User login
Pneumonia risk soars in heart failure patients, especially HFpEF
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Medtronic recall of almost 240,000 ICDs is class I, FDA says
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
Remote cardio visits expand access for underserved during COVID
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
OCS heart system earns hard-won backing of FDA panel
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
Eating fish tied to fewer CVD events in high-risk people
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
Inpatient sodium imbalances linked to adverse COVID-19 outcomes
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity: A ‘double hit’ in pregnant women with heart disease
Being obese and pregnant raises the risk for cardiac complications in women with preexisting heart disease, new research suggests, highlighting the need for earlier interventions in this high-risk population.
The analysis of 790 pregnancies revealed that 23% of women with obesity, defined as body mass index greater than 30 kg/m2, had a cardiac event during pregnancy versus 14% of women with normal body weight (P = .006).
The difference was driven largely by an increase in heart failure (8% vs. 3%; P = .02), although arrhythmias also trended higher in obese women (14% vs. 10%; P = .19).
Nearly half of the women with obesity and a cardiac event presented in the postpartum period (47%).
In multivariate analysis, both obesity and Canadian Cardiac Disease in Pregnancy Study (CARPREG) II risk score were independent predictors of cardiac events (odds ratios for both, 1.7), the investigators, led by Birgit Pfaller, MD, University of Toronto, reported in the Journal of the American College of Cardiology.
Although obesity has been linked to worse pregnancy outcomes and higher cardiovascular risk after delivery in the general population, the authors noted that this is the first study to examine its effect on outcomes in women with heart disease.
“We wanted to look at this high-risk group of women that had preexisting heart disease, but in addition had obesity, to try and find out if there was a kind of double hit for these women – and that, in the end, is what we found. It’s not just simply having heart disease, not simply having obesity, but the combination that’s problematic,” senior author and cardiologist Candice Silversides, MD, University of Toronto, said in an interview.
The findings are concerning given the rising prevalence of obesity worldwide. National data from 2018 show that slightly more than half of women who gave birth in the United States were significantly overweight or obese before becoming pregnant.
Similarly, in the present analysis of 600 women in the CARPREG study who gave birth from 2004 to 2014, nearly 1 in 5 pregnancies (19%) occurred in women with obesity and 25% were in overweight women.
Obese women were significantly more likely than those without obesity to have coronary artery disease (6% vs. 2%), cardiomyopathies (19% vs. 8%) and left ventricular dysfunction (19% vs. 12%) and to be hypertensive or have a hypertensive disorder of pregnancy (13% vs. 3%).
Preeclampsia developed in 32 women during the index pregnancy and 69% of these women were obese or overweight. Cardiac event rates were similar in women with or without preeclampsia but trended higher in women with preeclampsia with versus without obesity (36% vs. 14%; P = .20).
The ill effects of obesity were also reflected in fetal and neonatal events. Overall, 43% of women with obesity and 33% of normal-weight women had at least one fetal event (P = .02), with higher rates of preterm birth (19% vs. 10%; P = .005) and respiratory distress syndrome (8% vs. 3%; P = .02) in women with obesity. Congenital cardiac malformations were present in 6% of women in both groups.
Taken together, the composite of cardiac events, preeclampsia, or fetal events was significantly more common in women with obesity than in normal-weight women (56% vs. 41%; P = .002).
“We’ve spent the last number of years trying to research and understand what the drivers of these adverse outcomes are in this high-risk pregnant cohort, but on a bigger picture the real issue is how do we start intervening in a meaningful way,” Dr. Silversides said.
Like many in the burgeoning field of cardio-obstetrics, the team proposed a multidisciplinary approach that stresses preconception counseling, educating pregnant women with heart disease and obesity about their risks, ensuring that dietary advice, weight-gain recommendations, and comorbidities are addressed as part of routine care, and providing postpartum surveillance.
Preconception screening “has been the recommendation for a long, long time; it’s just that it doesn’t always happen in reality,” she said. “Many pregnancies aren’t planned and not all women are filtered into preconception counseling. So sometimes you’ll do it at the first antenatal visit and try to ensure women are educated but optimally you want to do it well in advance of pregnancy.”
Part of that preconception counseling “should also include giving them appropriate advice for contraception, if what they want to do is avoid pregnancy,” added Dr. Silversides.
