User login
Incident heart failure linked to HIV infection
AMSTERDAM –
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“HIV infection is independently associated with a higher risk for developing heart failure, and this excess risk does not appear mediated through atherosclerotic disease pathways or differential use of cardioprotective medications,” Alan S. Go, MD, said at the 22nd International AIDS Conference.
The finding sends two important messages to physicians who care for people living with HIV, Dr. Go said in a video interview. First, have “greater awareness for the risk of heart failure” in people living with HIV, even in those who have excellent [HIV] treatment. Be on the lookout, he recommended, for classic symptoms of heart failure like dyspnea and fatigue, and if found follow-up with an assessment of heart function, usually by echocardiography. The second message is to pay attention to and aggressively treat risk factors for heart failure, such as hypertension, smoking, obesity, diabetes, and hypercholesterolemia, said Dr. Go, director of the Comprehensive Clinical Research Unit of Kaiser Permanente in Oakland, Calif.
Results from a small number of prior studies also suggested an increased heart failure rate in people infected with HIV, but those reports had not been able to untangle this observed increase from a possible relationship to the elevated rate of MIs among people living with HIV. The study led by Dr. Go adjusted for acute coronary syndrome events that occurred during follow-up in the analysis and this showed that the increased incidence of heart failure occurred independently of any preceding MI or unstable angina event.
Dr. Go proposed several potential mechanisms that could tie HIV infection to an elevated heart failure risk that was not linked to a prior ischemic heart disease event. The virus could directly damage cardiac myocytes to produce fibrosis, the virus could trigger cardiac inflammation, and the infected person could have an increased susceptibility to infection by a pathogen know to potentially cause cardiac damage and myocarditis such as coxsackievirus.
For the time being, patients infected by HIV who develop heart failure should receive the same treatments that are recommended for the general population, Dr. Go said, but he also highlighted the need for further study to determine the effectiveness of standard heart failure treatments specifically in people living with HIV. He and his associates are also currently analyzing the relationship of several other variables to the risk for heart failure in HIV-infected people, such as the degree of HIV control, and the types of antiretroviral therapy that patients receive. So far the study has not shown a relationship between HIV infection and any specific type of heart failure. About a quarter of the HIV-infected people who developed heart failure in this study had reduced left ventricular ejection fraction, about a quarter had preserved ejection fraction, and for the remaining patients information on their left ventricular ejection fraction was not available, Dr. Go said.
The Kaiser Permanente HIV Heart Study used data from health records from about 13.5 million people enrolled in the health system during 2000-2016 at locations in northern California, southern California, or the mid-Atlantic region. From these records the researchers identified 38,868 people diagnosed with an HIV infection, free of a heart failure diagnosis, and at least 21 years old, and matched them by age, sex, and race with 386,586 people in the health system who were both uninfected and free of heart failure. At “baseline” in the analysis the two study groups had very similar rates of smoking, but those with HIV had somewhat more alcohol abuse and nearly twice the rate of illicit drug use, although even among those with HIV this rate was low at 4%.
Some clinical characteristics at baseline showed significant differences between the two groups. People living with HIV had substantially less hypertension, 7% compared with 12% in those without HIV; half the rate of dyslipidemia, 8% compared with 16% among the control group; and nearly half the prevalence of diabetes, 3% versus 5% among those without HIV. On the other hand, certain other clinical characteristics were more common among those with HIV. The prevalence at baseline of diagnosed dementia was 15% among people infected with HIV and essentially nonexistent (less than 1%) among controls, and the prevalence of diagnosed depression was 8% among people with HIV and 5% among those without the infection.
Baseline parameters also showed that at the time this review first identified a person with HIV and without heart failure in the system records only 18% of the HIV-infected individuals were on an antiretroviral therapy regimen. Dr. Go said that the study is currently analyzing subsequent HIV treatments that these patients may have received. Also at “baseline” 13% of people with documented HIV infection had a CD4 cell count of fewer than 200 cell/mm3, with 4% having fewer than 50 CD4 cells/mm3, and 29% of those with HIV had a blood level of at least 500 copies of HIV RNA/mL. In addition, information on CD4 cell counts was unavailable for 43% of these people, and information on viral load was unavailable for about half.
