User login
Danish endocarditis strategy halved hospital days
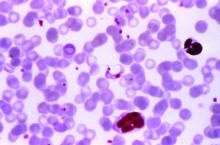
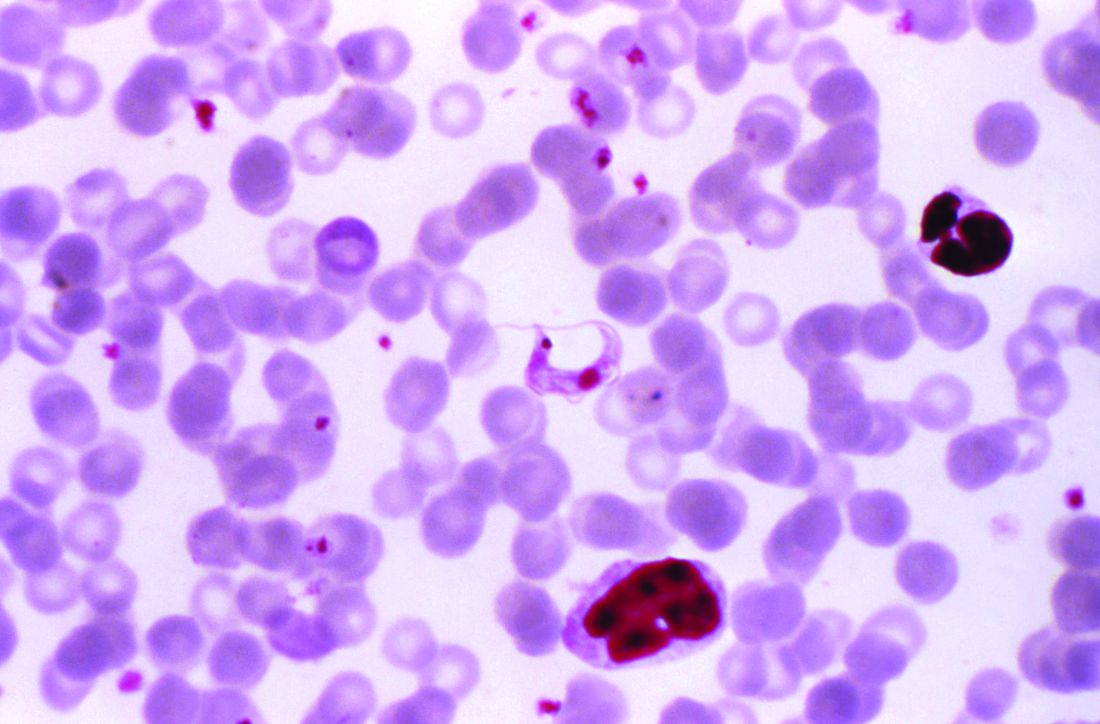
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Clinically stable patients with left-sided infectious endocarditis can safely and effectively be discharged on oral antibiotics after completing half of a full course of intravenous antibiotics.
Major finding: Key 6-month outcomes were similar in patients with left-sided infectious endocarditis regardless of whether they were discharged early on carefully selected oral antibiotics or remained in hospital to complete a full course of intravenous antibiotics.
Study details: This prospective, multicenter, Danish randomized trial included 400 patients with left-sided infectious endocarditis.
Disclosures: The presenter reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
ATTR-ACT shows treatment breakthrough in amyloid cardiomyopathy
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Tafamidis is the first-ever proven disease-modifying therapy for patients with a rapidly progressive form of cardiomyopathy.
Major finding: .
Study details: This 13-country, randomized, phase 3, double-blind trial included 441 patients with transthyretin amyloid cardiomyopathy.
Disclosures: The presenter reported receiving research grants, speaker honoraria, and consultant fees from Pfizer, which sponsored the ATTR-ACT trial.
Declining lung function linked to heart failure, stroke
Rapid declines in spirometric measures of lung function were associated with higher risks of cardiovascular disease, according to a recent analysis of a large, prospective cohort study.
Rapid declines in forced expiratory volume in 1 second (FEV1) were associated with increased incidence of heart failure, stroke, and death in the analysis of the ARIC (Atherosclerosis Risk in Communities) study.
The risk of incident heart failure among FEV1 rapid decliners was particularly high, with a fourfold increase within 12 months. That suggests clinicians should carefully consider incipient heart failure in patients with rapid changes in FEV1, investigators reported in the Journal of the American College of Cardiology.
Rapid declines in forced vital capacity (FVC) were also associated with higher incidences of heart failure and death in the analysis by Odilson M. Silvestre, MD, of the division of cardiovascular medicine at Brigham and Women’s Hospital, Boston, and colleagues.
The analysis included a total of 10,351 ARIC participants with a mean follow-up of 17 years. All had undergone spirometry at the first study visit between 1987 and 1989, and on the second visit between 1990 and 1992.
One-quarter of participants were classified as FEV1 rapid decliners, defined by an FEV1 decrease of at least 1.9% per year. Likewise, one-quarter of participants were classified as FVC rapid decliners, based on an FVC decrease of at least 2.1%.