Garima Sharma, MD, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, and colleagues wrote in an accompanying editorial that the adverse events observed in this high-risk cohort have “important implications for cardio-obstetricians and should be incorporated in routine prepregnancy and antenatal counseling, monitoring, and risk stratification for women with existing cardiovascular disease.”
They pointed to a paucity of data incorporating maternal prepregnancy obesity and gestational weight gain in risk prediction and called for larger population-based studies on the additive impact of obesity severity on predicting adverse cardiac events in women with existing cardiovascular disease.
Randomized trials are also urgently needed to evaluate the effect of nutritional and behavioral interventions in pregnancy on short- and long-term outcomes in mother and child.
“As the obesity epidemic continues to grow and public health interventions promoting lifestyle changes for obesity management remain a major challenge, maternal obesity may prove to be the ‘Achilles’ heel’ of sustainable national efforts to reduce maternal mortality and improve health equity. This is a call to action,” Dr. Sharma and colleagues concluded.
The investigators noted that the study was conducted at a single center and used self-reported pregnancy weight collected at the first antenatal visit, which may have underestimated obesity rates. Other limitations are that weight changes over the course of pregnancy were not studied and there was a limited number of women with a body mass index of 40 or higher.
The study was supported by a grant from the Allan E. Tiffin Trust, Toronto General and Western Hospital Foundation, and by a donation from Mrs. Josephine Rogers, Toronto General Hospital. Dr. Silversides is supported by the Miles Nadal Chair in Pregnancy and Heart Disease. Dr. Sharma and colleagues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Being obese and pregnant raises the risk for cardiac complications in women with preexisting heart disease, new research suggests, highlighting the need for earlier interventions in this high-risk population.
The analysis of 790 pregnancies revealed that 23% of women with obesity, defined as body mass index greater than 30 kg/m2, had a cardiac event during pregnancy versus 14% of women with normal body weight (P = .006).
The difference was driven largely by an increase in heart failure (8% vs. 3%; P = .02), although arrhythmias also trended higher in obese women (14% vs. 10%; P = .19).
Nearly half of the women with obesity and a cardiac event presented in the postpartum period (47%).
In multivariate analysis, both obesity and Canadian Cardiac Disease in Pregnancy Study (CARPREG) II risk score were independent predictors of cardiac events (odds ratios for both, 1.7), the investigators, led by Birgit Pfaller, MD, University of Toronto, reported in the Journal of the American College of Cardiology.
Although obesity has been linked to worse pregnancy outcomes and higher cardiovascular risk after delivery in the general population, the authors noted that this is the first study to examine its effect on outcomes in women with heart disease.
“We wanted to look at this high-risk group of women that had preexisting heart disease, but in addition had obesity, to try and find out if there was a kind of double hit for these women – and that, in the end, is what we found. It’s not just simply having heart disease, not simply having obesity, but the combination that’s problematic,” senior author and cardiologist Candice Silversides, MD, University of Toronto, said in an interview.
The findings are concerning given the rising prevalence of obesity worldwide. National data from 2018 show that slightly more than half of women who gave birth in the United States were significantly overweight or obese before becoming pregnant.
Similarly, in the present analysis of 600 women in the CARPREG study who gave birth from 2004 to 2014, nearly 1 in 5 pregnancies (19%) occurred in women with obesity and 25% were in overweight women.
Obese women were significantly more likely than those without obesity to have coronary artery disease (6% vs. 2%), cardiomyopathies (19% vs. 8%) and left ventricular dysfunction (19% vs. 12%) and to be hypertensive or have a hypertensive disorder of pregnancy (13% vs. 3%).
Preeclampsia developed in 32 women during the index pregnancy and 69% of these women were obese or overweight. Cardiac event rates were similar in women with or without preeclampsia but trended higher in women with preeclampsia with versus without obesity (36% vs. 14%; P = .20).
The ill effects of obesity were also reflected in fetal and neonatal events. Overall, 43% of women with obesity and 33% of normal-weight women had at least one fetal event (P = .02), with higher rates of preterm birth (19% vs. 10%; P = .005) and respiratory distress syndrome (8% vs. 3%; P = .02) in women with obesity. Congenital cardiac malformations were present in 6% of women in both groups.