During “follow-up” in the system’s medical records for a period of up to 17 years, diagnoses of incident heart failure accumulated significantly faster among people with HIV compared to those without HIV. After adjustment for demographic differences, the time of entry into the health system, cardiovascular and other medical differences, and differences in medication use, people living with HIV had a 75% higher rate of incident heart failure compared with those without HIV. Further adjustment based on incident first episodes of acute coronary syndrome during “follow-up” brought the excess rate of heart failure to 66% higher among people infected by HIV, Dr. Go reported. He cautioned that the findings came from a U.S. population that had access to comprehensive health care.
SOURCE: Go AS et al. AIDS 2018, Abstract 2778, THAB0103.
AMSTERDAM –
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“HIV infection is independently associated with a higher risk for developing heart failure, and this excess risk does not appear mediated through atherosclerotic disease pathways or differential use of cardioprotective medications,” Alan S. Go, MD, said at the 22nd International AIDS Conference.
The finding sends two important messages to physicians who care for people living with HIV, Dr. Go said in a video interview. First, have “greater awareness for the risk of heart failure” in people living with HIV, even in those who have excellent [HIV] treatment. Be on the lookout, he recommended, for classic symptoms of heart failure like dyspnea and fatigue, and if found follow-up with an assessment of heart function, usually by echocardiography. The second message is to pay attention to and aggressively treat risk factors for heart failure, such as hypertension, smoking, obesity, diabetes, and hypercholesterolemia, said Dr. Go, director of the Comprehensive Clinical Research Unit of Kaiser Permanente in Oakland, Calif.
Results from a small number of prior studies also suggested an increased heart failure rate in people infected with HIV, but those reports had not been able to untangle this observed increase from a possible relationship to the elevated rate of MIs among people living with HIV. The study led by Dr. Go adjusted for acute coronary syndrome events that occurred during follow-up in the analysis and this showed that the increased incidence of heart failure occurred independently of any preceding MI or unstable angina event.
Dr. Go proposed several potential mechanisms that could tie HIV infection to an elevated heart failure risk that was not linked to a prior ischemic heart disease event. The virus could directly damage cardiac myocytes to produce fibrosis, the virus could trigger cardiac inflammation, and the infected person could have an increased susceptibility to infection by a pathogen know to potentially cause cardiac damage and myocarditis such as coxsackievirus.
For the time being, patients infected by HIV who develop heart failure should receive the same treatments that are recommended for the general population, Dr. Go said, but he also highlighted the need for further study to determine the effectiveness of standard heart failure treatments specifically in people living with HIV. He and his associates are also currently analyzing the relationship of several other variables to the risk for heart failure in HIV-infected people, such as the degree of HIV control, and the types of antiretroviral therapy that patients receive. So far the study has not shown a relationship between HIV infection and any specific type of heart failure. About a quarter of the HIV-infected people who developed heart failure in this study had reduced left ventricular ejection fraction, about a quarter had preserved ejection fraction, and for the remaining patients information on their left ventricular ejection fraction was not available, Dr. Go said.
The Kaiser Permanente HIV Heart Study used data from health records from about 13.5 million people enrolled in the health system during 2000-2016 at locations in northern California, southern California, or the mid-Atlantic region. From these records the researchers identified 38,868 people diagnosed with an HIV infection, free of a heart failure diagnosis, and at least 21 years old, and matched them by age, sex, and race with 386,586 people in the health system who were both uninfected and free of heart failure. At “baseline” in the analysis the two study groups had very similar rates of smoking, but those with HIV had somewhat more alcohol abuse and nearly twice the rate of illicit drug use, although even among those with HIV this rate was low at 4%.