Rapid decline in FEV1 was associated with a higher risk of incident heart failure (hazard ratio, 1.17; 95% confidence interval, 1.04-1.33; P = .010), and was most prognostic in the first year of follow-up (HR, 4.22; 95% CI, 1.34-13.26; P = .01), investigators said.
Rapid decline in FVC was likewise associated with a greater heart failure risk (HR, 1.27; 95% CI, 1.12-1.44; P less than .001).
Increased heart failure risk persisted after excluding patients with incident coronary heart disease in both the FEV1 and FVC rapid decliners, the investigators said.
A rapid decline in FEV1 was also associated with a higher stroke risk (HR, 1.25; 95% CI, 1.04-1.50; P = .015).
FEV1 rapid decliners had a higher overall rate of incident cardiovascular disease than those without rapid decline, even after adjustment for baseline variables such as age, sex, race, body mass index, and heart rate (HR, 1.15; 95% CI, 1.04-1.26; P = .004), and FVC rapid decliners likewise had a 19% greater risk of the composite endpoint (HR, 1.19; 95% CI, 1.08-1.32; P less than .001).
The National Heart, Lung, and Blood Institute, the American Heart Association, and other sources supported the study. Dr. Silvestre reported having no relevant conflicts.
SOURCE: Silvestre OM et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1109-22.
Cardiologists and pulmonologists should closely collaborate to better identify the relationship between lung function decline and early cardiovascular disease detection, said the authors of an editorial accompanying the study.
Improved collaboration would help manage these conditions, stopping early disease progression and preventing overt cardiovascular disease, wrote Daniel A. Duprez, MD, PhD, and David R. Jacobs Jr., PhD.
“The resulting symptomatic and prognostic benefits outweigh those attainable by treating either condition alone,” noted Dr. Duprez and Dr. Jacobs.
The study by Dr. Silvestre and coauthors showed that rapid declines in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) among participants in the ARIC (Atherosclerosis Risk in Communities) study were associated with a higher incidence of composite cardiovascular disease, heart failure, and total death.
The association between FEV1 and new heart failure was substantially impacted by 22 heart failure events occurring in the first year, with a hazard ratio of 4.22 for predicting those cases, the editorial authors noted.
“We suggest that this association with early cases could be the result of reversed causality, reflecting heart failure undiagnosed at the second spirometry test,” they explained (J Am Coll Cardiol. 2018 Sep 4;72[10]:1123-5).
Dr. Duprez is with the cardiovascular division of the University of Minnesota, Minneapolis, and Dr. Jacobs is with the division of epidemiology and community health at the University of Minnesota, Minneapolis. Dr. Duprez and Dr. Jacobs reported they had no relevant disclosures.
Cardiologists and pulmonologists should closely collaborate to better identify the relationship between lung function decline and early cardiovascular disease detection, said the authors of an editorial accompanying the study.
Improved collaboration would help manage these conditions, stopping early disease progression and preventing overt cardiovascular disease, wrote Daniel A. Duprez, MD, PhD, and David R. Jacobs Jr., PhD.
“The resulting symptomatic and prognostic benefits outweigh those attainable by treating either condition alone,” noted Dr. Duprez and Dr. Jacobs.
The study by Dr. Silvestre and coauthors showed that rapid declines in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) among participants in the ARIC (Atherosclerosis Risk in Communities) study were associated with a higher incidence of composite cardiovascular disease, heart failure, and total death.
The association between FEV1 and new heart failure was substantially impacted by 22 heart failure events occurring in the first year, with a hazard ratio of 4.22 for predicting those cases, the editorial authors noted.
“We suggest that this association with early cases could be the result of reversed causality, reflecting heart failure undiagnosed at the second spirometry test,” they explained (J Am Coll Cardiol. 2018 Sep 4;72[10]:1123-5).
Dr. Duprez is with the cardiovascular division of the University of Minnesota, Minneapolis, and Dr. Jacobs is with the division of epidemiology and community health at the University of Minnesota, Minneapolis. Dr. Duprez and Dr. Jacobs reported they had no relevant disclosures.
Cardiologists and pulmonologists should closely collaborate to better identify the relationship between lung function decline and early cardiovascular disease detection, said the authors of an editorial accompanying the study.
Improved collaboration would help manage these conditions, stopping early disease progression and preventing overt cardiovascular disease, wrote Daniel A. Duprez, MD, PhD, and David R. Jacobs Jr., PhD.
“The resulting symptomatic and prognostic benefits outweigh those attainable by treating either condition alone,” noted Dr. Duprez and Dr. Jacobs.
The study by Dr. Silvestre and coauthors showed that rapid declines in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) among participants in the ARIC (Atherosclerosis Risk in Communities) study were associated with a higher incidence of composite cardiovascular disease, heart failure, and total death.
The association between FEV1 and new heart failure was substantially impacted by 22 heart failure events occurring in the first year, with a hazard ratio of 4.22 for predicting those cases, the editorial authors noted.
“We suggest that this association with early cases could be the result of reversed causality, reflecting heart failure undiagnosed at the second spirometry test,” they explained (J Am Coll Cardiol. 2018 Sep 4;72[10]:1123-5).