Taken together, the composite of cardiac events, preeclampsia, or fetal events was significantly more common in women with obesity than in normal-weight women (56% vs. 41%; P = .002).
“We’ve spent the last number of years trying to research and understand what the drivers of these adverse outcomes are in this high-risk pregnant cohort, but on a bigger picture the real issue is how do we start intervening in a meaningful way,” Dr. Silversides said.
Like many in the burgeoning field of cardio-obstetrics, the team proposed a multidisciplinary approach that stresses preconception counseling, educating pregnant women with heart disease and obesity about their risks, ensuring that dietary advice, weight-gain recommendations, and comorbidities are addressed as part of routine care, and providing postpartum surveillance.
Preconception screening “has been the recommendation for a long, long time; it’s just that it doesn’t always happen in reality,” she said. “Many pregnancies aren’t planned and not all women are filtered into preconception counseling. So sometimes you’ll do it at the first antenatal visit and try to ensure women are educated but optimally you want to do it well in advance of pregnancy.”
Part of that preconception counseling “should also include giving them appropriate advice for contraception, if what they want to do is avoid pregnancy,” added Dr. Silversides.
Garima Sharma, MD, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, and colleagues wrote in an accompanying editorial that the adverse events observed in this high-risk cohort have “important implications for cardio-obstetricians and should be incorporated in routine prepregnancy and antenatal counseling, monitoring, and risk stratification for women with existing cardiovascular disease.”
They pointed to a paucity of data incorporating maternal prepregnancy obesity and gestational weight gain in risk prediction and called for larger population-based studies on the additive impact of obesity severity on predicting adverse cardiac events in women with existing cardiovascular disease.
Randomized trials are also urgently needed to evaluate the effect of nutritional and behavioral interventions in pregnancy on short- and long-term outcomes in mother and child.
“As the obesity epidemic continues to grow and public health interventions promoting lifestyle changes for obesity management remain a major challenge, maternal obesity may prove to be the ‘Achilles’ heel’ of sustainable national efforts to reduce maternal mortality and improve health equity. This is a call to action,” Dr. Sharma and colleagues concluded.
The investigators noted that the study was conducted at a single center and used self-reported pregnancy weight collected at the first antenatal visit, which may have underestimated obesity rates. Other limitations are that weight changes over the course of pregnancy were not studied and there was a limited number of women with a body mass index of 40 or higher.
The study was supported by a grant from the Allan E. Tiffin Trust, Toronto General and Western Hospital Foundation, and by a donation from Mrs. Josephine Rogers, Toronto General Hospital. Dr. Silversides is supported by the Miles Nadal Chair in Pregnancy and Heart Disease. Dr. Sharma and colleagues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Being obese and pregnant raises the risk for cardiac complications in women with preexisting heart disease, new research suggests, highlighting the need for earlier interventions in this high-risk population.
The analysis of 790 pregnancies revealed that 23% of women with obesity, defined as body mass index greater than 30 kg/m2, had a cardiac event during pregnancy versus 14% of women with normal body weight (P = .006).
The difference was driven largely by an increase in heart failure (8% vs. 3%; P = .02), although arrhythmias also trended higher in obese women (14% vs. 10%; P = .19).
Nearly half of the women with obesity and a cardiac event presented in the postpartum period (47%).
In multivariate analysis, both obesity and Canadian Cardiac Disease in Pregnancy Study (CARPREG) II risk score were independent predictors of cardiac events (odds ratios for both, 1.7), the investigators, led by Birgit Pfaller, MD, University of Toronto, reported in the Journal of the American College of Cardiology.
Although obesity has been linked to worse pregnancy outcomes and higher cardiovascular risk after delivery in the general population, the authors noted that this is the first study to examine its effect on outcomes in women with heart disease.
“We wanted to look at this high-risk group of women that had preexisting heart disease, but in addition had obesity, to try and find out if there was a kind of double hit for these women – and that, in the end, is what we found. It’s not just simply having heart disease, not simply having obesity, but the combination that’s problematic,” senior author and cardiologist Candice Silversides, MD, University of Toronto, said in an interview.