Some clinical characteristics at baseline showed significant differences between the two groups. People living with HIV had substantially less hypertension, 7% compared with 12% in those without HIV; half the rate of dyslipidemia, 8% compared with 16% among the control group; and nearly half the prevalence of diabetes, 3% versus 5% among those without HIV. On the other hand, certain other clinical characteristics were more common among those with HIV. The prevalence at baseline of diagnosed dementia was 15% among people infected with HIV and essentially nonexistent (less than 1%) among controls, and the prevalence of diagnosed depression was 8% among people with HIV and 5% among those without the infection.
Baseline parameters also showed that at the time this review first identified a person with HIV and without heart failure in the system records only 18% of the HIV-infected individuals were on an antiretroviral therapy regimen. Dr. Go said that the study is currently analyzing subsequent HIV treatments that these patients may have received. Also at “baseline” 13% of people with documented HIV infection had a CD4 cell count of fewer than 200 cell/mm3, with 4% having fewer than 50 CD4 cells/mm3, and 29% of those with HIV had a blood level of at least 500 copies of HIV RNA/mL. In addition, information on CD4 cell counts was unavailable for 43% of these people, and information on viral load was unavailable for about half.
During “follow-up” in the system’s medical records for a period of up to 17 years, diagnoses of incident heart failure accumulated significantly faster among people with HIV compared to those without HIV. After adjustment for demographic differences, the time of entry into the health system, cardiovascular and other medical differences, and differences in medication use, people living with HIV had a 75% higher rate of incident heart failure compared with those without HIV. Further adjustment based on incident first episodes of acute coronary syndrome during “follow-up” brought the excess rate of heart failure to 66% higher among people infected by HIV, Dr. Go reported. He cautioned that the findings came from a U.S. population that had access to comprehensive health care.
SOURCE: Go AS et al. AIDS 2018, Abstract 2778, THAB0103.
AMSTERDAM –
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“HIV infection is independently associated with a higher risk for developing heart failure, and this excess risk does not appear mediated through atherosclerotic disease pathways or differential use of cardioprotective medications,” Alan S. Go, MD, said at the 22nd International AIDS Conference.
The finding sends two important messages to physicians who care for people living with HIV, Dr. Go said in a video interview. First, have “greater awareness for the risk of heart failure” in people living with HIV, even in those who have excellent [HIV] treatment. Be on the lookout, he recommended, for classic symptoms of heart failure like dyspnea and fatigue, and if found follow-up with an assessment of heart function, usually by echocardiography. The second message is to pay attention to and aggressively treat risk factors for heart failure, such as hypertension, smoking, obesity, diabetes, and hypercholesterolemia, said Dr. Go, director of the Comprehensive Clinical Research Unit of Kaiser Permanente in Oakland, Calif.
Results from a small number of prior studies also suggested an increased heart failure rate in people infected with HIV, but those reports had not been able to untangle this observed increase from a possible relationship to the elevated rate of MIs among people living with HIV. The study led by Dr. Go adjusted for acute coronary syndrome events that occurred during follow-up in the analysis and this showed that the increased incidence of heart failure occurred independently of any preceding MI or unstable angina event.
Dr. Go proposed several potential mechanisms that could tie HIV infection to an elevated heart failure risk that was not linked to a prior ischemic heart disease event. The virus could directly damage cardiac myocytes to produce fibrosis, the virus could trigger cardiac inflammation, and the infected person could have an increased susceptibility to infection by a pathogen know to potentially cause cardiac damage and myocarditis such as coxsackievirus.
For the time being, patients infected by HIV who develop heart failure should receive the same treatments that are recommended for the general population, Dr. Go said, but he also highlighted the need for further study to determine the effectiveness of standard heart failure treatments specifically in people living with HIV. He and his associates are also currently analyzing the relationship of several other variables to the risk for heart failure in HIV-infected people, such as the degree of HIV control, and the types of antiretroviral therapy that patients receive. So far the study has not shown a relationship between HIV infection and any specific type of heart failure. About a quarter of the HIV-infected people who developed heart failure in this study had reduced left ventricular ejection fraction, about a quarter had preserved ejection fraction, and for the remaining patients information on their left ventricular ejection fraction was not available, Dr. Go said.