Dr. Duprez is with the cardiovascular division of the University of Minnesota, Minneapolis, and Dr. Jacobs is with the division of epidemiology and community health at the University of Minnesota, Minneapolis. Dr. Duprez and Dr. Jacobs reported they had no relevant disclosures.
Rapid declines in spirometric measures of lung function were associated with higher risks of cardiovascular disease, according to a recent analysis of a large, prospective cohort study.
Rapid declines in forced expiratory volume in 1 second (FEV1) were associated with increased incidence of heart failure, stroke, and death in the analysis of the ARIC (Atherosclerosis Risk in Communities) study.
The risk of incident heart failure among FEV1 rapid decliners was particularly high, with a fourfold increase within 12 months. That suggests clinicians should carefully consider incipient heart failure in patients with rapid changes in FEV1, investigators reported in the Journal of the American College of Cardiology.
Rapid declines in forced vital capacity (FVC) were also associated with higher incidences of heart failure and death in the analysis by Odilson M. Silvestre, MD, of the division of cardiovascular medicine at Brigham and Women’s Hospital, Boston, and colleagues.
The analysis included a total of 10,351 ARIC participants with a mean follow-up of 17 years. All had undergone spirometry at the first study visit between 1987 and 1989, and on the second visit between 1990 and 1992.
One-quarter of participants were classified as FEV1 rapid decliners, defined by an FEV1 decrease of at least 1.9% per year. Likewise, one-quarter of participants were classified as FVC rapid decliners, based on an FVC decrease of at least 2.1%.
Rapid decline in FEV1 was associated with a higher risk of incident heart failure (hazard ratio, 1.17; 95% confidence interval, 1.04-1.33; P = .010), and was most prognostic in the first year of follow-up (HR, 4.22; 95% CI, 1.34-13.26; P = .01), investigators said.
Rapid decline in FVC was likewise associated with a greater heart failure risk (HR, 1.27; 95% CI, 1.12-1.44; P less than .001).
Increased heart failure risk persisted after excluding patients with incident coronary heart disease in both the FEV1 and FVC rapid decliners, the investigators said.
A rapid decline in FEV1 was also associated with a higher stroke risk (HR, 1.25; 95% CI, 1.04-1.50; P = .015).
FEV1 rapid decliners had a higher overall rate of incident cardiovascular disease than those without rapid decline, even after adjustment for baseline variables such as age, sex, race, body mass index, and heart rate (HR, 1.15; 95% CI, 1.04-1.26; P = .004), and FVC rapid decliners likewise had a 19% greater risk of the composite endpoint (HR, 1.19; 95% CI, 1.08-1.32; P less than .001).
The National Heart, Lung, and Blood Institute, the American Heart Association, and other sources supported the study. Dr. Silvestre reported having no relevant conflicts.
SOURCE: Silvestre OM et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1109-22.
Rapid declines in spirometric measures of lung function were associated with higher risks of cardiovascular disease, according to a recent analysis of a large, prospective cohort study.
Rapid declines in forced expiratory volume in 1 second (FEV1) were associated with increased incidence of heart failure, stroke, and death in the analysis of the ARIC (Atherosclerosis Risk in Communities) study.
The risk of incident heart failure among FEV1 rapid decliners was particularly high, with a fourfold increase within 12 months. That suggests clinicians should carefully consider incipient heart failure in patients with rapid changes in FEV1, investigators reported in the Journal of the American College of Cardiology.
Rapid declines in forced vital capacity (FVC) were also associated with higher incidences of heart failure and death in the analysis by Odilson M. Silvestre, MD, of the division of cardiovascular medicine at Brigham and Women’s Hospital, Boston, and colleagues.
The analysis included a total of 10,351 ARIC participants with a mean follow-up of 17 years. All had undergone spirometry at the first study visit between 1987 and 1989, and on the second visit between 1990 and 1992.
One-quarter of participants were classified as FEV1 rapid decliners, defined by an FEV1 decrease of at least 1.9% per year. Likewise, one-quarter of participants were classified as FVC rapid decliners, based on an FVC decrease of at least 2.1%.
Rapid decline in FEV1 was associated with a higher risk of incident heart failure (hazard ratio, 1.17; 95% confidence interval, 1.04-1.33; P = .010), and was most prognostic in the first year of follow-up (HR, 4.22; 95% CI, 1.34-13.26; P = .01), investigators said.
Rapid decline in FVC was likewise associated with a greater heart failure risk (HR, 1.27; 95% CI, 1.12-1.44; P less than .001).
Increased heart failure risk persisted after excluding patients with incident coronary heart disease in both the FEV1 and FVC rapid decliners, the investigators said.
A rapid decline in FEV1 was also associated with a higher stroke risk (HR, 1.25; 95% CI, 1.04-1.50; P = .015).
FEV1 rapid decliners had a higher overall rate of incident cardiovascular disease than those without rapid decline, even after adjustment for baseline variables such as age, sex, race, body mass index, and heart rate (HR, 1.15; 95% CI, 1.04-1.26; P = .004), and FVC rapid decliners likewise had a 19% greater risk of the composite endpoint (HR, 1.19; 95% CI, 1.08-1.32; P less than .001).
The National Heart, Lung, and Blood Institute, the American Heart Association, and other sources supported the study. Dr. Silvestre reported having no relevant conflicts.