The findings are concerning given the rising prevalence of obesity worldwide. National data from 2018 show that slightly more than half of women who gave birth in the United States were significantly overweight or obese before becoming pregnant.
Similarly, in the present analysis of 600 women in the CARPREG study who gave birth from 2004 to 2014, nearly 1 in 5 pregnancies (19%) occurred in women with obesity and 25% were in overweight women.
Obese women were significantly more likely than those without obesity to have coronary artery disease (6% vs. 2%), cardiomyopathies (19% vs. 8%) and left ventricular dysfunction (19% vs. 12%) and to be hypertensive or have a hypertensive disorder of pregnancy (13% vs. 3%).
Preeclampsia developed in 32 women during the index pregnancy and 69% of these women were obese or overweight. Cardiac event rates were similar in women with or without preeclampsia but trended higher in women with preeclampsia with versus without obesity (36% vs. 14%; P = .20).
The ill effects of obesity were also reflected in fetal and neonatal events. Overall, 43% of women with obesity and 33% of normal-weight women had at least one fetal event (P = .02), with higher rates of preterm birth (19% vs. 10%; P = .005) and respiratory distress syndrome (8% vs. 3%; P = .02) in women with obesity. Congenital cardiac malformations were present in 6% of women in both groups.
Taken together, the composite of cardiac events, preeclampsia, or fetal events was significantly more common in women with obesity than in normal-weight women (56% vs. 41%; P = .002).
“We’ve spent the last number of years trying to research and understand what the drivers of these adverse outcomes are in this high-risk pregnant cohort, but on a bigger picture the real issue is how do we start intervening in a meaningful way,” Dr. Silversides said.
Like many in the burgeoning field of cardio-obstetrics, the team proposed a multidisciplinary approach that stresses preconception counseling, educating pregnant women with heart disease and obesity about their risks, ensuring that dietary advice, weight-gain recommendations, and comorbidities are addressed as part of routine care, and providing postpartum surveillance.
Preconception screening “has been the recommendation for a long, long time; it’s just that it doesn’t always happen in reality,” she said. “Many pregnancies aren’t planned and not all women are filtered into preconception counseling. So sometimes you’ll do it at the first antenatal visit and try to ensure women are educated but optimally you want to do it well in advance of pregnancy.”
Part of that preconception counseling “should also include giving them appropriate advice for contraception, if what they want to do is avoid pregnancy,” added Dr. Silversides.
Garima Sharma, MD, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, and colleagues wrote in an accompanying editorial that the adverse events observed in this high-risk cohort have “important implications for cardio-obstetricians and should be incorporated in routine prepregnancy and antenatal counseling, monitoring, and risk stratification for women with existing cardiovascular disease.”
They pointed to a paucity of data incorporating maternal prepregnancy obesity and gestational weight gain in risk prediction and called for larger population-based studies on the additive impact of obesity severity on predicting adverse cardiac events in women with existing cardiovascular disease.
Randomized trials are also urgently needed to evaluate the effect of nutritional and behavioral interventions in pregnancy on short- and long-term outcomes in mother and child.
“As the obesity epidemic continues to grow and public health interventions promoting lifestyle changes for obesity management remain a major challenge, maternal obesity may prove to be the ‘Achilles’ heel’ of sustainable national efforts to reduce maternal mortality and improve health equity. This is a call to action,” Dr. Sharma and colleagues concluded.
The investigators noted that the study was conducted at a single center and used self-reported pregnancy weight collected at the first antenatal visit, which may have underestimated obesity rates. Other limitations are that weight changes over the course of pregnancy were not studied and there was a limited number of women with a body mass index of 40 or higher.
The study was supported by a grant from the Allan E. Tiffin Trust, Toronto General and Western Hospital Foundation, and by a donation from Mrs. Josephine Rogers, Toronto General Hospital. Dr. Silversides is supported by the Miles Nadal Chair in Pregnancy and Heart Disease. Dr. Sharma and colleagues disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Semaglutide for meaningful weight loss in obesity and diabetes?
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
A 2.4-mg weekly injection of the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide led to a clinically meaningful 5% loss in weight for roughly two-thirds of patients with both overweight/obesity and type 2 diabetes, researchers report.