The Kaiser Permanente HIV Heart Study used data from health records from about 13.5 million people enrolled in the health system during 2000-2016 at locations in northern California, southern California, or the mid-Atlantic region. From these records the researchers identified 38,868 people diagnosed with an HIV infection, free of a heart failure diagnosis, and at least 21 years old, and matched them by age, sex, and race with 386,586 people in the health system who were both uninfected and free of heart failure. At “baseline” in the analysis the two study groups had very similar rates of smoking, but those with HIV had somewhat more alcohol abuse and nearly twice the rate of illicit drug use, although even among those with HIV this rate was low at 4%.
Some clinical characteristics at baseline showed significant differences between the two groups. People living with HIV had substantially less hypertension, 7% compared with 12% in those without HIV; half the rate of dyslipidemia, 8% compared with 16% among the control group; and nearly half the prevalence of diabetes, 3% versus 5% among those without HIV. On the other hand, certain other clinical characteristics were more common among those with HIV. The prevalence at baseline of diagnosed dementia was 15% among people infected with HIV and essentially nonexistent (less than 1%) among controls, and the prevalence of diagnosed depression was 8% among people with HIV and 5% among those without the infection.
Baseline parameters also showed that at the time this review first identified a person with HIV and without heart failure in the system records only 18% of the HIV-infected individuals were on an antiretroviral therapy regimen. Dr. Go said that the study is currently analyzing subsequent HIV treatments that these patients may have received. Also at “baseline” 13% of people with documented HIV infection had a CD4 cell count of fewer than 200 cell/mm3, with 4% having fewer than 50 CD4 cells/mm3, and 29% of those with HIV had a blood level of at least 500 copies of HIV RNA/mL. In addition, information on CD4 cell counts was unavailable for 43% of these people, and information on viral load was unavailable for about half.
During “follow-up” in the system’s medical records for a period of up to 17 years, diagnoses of incident heart failure accumulated significantly faster among people with HIV compared to those without HIV. After adjustment for demographic differences, the time of entry into the health system, cardiovascular and other medical differences, and differences in medication use, people living with HIV had a 75% higher rate of incident heart failure compared with those without HIV. Further adjustment based on incident first episodes of acute coronary syndrome during “follow-up” brought the excess rate of heart failure to 66% higher among people infected by HIV, Dr. Go reported. He cautioned that the findings came from a U.S. population that had access to comprehensive health care.
SOURCE: Go AS et al. AIDS 2018, Abstract 2778, THAB0103.
REPORTING FROM AIDS 2018
Key clinical point: HIV infection may be an independent trigger for heart failure.
Major finding: After extensive adjustment for potential confounders, HIV infection linked with a 66% increased rate of incident heart failure.
Study details: The Kaiser Permanente HIV Heart Study, which included medical records for 425,454 people.
Disclosures: Dr. Go had no disclosures.
Source: Go AS et al. AIDS 2018, Abstract 2778, THAB0103.
Ten tips for managing patients with both heart failure and COPD
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
EXPERT ANALYSIS FROM CHEST JOURNAL
Medicare’s bundled pay plan didn’t deliver big cost savings
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Participation in Medicare’s Bundled Payments for Care Improvement (BPCI) initiative didn’t significantly change payments per episode for the top five medical conditions selected under the program.
Major finding: Baseline payments per episode for those conditions were a mean of $24,280 before participation in the BPCI, and $23,993 after adoption (P = .41).
Study details: A retrospective analysis of Medicare data for 125 hospitals participating in the program and matched control hospitals.
Disclosures: The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine.
Source: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
ICD use curbed in hospitals named in federal lawsuit
A 2005 update in Medicare reimbursement policy had a modest effect on the use of implantable cardioverter defibrillators for primary prevention, but it took a whistle-blower and a federal lawsuit to bring the numbers down substantially.
Usage dropped just slightly from 2007 to 2009, after Medicare updated its appropriate use criteria, Nihar R. Desai, MD, MPH, and his colleagues reported in JAMA. From 2010 to 2011, after the Department of Justice suit became public knowledge, the declines were significantly greater: 7.4% in hospitals that eventually settled for a total of $280 million, and 4.7% in hospitals that weren’t named in the suit.