SOURCE: Silvestre OM et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1109-22.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Rapid declines in spirometric measures of lung function were associated with higher risks of heart failure, among other adverse cardiovascular outcomes.
Major finding: Rapid decline in forced expiratory volume in 1 second was associated with higher risk of incident heart failure (HR, 1.17; 95% CI, 1.04-1.33; P = .010), and was most prognostic in the first year of follow-up (HR, 4.22; 95% CI, 1.34-13.26; P = .01).
Study details: An analysis including a total of 10,351 participants in a large, prospective cohort study with a mean follow-up of 17 years.
Disclosures: The National Heart, Lung, and Blood Institute, the American Heart Association, and other sources supported the study. Dr. Silvestre reported having no relevant conflicts.
Source: Silvestre OM et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1109-22.
Rivaroxaban no help for heart failure outcomes
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
cardnews@mdedge.com
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
cardnews@mdedge.com
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
cardnews@mdedge.com
SOURCE: Zannad F et al. NEJM/ESC.
.
Key clinical point: For patients with heart failure and coronary artery disease, rivaroxaban does not significantly reduce the risk of death, myocardial infarction, or stroke.
Major finding: Death, myocardial failure, or stroke occurred in 25.0% of patients in the rivaroxaban group compared with 26.2% of patients in the placebo group (P = .27).
Study details: The COMMANDER study was a double-blind, randomized trial involving 5,022 patients. Patients had heart failure, normal sinus rhythm, and coronary artery disease.
Disclosures: Funding was provided by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
Source: Zannad F et al. NEJM/ESC.
FDA alert: Artificial heart driver linked to higher mortality
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
Postapproval results for SynCardia Systems’ Companion 2 (C2) driver system for temporary total artificial hearts (TAH-t) have shown higher mortality and stroke rates than were seen with the previous system, the circulatory support system. As a result, the Food and Drug Administration has issued a safety alert cautioning them to weigh the risks and benefits carefully. The alert, issued on August 17, is based on a postapproval study conducted by SynCardia Systems.
Furthermore, patients and health care professionals are encouraged to report any adverse events using the FDA’s Medwatch reporting form, as well as return any devices associated with adverse events to the SynCardia Systems to help them and the FDA better understand the issue.
The C2 driver system is an external pneumatic system that activates an implanted TAH-t in eligible heart failure patients who have severe biventricular failure and are waiting for transplant. It is smaller than its predecessor, but per the device’s approved use, patients must still remain in the hospital while on the device. Since its approval in 2012, the Freedom driver system was approved in 2014, which allows patients to return home.
The full safety alert can be found on the FDA website.
AHA: Chagas disease and its heart effects have come to the U.S.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
FROM CIRCULATION
Prolonged antimalarial therapy linked to elevated cardiac biomarkers, cardiomyopathy
People with systemic lupus erythematosus (SLE) taking antimalarials, particularly for prolonged periods, could be at risk for cardiomyopathy, and measuring specific myocardial biomarkers could help identify patients at particularly high risk, researchers have suggested.
Writing in the Journal of Rheumatology, the research team led by Konstantinos Tselios, MD, PhD, of the University of Toronto Lupus Clinic at the Centre for Prognosis Studies in the Rheumatic Diseases, noted that antimalarial-induced cardiomyopathy (AMIC) had been reported in 47 patients to date, 19 of whom had SLE, with duration and cumulative use of antimalarials thought to be the cause.
The authors suggested that AMIC could be underrecognized because antimalarials were currently recommended for all patients with SLE (without known contraindications) for prolonged periods. It was speculated that the mechanism by which AMIC occurred involved the deposition of antimalarials in the myocardial fibers and that this could lead to chronic, subclinical tissue necrosis, a process which, if not reversed, could lead to heart failure.
“Heart-specific biomarkers, such as cardiac troponin (for the assessment of myocardial necrosis) and brain natriuretic peptide (BNP; for the assessment of volume and/or pressure overload), may be of value in identifying subclinical heart damage,” the research team suggested.
The current study involved 151 consecutive patients with SLE who had no prior cardiac disease and were attending the University of Toronto Lupus Clinic from March to May 2016.
During the course of the disease, 28 of the patients had been taking chloroquine, and 137 had been taking hydroxychloroquine (14 patients had been treated with both drugs at different time periods). In the study, normal range was less than 26 ng/mL for high-sensitivity cardiac troponin I (cTnI) and less than 100 pg/mL for BNP.
Overall, 16 patients (10.6%) had abnormal BNP, of whom 9 (6%) also had abnormal cTnI. Prolonged antimalarial use (greater than 5.6 yrs) was associated with increased risk for these elevated biomarkers, regardless of age and SLE disease duration.
“Because duration of AM [antimalarial] treatment is the critical predictor of AMIC, the majority of patients with SLE should be considered at risk because most of them receive long-term maintenance therapy,” the researchers wrote.
They said that elevations of the biomarkers implicated possible active myocardial necrosis (elevated cTnI) and/or increased intracardiac ventricular pressure (elevated BNP).
“In this context, AM deposition in the myocardium, leading over time to overt cardiomyopathy, may be an important contributing factor. Indeed, 6 out of 16 of these patients were ultimately diagnosed with definite or possible AMIC, while 3 of them were completely asymptomatic, and their investigation was initiated because of the abnormal biomarkers,” they wrote.