These findings from the Semaglutide Treatment Effect in People With Obesity 2 (STEP 2) trial, one of four phase 3 trials of this drug, which is currently under regulatory review for weight loss, were published March 2 in The Lancet.
More than 1,000 patients (mean initial weight, 100 kg [220 pounds]) were randomly assigned to receive a lifestyle intervention plus a weekly injection of semaglutide 2.4 mg or semaglutide 1.0 mg or placebo. At 68 weeks, they had lost a mean of 9.6%, 7.0%, and 3.4%, respectively, of their starting weight.
In addition, 69% of patients who had received semaglutide 2.4 mg experienced a clinically meaningful 5% loss of weight, compared with 57% of patients who had received the lower dose and 29% of patients who had received placebo.
The higher dose of semaglutide was associated with a greater improvement in cardiometabolic risk factors. The safety profile was similar to that seen with other drugs in this class.
“By far the best results with any weight loss medicine in diabetes”
Importantly, “more than a quarter of participants lost over 15% of their body weight,” senior author Ildiko Lingvay, MD, stressed. This “is by far the best result we had with any weight loss medicine in patients with diabetes,” Dr. Lingvay, of the University of Texas, Dallas, said in a statement from the university.
“The drug works by suppressing appetite centers in the brain to reduce caloric intake,” she explained. “The medication continually tells the body that you just ate, you’re full.”
Similarly, lead author Melanie J. Davies, MD, said that the STEP 2 results “are exciting and represent a new era in weight management in people with type 2 diabetes.
“They mark a real paradigm shift in our ability to treat obesity,” with results closer to those achieved with bariatric surgery, Dr. Davies, of the University of Leicester, England, said in a statement from her institution.
“It is really encouraging,” she continued, “that along with the weight loss we saw real improvements in general health, with significant improvement in physical functioning scores, blood pressure, and blood glucose control.”
Dr. Lingvay noted that on average, patients in the four STEP clinical trials lost 10%-17% of their body weight, “which is a huge step forward compared with all other medications currently available to treat obesity.” She stressed that these results are comparable to the 20%-30% weight loss seen with bariatric surgery.
One of four trials under review
More than 90% of people with type 2 diabetes are overweight or have obesity, and more than 20% of people with obesity have diabetes, wrote Dr. Davies and colleagues.
Semaglutide (Ozempic), administered subcutaneously at a dose of 0.5 mg to 1 mg weekly, is approved by the Food and Drug Administration for the treatment of type 2 diabetes. Dosing studies indicated that it is associated with weight loss.
As previously reported, four trials of the use of semaglutide for weight loss (STEP 1, 2, 3, and 4) have been completed. The combined data were submitted to the FDA on Dec. 4, 2020 (a decision is expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
The STEP 1 and STEP 3 trials of semaglutide 2.4 mg vs. placebo were recently published. The STEP 1 trial involved 1,961 adults with obesity or overweight; the STEP 3 trial, 611 adults with obesity or overweight. In each of the trials, some patients also underwent an intensive lifestyle intervention, and some did not. In both trials, patients with type 2 diabetes were excluded.
Topline results from STEP 2 were reported in June 2020.
STEP 2 enrolled patients with type 2 diabetes
STEP 2 involved 1,210 adults in 149 outpatient clinics in 12 countries in Europe, North America, South America, the Middle East, South Africa, and Asia. All participants had type 2 diabetes.
For all patients, the body mass index was ≥27 kg/m2, and the A1c concentration was 7%-10%. The mean BMI was 35.7 kg/m2, and the mean A1c was 8.1%.
The mean age of the patients was 55 years, and 51% were women; 62% were White, 26% were Asian, 13% were Hispanic, 8% were Black, and 4% were of other ethnicity.
Participants were managed with diet and exercise alone or underwent treatment with a stable dose of up to three oral glucose-lowering agents (metformin, sulfonylureas, SGLT2 inhibitors, or thiazolidinediones) for at least 90 days. They were then randomly assigned in 1:1:1 ratio to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo.
The starting dose of semaglutide was 0.25 mg/wk; the dose was escalated every 4 weeks to reach the target dose.
All patients received monthly counseling from a dietitian about calories (the goal was a 500-calorie/day deficit) and activity (the goal was 150 minutes of walking or stair climbing per week).
