at night, or over 24 hours – and weren’t dependent on baseline BP levels.
The 224-patient RADIANCE II Pivotal Study follows two earlier successful sham-controlled trials that used the same renal denervation catheter in other types of patients with HTN. They were RADIANCE-HTN SOLO, which entered patients with mild to moderate HTN not taking medication, and RADIANCE-HTN TRIO, which included patients with HTN despite fixed-dose, single-pill, triple-antihypertensive therapy.
The consistent results of all three trials suggest that the ultrasound renal denervation (uRDN) technique “lowers blood pressure across the spectrum of hypertension,” concluded co–principal investigator Ajay J. Kirtane, MD, SM, Columbia University Irving Medical Center, New York–Presbyterian Hospital, when presenting RADIANCE II at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
RADIANCE II, the largest of the three studies, met its prespecified primary efficacy endpoint of change in daytime ambulatory systolic BP at 2 months by showing a significant 6.3–mm Hg greater reduction in the uRDN group, compared with the sham-control group. There were no major adverse events at 30 days in either group.
The trial was similarly successful for the secondary endpoints of change in systolic BP measured in various other settings, including over 24 hours. Reductions after uRDN averaged 5-7 mm Hg greater than in the control group.
Sparse top-line results of the RADIANCE II pivotal trial were announced in July by the study’s sponsor, ReCor Medical.
Dr. Kirtane stressed in an interview that uRDN and likely any form of HTN renal denervation therapy is not a substitute for standard management. “This is really for patients in whom you’ve made best efforts to do the traditional things – lifestyle modification, medications, all of that – and yet they’re still uncontrolled.” At that point, assuming denervation therapy is available in practice, “it would be something to potentially consider.”
As a panelist after Dr. Kirtane’s formal presentation of RADIANCE II at the conference, Naomi D. Fisher, MD, who was a RADIANCE-HTN TRIO investigator, described how the treatment’s perceived intended patient population evolved over time.
“We all began with the idea that we were going to treat patients with resistant hypertension, that was going to be the first target. We have learned that those patients are far fewer than we thought,” said Dr. Fisher, who directs the hypertension service at Brigham and Women’s Hospital, Boston.
Initial estimates were that such patients with the resistant form, “meaning they require more than three drugs to control their blood pressure,” would represent 15%-20% of patients with HTN.
“We learned from our TRIO data that if you give these patients one single combined pill, lo and behold, many of them become controlled,” she said. “There is so much nonadherence out there in the world, about 50% of our patients aren’t taking their pills. It’s a hard and true fact.”
Exclude patients who aren’t adherent and “our true resistance population becomes minuscule. So, I don’t think that’s going to be the main population” for renal denervation therapy.
More likely, she said, it would be “patients who are uncontrolled and unable to take their medications. So that is going to include nonadherence, intolerance. It’s a very large category of patients. And the priorities can be stacked in favor of those who have higher cardiovascular risk.”
RADIANCE II can show the persistence of uRDN’s BP-lowering effect only out to 2 months so far, but the effect’s durability based on the RADIANCE program’s combined experience appears to be at least 2 years, Dr. Kirtane said in an interview.
“The RADIANCE II pivotal trial is a powerful, well-designed study attesting to the efficacy of renal denervation in BP lowering,” Franz H. Messerli, MD, Swiss Cardiovascular Center, University Hospital Bern, said in an interview.
The trial “shows the well-known unpredictability of antihypertensive response. We cannot predict who responds to renal denervation and who does not, and who even has a paradoxical increase in BP,” Dr. Messerli, an international hypertension expert not associated with the trial, said in an interview.
“As long as we cannot predict the antihypertensive response to renal denervation therapy, potential synergism/antagonism with drug therapy remains an educated guess,” he said.
“Hypertension is a disease that lasts years and decades. As impressive as RADIANCE II’s 2-month snapshot is, I look forward to similar or better BP data 12 and 24 months after renal denervation,” Dr. Messerli added.
RADIANCE II entered patients with mild to moderate uncontrolled HTN, that is, a systolic BP at least 140/90 mm Hg and less than 180/120 mm Hg, who were receiving no more than two antihypertensive medications. They could have no history of cardiovascular or cerebrovascular events or uncontrolled diabetes, and their estimated glomerular filtration rate (eGFR) had to be at least 40 mL/min per 1.73 m2.
After a 4-week drug washout period, patients who were clinically stable with an ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg underwent CT and renal angiography. Then, the 224 patients still anatomically eligible for the procedure were randomly assigned 2:1 to uRDN or a sham-control procedure: 150 and 74 patients, respectively.
At 2 months, daytime ambulatory systolic BP on average fell 7.9 mm Hg in the uRDN group and 1.8 mm Hg in the sham-control group, for a drop that was steeper by 6.3 mm Hg (P < .0001) after uRDN.
Also in the uRDN group, there was a 6.2–mm Hg larger decrease in 24-hour ambulatory systolic BP (P < .0001), a 5.8–mm Hg greater decline in nighttime ambulatory systolic BP (P < .0004), a 7.6–mm Hg steeper drop in mean home systolic BP (P < .0001), and 5.4 mm Hg more of a decrease in office-based systolic BP (P = .0035).
No significant differences were seen in subgroup analyses by sex, age, higher versus lower baseline systolic pressures, high versus low baseline eGFR, degree of abdominal obesity, U.S. versus European site, or whether patients entered before or during the COVID pandemic
Regulators have been accepting change in systolic BP as a surrogate for clinical endpoints in trials of antihypertensive therapy, whether pharmacologic or interventional, under consideration for approval. “That’s why safety endpoints are important to investigate” in these clinical trials, especially for invasive therapies like renal denervation, Dr. Kirtane observed.
That said, “in the longer-term follow-ups of the renal denervation therapies that are out there, including this one, there does not appear to be an appreciable decline in glomerular filtration rate, or any adverse safety signals that we see to date,” Dr. Kirtane said in an interview. “But we know that these are low-frequency events, so we have to be very vigilant, and we can’t get complacent about it.”
In RADIANCE II, there were zero adverse events within 30 days in both groups; the endpoint included death, new myocardial infarction, renal artery complications requiring invasive intervention, and hospitalization for major cardiovascular or hemodynamic-related events. Nor were there instances of new-onset renal artery stenosis greater than 70% documented by imaging at 6 months.
The ReCor uRDN catheter uses ultrasound energy to disrupt renal nerve signaling, a technology thought to deliver safer “burns,” compared with other renal denervation catheter technologies. It features an axially stabilizing balloon that transmits ultrasound energy – two to three sonications, each lasting 7 seconds, Dr. Kirtane said – outward through the arterial wall. The design is intended to ensure consistently circumferential ablation. Circulating saline within the balloon, Kirtane noted, directly cools the adjacent vessel wall to help it avoid thermal damage.
Dr. Kirtane reported receiving institutional funding from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck; consulting for IMDS; and receiving travel and meal expenses from Medtronic, Boston Scientific, Abbott Vascular, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr. Fisher disclosed receiving honoraria or fees for consulting or serving on a speaker’s bureau for Medtronic, ReCor Medical, and Aktiia and receiving grant support or holding research contracts for Recor Medical and Aktiia. Dr. Messerli disclosed receiving honoraria from Medtronic, Menarini, Krka, and Ipca.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
TAVR now used in almost 50% of younger severe aortic stenosis patients
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
In cardiogenic shock, edge-to-edge mitral valve repair improves outcome
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Walking intensity and step count are linked to health benefits
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
FROM JAMA INTERNAL MEDICINE
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
Extravascular ICD surpasses goals in pivotal trial
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
AT ESC CONGRESS 2022