The researchers also found that persistent creatine phosphokinase (CPK) elevation was also an important predictor for elevated cardiac biomarkers.
“We showed that chronic AM use is associated with a more than threefold increased risk for elevated CPK (after excluding patients with active myositis and statin therapy). ... It is not known whether elevated CPK may predict patients at risk for development of AMIC or whether certain patients are predisposed to AM-related muscle damage,” they said.
“Prolonged AM therapy and persistent CPK elevation conferred an increased risk for abnormal BNP and cTnI, which might predict AMIC,” they concluded.
Nevertheless, the authors acknowledged that expensive and invasive investigation was unjustified in asymptomatic individuals. They therefore suggested that identifying heart-specific biomarkers for the detection of subclinical heart damage could allow patients to be stratified according to their risk.
“Cardiac biomarkers could become a screening test for patients with SLE using AM for longer than 5.6 years and/or who have persistently elevated CPK levels. Further research is needed to delineate the differential toxicity of CQ [chloroquine] and HCQ [hydroxychloroquine],” they suggested.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario. Dr. Tselios is financially supported by Lupus Ontario.
SOURCE: Tselios K et al. J Rheumatol. 2018. doi: 10.3899/jrheum.171436.
People with systemic lupus erythematosus (SLE) taking antimalarials, particularly for prolonged periods, could be at risk for cardiomyopathy, and measuring specific myocardial biomarkers could help identify patients at particularly high risk, researchers have suggested.
Writing in the Journal of Rheumatology, the research team led by Konstantinos Tselios, MD, PhD, of the University of Toronto Lupus Clinic at the Centre for Prognosis Studies in the Rheumatic Diseases, noted that antimalarial-induced cardiomyopathy (AMIC) had been reported in 47 patients to date, 19 of whom had SLE, with duration and cumulative use of antimalarials thought to be the cause.
The authors suggested that AMIC could be underrecognized because antimalarials were currently recommended for all patients with SLE (without known contraindications) for prolonged periods. It was speculated that the mechanism by which AMIC occurred involved the deposition of antimalarials in the myocardial fibers and that this could lead to chronic, subclinical tissue necrosis, a process which, if not reversed, could lead to heart failure.
“Heart-specific biomarkers, such as cardiac troponin (for the assessment of myocardial necrosis) and brain natriuretic peptide (BNP; for the assessment of volume and/or pressure overload), may be of value in identifying subclinical heart damage,” the research team suggested.
The current study involved 151 consecutive patients with SLE who had no prior cardiac disease and were attending the University of Toronto Lupus Clinic from March to May 2016.
During the course of the disease, 28 of the patients had been taking chloroquine, and 137 had been taking hydroxychloroquine (14 patients had been treated with both drugs at different time periods). In the study, normal range was less than 26 ng/mL for high-sensitivity cardiac troponin I (cTnI) and less than 100 pg/mL for BNP.
Overall, 16 patients (10.6%) had abnormal BNP, of whom 9 (6%) also had abnormal cTnI. Prolonged antimalarial use (greater than 5.6 yrs) was associated with increased risk for these elevated biomarkers, regardless of age and SLE disease duration.
“Because duration of AM [antimalarial] treatment is the critical predictor of AMIC, the majority of patients with SLE should be considered at risk because most of them receive long-term maintenance therapy,” the researchers wrote.
They said that elevations of the biomarkers implicated possible active myocardial necrosis (elevated cTnI) and/or increased intracardiac ventricular pressure (elevated BNP).
“In this context, AM deposition in the myocardium, leading over time to overt cardiomyopathy, may be an important contributing factor. Indeed, 6 out of 16 of these patients were ultimately diagnosed with definite or possible AMIC, while 3 of them were completely asymptomatic, and their investigation was initiated because of the abnormal biomarkers,” they wrote.
The researchers also found that persistent creatine phosphokinase (CPK) elevation was also an important predictor for elevated cardiac biomarkers.
“We showed that chronic AM use is associated with a more than threefold increased risk for elevated CPK (after excluding patients with active myositis and statin therapy). ... It is not known whether elevated CPK may predict patients at risk for development of AMIC or whether certain patients are predisposed to AM-related muscle damage,” they said.
“Prolonged AM therapy and persistent CPK elevation conferred an increased risk for abnormal BNP and cTnI, which might predict AMIC,” they concluded.
Nevertheless, the authors acknowledged that expensive and invasive investigation was unjustified in asymptomatic individuals. They therefore suggested that identifying heart-specific biomarkers for the detection of subclinical heart damage could allow patients to be stratified according to their risk.
“Cardiac biomarkers could become a screening test for patients with SLE using AM for longer than 5.6 years and/or who have persistently elevated CPK levels. Further research is needed to delineate the differential toxicity of CQ [chloroquine] and HCQ [hydroxychloroquine],” they suggested.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario. Dr. Tselios is financially supported by Lupus Ontario.
SOURCE: Tselios K et al. J Rheumatol. 2018. doi: 10.3899/jrheum.171436.