The mean A1c dropped by 1.6% and 1.5% in the semaglutide groups and by 0.4% in the placebo group.
Adverse events were more frequent among the patients who received semaglutide (88% and 82%) than in the placebo group (77%).
Gastrointestinal events that were mainly mild to moderate in severity were reported by 64% of patients in the 2.4-mg semaglutide group, 58% in the 1.0-mg semaglutide group, and 34% in the placebo group.
Semaglutide (Rybelsus) is approved in the United States as a once-daily oral agent for use in type 2 diabetes in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
The study was supported by Novo Nordisk. The authors’ relevant financial relationships are listed in the original article.
A version of this article first appeared on Medscape.com.
Heart failure redefined with new classifications, staging
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
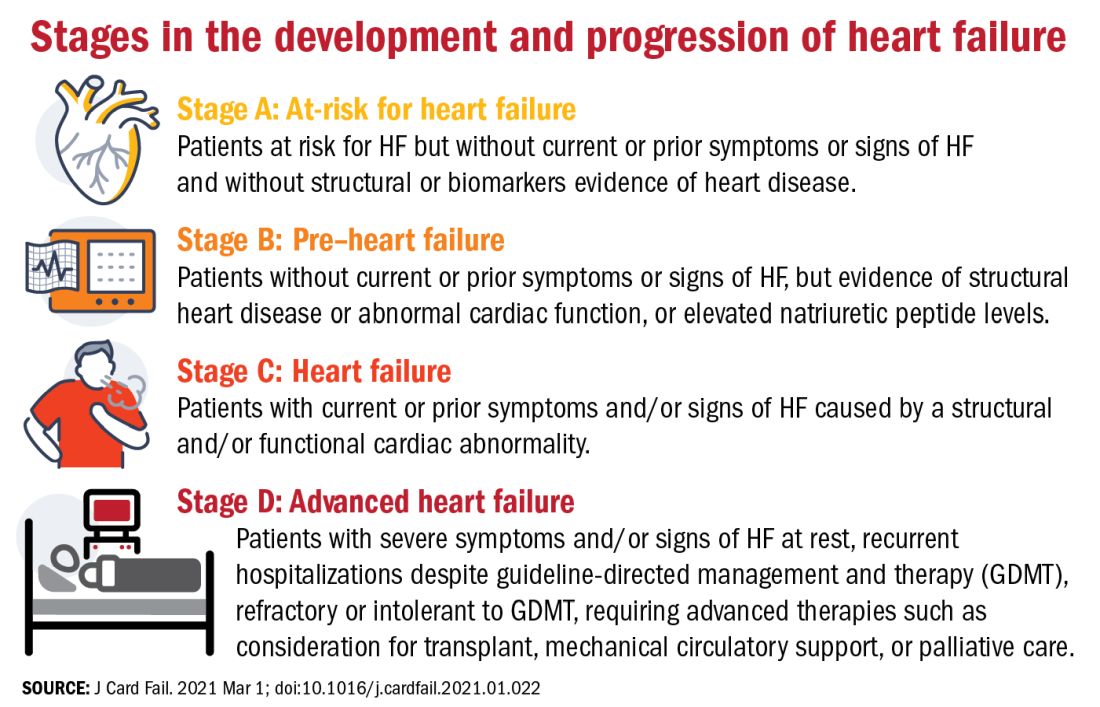
One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
FROM THE JOURNAL OF CARDIAC FAILURE
More from DAPA-HF: Dapagliflozin quickly reduces heart failure events
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
Dapagliflozin’s benefits in patients with heart failure with reduced ejection fraction appeared quickly after treatment began, and patients who had been hospitalized for heart failure within the prior year got the biggest boost from the drug, according to secondary analyses of the more than 4,700-patient DAPA-HF trial.
Dapagliflozin’s significant reduction of the incidence of cardiovascular death or worsening heart failure became apparent in DAPA-HF within 28 days after patients started treatment, by which time those on the study drug had a 49% cut in this combined endpoint, compared with patients on placebo, David D. Berg, MD, and associates said in a recent report published in JAMA Cardiology.