People with systemic lupus erythematosus (SLE) taking antimalarials, particularly for prolonged periods, could be at risk for cardiomyopathy, and measuring specific myocardial biomarkers could help identify patients at particularly high risk, researchers have suggested.
Writing in the Journal of Rheumatology, the research team led by Konstantinos Tselios, MD, PhD, of the University of Toronto Lupus Clinic at the Centre for Prognosis Studies in the Rheumatic Diseases, noted that antimalarial-induced cardiomyopathy (AMIC) had been reported in 47 patients to date, 19 of whom had SLE, with duration and cumulative use of antimalarials thought to be the cause.
The authors suggested that AMIC could be underrecognized because antimalarials were currently recommended for all patients with SLE (without known contraindications) for prolonged periods. It was speculated that the mechanism by which AMIC occurred involved the deposition of antimalarials in the myocardial fibers and that this could lead to chronic, subclinical tissue necrosis, a process which, if not reversed, could lead to heart failure.
“Heart-specific biomarkers, such as cardiac troponin (for the assessment of myocardial necrosis) and brain natriuretic peptide (BNP; for the assessment of volume and/or pressure overload), may be of value in identifying subclinical heart damage,” the research team suggested.
The current study involved 151 consecutive patients with SLE who had no prior cardiac disease and were attending the University of Toronto Lupus Clinic from March to May 2016.
During the course of the disease, 28 of the patients had been taking chloroquine, and 137 had been taking hydroxychloroquine (14 patients had been treated with both drugs at different time periods). In the study, normal range was less than 26 ng/mL for high-sensitivity cardiac troponin I (cTnI) and less than 100 pg/mL for BNP.
Overall, 16 patients (10.6%) had abnormal BNP, of whom 9 (6%) also had abnormal cTnI. Prolonged antimalarial use (greater than 5.6 yrs) was associated with increased risk for these elevated biomarkers, regardless of age and SLE disease duration.
“Because duration of AM [antimalarial] treatment is the critical predictor of AMIC, the majority of patients with SLE should be considered at risk because most of them receive long-term maintenance therapy,” the researchers wrote.
They said that elevations of the biomarkers implicated possible active myocardial necrosis (elevated cTnI) and/or increased intracardiac ventricular pressure (elevated BNP).
“In this context, AM deposition in the myocardium, leading over time to overt cardiomyopathy, may be an important contributing factor. Indeed, 6 out of 16 of these patients were ultimately diagnosed with definite or possible AMIC, while 3 of them were completely asymptomatic, and their investigation was initiated because of the abnormal biomarkers,” they wrote.
The researchers also found that persistent creatine phosphokinase (CPK) elevation was also an important predictor for elevated cardiac biomarkers.
“We showed that chronic AM use is associated with a more than threefold increased risk for elevated CPK (after excluding patients with active myositis and statin therapy). ... It is not known whether elevated CPK may predict patients at risk for development of AMIC or whether certain patients are predisposed to AM-related muscle damage,” they said.
“Prolonged AM therapy and persistent CPK elevation conferred an increased risk for abnormal BNP and cTnI, which might predict AMIC,” they concluded.
Nevertheless, the authors acknowledged that expensive and invasive investigation was unjustified in asymptomatic individuals. They therefore suggested that identifying heart-specific biomarkers for the detection of subclinical heart damage could allow patients to be stratified according to their risk.
“Cardiac biomarkers could become a screening test for patients with SLE using AM for longer than 5.6 years and/or who have persistently elevated CPK levels. Further research is needed to delineate the differential toxicity of CQ [chloroquine] and HCQ [hydroxychloroquine],” they suggested.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario. Dr. Tselios is financially supported by Lupus Ontario.
SOURCE: Tselios K et al. J Rheumatol. 2018. doi: 10.3899/jrheum.171436.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Patients with SLE who receive prolonged antimalarial treatment (greater than 5.6 years) are at increased risk for elevated cardiac biomarkers (BNP/cTnI), particularly when persistently elevated creatine phosphokinase is present. These biomarkers could be used to predict antimalarial-induced cardiomyopathy.
Major finding: Of a cohort of 151 patients with SLE, 10% had elevated myocardial biomarkers in the absence of prior cardiac disease or pulmonary arterial hypertension. One-third of the patients were diagnosed with antimalarial-induced cardiomyopathy.
Study details: The study enrolled 151 consecutive patients attending the University of Toronto Lupus Clinic from March to May 2016.
Disclosures: The University of Toronto Lupus Research Program is supported by the University Health Network, Lou and Marissa Rocca, and the Lupus Foundation of Ontario. Dr. Tselios is financially supported by Lupus Ontario.
Source: Tselios K et al. J Rheumatol. 2018. doi: 10.3899/jrheum.171436.
Herceptin linked to doubling of HF risk in women with breast cancer
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
While trastuzumab clearly benefits patients with HER2-positive breast cancer at various stages of progression, concerns about heart failure persist. Studies have suggested that the drug doesn’t boost the risk of late cardiac events, but it’s not clear if this is due to mandated screening in these trials. The new study provides more evidence that adherence to screening guidelines is limited, and recent trials offer evidence that the general cardiac risk may be overblown. Future studies could be designed to offer insight into the wisdom of adjusting screening regimens based on stratification of risk. A meta-analysis could also be helpful, and the upcoming results of the SAFE-HEART study will provide information about the safety of anti-HER2 antibody therapy in patients with low but asymptomatic left-ventricular ejection fraction.
These comments are excerpted from a commentary by Chau T. Dang, MD, of Memorial Sloan Kettering Cancer Center, New York, and her associates (JACC: Cardiovasc Imaging. 2018 Aug;11[8]:1094-7). Most of the commentary authors report various disclosures.
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
Adding more evidence to an ongoing debate, a large new study suggests that patients with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of developing heart failure, with older women at highest risk.
The study also found that most patients who took trastuzumab didn’t receive recommended cardiac screening.
The researchers said their findings are unique because they tracked both younger and older patients. “By examining the rates of both cardiac monitoring and cardiotoxicity, we could begin to address the controversial issue of whether cardiac monitoring is warranted in young breast cancer patients,” wrote Mariana Chavez-MacGregor, MD, MSC, of the University of Texas MD Anderson Cancer Center, and her associates. The report was published in JACC: Cardiovascular Imaging.
While Trastuzumab has boosted breast cancer survival rates for patients with HER2-positive tumors, it’s also raised concerns about cardiotoxicity that could be an indicator of subsequent congestive heart failure (Cochrane Database Syst Rev. 2014 Jun 12;(6):CD006242).
According to the new study, the risk of trastuzumab risk is linked to damage to cardiac myocytes that can cause reversible cardiotoxicity.
The prescribing information for trastuzumab advises patients to undergo cardiac monitoring before treatment with trastuzumab and every 3 months during treatment. Recommendations by medical organizations have varied.
Now, as a 2016 report put it, it’s “increasingly unclear” whether frequent routine monitoring is appropriate for all patients (J Clin Oncol. 2016 Apr 1;34[10]:1030-3).
For the current study, Dr. Chavez-MacGregor and her associates identified 16,456 adult women in the United States who were diagnosed with nonmetastatic invasive breast cancer from 2009 to 2014. Researchers tracked the group, with a median age of 56, through as late as 2015.
The women were treated with chemotherapy within 6 months of diagnosis, and 4,325 received trastuzumab.
Of all the subjects, 692 patients (4.2%) developed heart failure following chemotherapy. The rate among patients treated with trastuzumab was higher, at 8.3%, compared with 2.7% for those not treated with trastuzumab (P less than .001).
The researchers also looked at anthracycline users and found that they were slightly more likely to develop HF (4.6% vs. 4.0% among nonusers, P = .048).
Increased age boosted the risk of HF in the trastuzumab-treated patients, and the risk was highest in those treated with both anthracyclines and trastuzumab. Other factors linked to more risk were comorbidities, hypertension, and valve disease.
After adjusting for confounders, the researchers estimated that those treated with trastuzumab were 2.01 times more likely to develop HF (HR, 2.01; 95% confidence interval, 1.72-2.36), and those who took anthracycline were 1.53 times more likely (HR, 1.53; 95% CI, 1.30-1.80)
The researchers also examined medical records for evidence that subjects underwent cardiac screening at least once every 4 months, not 3 months, as the prescribing information recommends. The study team chose to focus on 4-month intervals “to compensate for differences in scheduling, resources, or levels of accessibility to medical care.”
Medical records suggest that 73.5% of patients who took trastuzumab underwent cardiac screening at the beginning of therapy, but only 46.2% continued to do so at least every 4 months.
An adjusted model linked more screening to the use of anthracyclines and taxanes, radiation treatment, and living in the Northeast vs. the West.
“HF was more frequently identified among patients undergoing recommended cardiac monitoring (10.4% compared with 6.5%, respectively; P less than.001), suggesting that, as more patients are screened, more patients are likely to be found having HF,” the researchers reported.
However, they added that “our sensitivity analysis using inpatient claims allowed us to determine that the HF identified using cardiac monitoring was not severe enough to require hospitalization and was likely asymptomatic. The clinical implications of the diagnosis of asymptomatic HF are hard to determine and are beyond the scope of this study.”
The researchers also noted that the findings suggest that screening has become more common in recent years.
“The number of cancer survivors is expected to increase over time, and we will continue to see patients develop treatment-related cardiotoxicity,” the researchers wrote. “Thus, more research, evidence-based guidelines, and tools for prediction of cancer treatment–related cardiotoxicity are needed.”
The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors reported grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors reported no disclosures.
SOURCE: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging. 2018 Aug;11[8]1084-93.
FROM JACC: CARDIOVASCULAR IMAGING
Key clinical point: Women with breast cancer who take trastuzumab (Herceptin) may face double the adjusted risk of heart failure, but most aren’t screened frequently.
Major finding: Patients who took trastuzumab were 2.01 times more likely to develop HF (HR, 95% CI, 1.72-2.36) than were those who didn’t. Of all patients who took the drug, fewer than half received recommended frequency of screening.
Study details: Analysis of 16,456 U.S. adult women with nonmetastatic breast cancer diagnosed from 2009 to 2014 and tracked through 2015. Of those, 4.2% developed HF.