Their analyses also showed that the absolute reduction linked with dapagliflozin treatment for this primary endpoint of the study (which classified worsening heart failure as either hospitalization for heart failure or an urgent visit because of heart failure that required intravenous therapy) was greatest, 10% during 2 years of follow-up, among the roughly one-quarter of enrolled patients who had been hospitalized for heart failure within 12 months of entering the study. Patients previously hospitalized for heart failure more than 12 months before they entered DAPA-HF had a 4% absolute cut in their primary-outcome events during the trial, and those who had never been hospitalized for heart failure had a 2% absolute benefit, compared with placebo, during 2 years of follow-up.
These findings were consistent with the timing of benefits for patients with heart failure with reduced ejection fraction (HFrEF) in recent studies of two other drugs from the same class, the sodium-glucose cotransporter (SGLT) inhibitors, including empagliflozin (Jardiance, which inhibits SGLT-2) in the EMPEROR-Reduced trial, and sotagliflozin (Zynquista, which inhibits both SGLT1 and -2) in the SOLOIST-WHF trial, noted Gregg C. Fonarow, MD, and Clyde W. Yancy, MD, in an editor’s note that accompanied the new report.
The new findings show “the opportunity to expeditiously implement this remarkable class of therapy for HFrEF is now compelling and deserves disruptive efforts to ensure comprehensive treatment and the best patient outcomes,” wrote Dr. Fonarow, a professor of medicine at the University of California, Los Angeles, and Dr. Yancy, a professor of medicine at Northwestern University, Chicago.
But despite these new findings, their exact meaning remains unclear in terms of when to start dapagliflozin (or a different drug from the same class), compared with the other drug classes that have proven highly effective in patients with HFrEF, and exactly how long after hospitalization for heart failure dapagliflozin can safely and effectively begin.
Data needed on starting an SGLT inhibitor soon after hospitalization in patients without diabetes
“DAPA-HF showed that, in patients with or without diabetes, an SGLT2 inhibitor reduced the risk of cardiovascular death or worsening heart failure in patients with stable HFrEF. SOLOIST-WHF looked strictly at patients with diabetes, and showed that a combined SGLT1 and SGLT2 inhibitor could reduce the risk of cardiovascular death or worsening heart failure in patients with recently decompensated heart failure,” Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston, noted in an interview. “What we don’t have is a trial focused exclusively on enrolling patients while hospitalized with acute heart failure, irrespective of whether they have diabetes, and testing the immediate clinical efficacy and safety of starting an SGLT2 inhibitor. That is what we are testing with the ongoing DAPA ACT HF-TIMI 68 trial.”
In addition, updated recommendations from the American College of Cardiology on initiating drug therapy in patients newly diagnosed with HFrEF that appeared in early 2021 promoted a sequence that starts most patients on sacubitril/valsartan (Entresto) and a beta-blocker, followed by a diuretic (when needed), a mineralocorticoid receptor agonist, and then an SGLT inhibitor. The recommendations note that starting a patient on all these drug classes could take 3-6 months.
“There are intense debates about the optimal sequence for introducing these therapies, and I don’t think we have solid data to suggest that one sequence is clearly better than another,” noted Dr. Berg. “A one-size-fits-all approach probably doesn’t make sense. For example, each of these therapies has a different set of effects on heart rate and blood pressure, and each has a unique side effect profile, so clinicians will often need to tailor the treatment approach to the patient. And, of course, cost is an important consideration. Although the optimal time to start an SGLT2 inhibitor remains uncertain, the results of our analysis suggest that waiting may result in preventable adverse heart failure events.”
DAPA-HF randomized 4,744 patients with HFrEF and in New York Heart Association functional class II-IV at 410 sites in 20 countries. The incidence of the primary, combined endpoint fell by 26% with dapagliflozin treatment, compared with placebo, during a median 18-month follow-up. Among the study cohort 27% of patients had been hospitalized for heart failure within a year of their entry, 20% had been hospitalized for heart failure more than 1 year before entry, and 53% had no history of a hospitalization for heart failure.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Berg has received research support through his institution from AstraZeneca. Dr. Fonarow has received personal fees from AstraZeneca and from numerous other companies. Dr. Yancy’s spouse works for Abbott Laboratories.
FROM JAMA CARDIOLOGY



