Disclosures: The National Cancer Institute and Cancer Prevention and Research Institute of Texas funded the study. Two study authors report grant funding from the Susan G. Komen Breast Cancer Foundation, and one reports consulting for Pfizer and Roche. The other authors report no disclosures.
Source: Chavez-MacGregor M et al. JACC: Cardiovasc Imaging 2018 Aug;11[8]1084-93.
More female authors than ever in cardiology journals
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
Also this week, valsartan recall and risk, synergy DES shines in acute MI, and incident heart failure is linked to HIV infection.
FDA: Cancer risk low with recalled valsartan
The risk of cancer from N-nitrosodimethylamine (NDMA) contained in impure valsartan is real but very low, the Food and Drug Administration said in a July 27 statement.
The agency had announced a voluntary recall of valsartan from Major Pharmaceuticals, Solco Healthcare, and Teva Pharmaceuticals, as well as valsartan/hydrochlorothiazide from Solco and Teva, on July 13 after detection of NDMA, a semi-volatile organic compound. The manufacturer, Zhejiang Huahai Pharmaceuticals in Linhai, China, has since stopped distribution. Contamination probably is tied to a change in the manufacturing process.
NDMA has been linked to cancer in animal studies but at levels “much higher than the impurity levels in recalled valsartan batches.” Even so, the agency “wanted to put some context around the actual potential risk posed to patients who used versions of valsartan that may have contained high levels of NDMA,” the FDA said in its updated press release.
Based on records from the manufacturer, “some levels of the impurity may have been in the valsartan-containing products for as long as 4 years. FDA scientists estimate that if 8,000 people took the highest valsartan dose (320 mg) from the recalled batches daily for the full 4 years, there may be one additional case of cancer over the lifetimes of these 8,000 people,” the agency said.
“To put this in context, currently one out of every three people in the U.S. will experience cancer in their lifetime,” it said.
The FDA advised patients to check their prescriptions to see if they originate from one of the recalled batches, and to let their doctors and pharmacists know if they are.
To help, the FDA has posted a list of products included in the recall and a list of products not included in the recall.
They should also follow the recall instructions provided by the specific companies, the FDA said.
The risk of cancer from N-nitrosodimethylamine (NDMA) contained in impure valsartan is real but very low, the Food and Drug Administration said in a July 27 statement.
The agency had announced a voluntary recall of valsartan from Major Pharmaceuticals, Solco Healthcare, and Teva Pharmaceuticals, as well as valsartan/hydrochlorothiazide from Solco and Teva, on July 13 after detection of NDMA, a semi-volatile organic compound. The manufacturer, Zhejiang Huahai Pharmaceuticals in Linhai, China, has since stopped distribution. Contamination probably is tied to a change in the manufacturing process.
NDMA has been linked to cancer in animal studies but at levels “much higher than the impurity levels in recalled valsartan batches.” Even so, the agency “wanted to put some context around the actual potential risk posed to patients who used versions of valsartan that may have contained high levels of NDMA,” the FDA said in its updated press release.
Based on records from the manufacturer, “some levels of the impurity may have been in the valsartan-containing products for as long as 4 years. FDA scientists estimate that if 8,000 people took the highest valsartan dose (320 mg) from the recalled batches daily for the full 4 years, there may be one additional case of cancer over the lifetimes of these 8,000 people,” the agency said.
“To put this in context, currently one out of every three people in the U.S. will experience cancer in their lifetime,” it said.
The FDA advised patients to check their prescriptions to see if they originate from one of the recalled batches, and to let their doctors and pharmacists know if they are.
To help, the FDA has posted a list of products included in the recall and a list of products not included in the recall.
They should also follow the recall instructions provided by the specific companies, the FDA said.
The risk of cancer from N-nitrosodimethylamine (NDMA) contained in impure valsartan is real but very low, the Food and Drug Administration said in a July 27 statement.
The agency had announced a voluntary recall of valsartan from Major Pharmaceuticals, Solco Healthcare, and Teva Pharmaceuticals, as well as valsartan/hydrochlorothiazide from Solco and Teva, on July 13 after detection of NDMA, a semi-volatile organic compound. The manufacturer, Zhejiang Huahai Pharmaceuticals in Linhai, China, has since stopped distribution. Contamination probably is tied to a change in the manufacturing process.
NDMA has been linked to cancer in animal studies but at levels “much higher than the impurity levels in recalled valsartan batches.” Even so, the agency “wanted to put some context around the actual potential risk posed to patients who used versions of valsartan that may have contained high levels of NDMA,” the FDA said in its updated press release.
Based on records from the manufacturer, “some levels of the impurity may have been in the valsartan-containing products for as long as 4 years. FDA scientists estimate that if 8,000 people took the highest valsartan dose (320 mg) from the recalled batches daily for the full 4 years, there may be one additional case of cancer over the lifetimes of these 8,000 people,” the agency said.
“To put this in context, currently one out of every three people in the U.S. will experience cancer in their lifetime,” it said.
The FDA advised patients to check their prescriptions to see if they originate from one of the recalled batches, and to let their doctors and pharmacists know if they are.
To help, the FDA has posted a list of products included in the recall and a list of products not included in the recall.
They should also follow the recall instructions provided by the specific companies, the FDA said.