The government launched the suit in 2010, after a whistle-blower used Medicare data to allege that many hospitals weren’t waiting the appropriate amount of time to implant an implantable cardioverter defibrillator (ICD) after a heart attack or coronary revascularization, wrote Dr. Desai of Yale University, New Haven, Conn., and his team. These procedures would have been against the 2005 Centers for Medicare & Medicaid Services National Coverage Determination (NCD), which required delaying implantation for 40 days after a heart attack and 90 days after a revascularization.
Just a year after the suit was filed, an independent investigator concluded that 22.5% of the ICDs implanted from 2006 to 2009 for primary prevention were not evidence based.
Dr. Desai and his coauthors used CMS data to examine changes in the proportion of primary-prevention ICD implantations at hospitals that eventually settled the suit, and those that did not. The study spanned 2007-2015 and comprised 1,809 U.S. hospitals in the National Cardiovascular Data Registry ICD Registry; of these, 452 hospitals that had done 99,591 procedures reached settlements.
After the steeper drops in 2010 and 2011, the number of procedures leveled off. From July 2011 to 2015, the proportions of ICDs not meeting the NCD criteria were similar and stable in both hospital settlement groups, with an annual change of −0.5% for settlement hospitals and −0.4% for nonsettlement hospitals, the team wrote.
Despite the changes, there was “persistent variation” among hospitals, with more than 14% of the primary-prevention ICDs not meeting NCD criteria at some of the worst-performing hospitals.
The decreases weren’t just in Medicare beneficiaries, though. Hospitals were rethinking this indication for ICD use in everyone, although the investigators found no evidence that the changing clinical landscape endangered the health of patients.
“The analyses of secondary prevention ICDs do not suggest that access to necessary procedures was negatively affected by the investigation. … These analyses offer some reassurance, but further research into hospital responses to the investigation could offer additional insights about possible unintended consequences,” the investigators wrote.
The study was sponsored by the Agency for Healthcare Research and Quality. Dr. Desai had no financial disclosures.
SOURCE: Desai NR et al. JAMA. 2018; 320: 63-71.
The federal investigation into inappropriate use of implanted cardioverter defibrillators (ICDs) appeared to be highly effective, both in recovering costs and changing behavior at hospitals.
Even though individuals were not the focus of the investigation, many physicians sensed a new exposure to civil liability, if not criminal penalties, and felt accused of providing substandard care. Did this investigation have the intended effect of improving care?
The numbers suggest it did.
The observed decrease in use raises the question of whether appropriate ICDs were also avoided, a potential unintended consequence of the investigation. The study by Desai et al noted that ICD implantations for secondary prevention remained relatively stable during this period, suggesting that appropriate ICD use likely did not decline substantially.
The investigation also clearly showed the power of a large, financially intimidating legal action.
The mere announcement of the investigation appeared to have a large and immediate influence on prompting hospitals to limit inappropriate ICD implantation for primary prevention. As a form of audit and feedback, the Department of Justice investigation appeared to be highly effective in changing practice. Past studies of audit and feedback show relatively modest effects on changing physician behavior, although these studies did not involve allegations of fraud with financial penalties. Clearly, the reward or penalty attached to the feedback influences clinician behavior with penalties likely more effective in promoting change.
Paul A. Heidenreich, MD, professor of cardiovascular medicine at Stanford (Calif.) University, made these comments in an accompanying editorial (JAMA. 2018; 320:40-2).
The federal investigation into inappropriate use of implanted cardioverter defibrillators (ICDs) appeared to be highly effective, both in recovering costs and changing behavior at hospitals.
Even though individuals were not the focus of the investigation, many physicians sensed a new exposure to civil liability, if not criminal penalties, and felt accused of providing substandard care. Did this investigation have the intended effect of improving care?
The numbers suggest it did.
The observed decrease in use raises the question of whether appropriate ICDs were also avoided, a potential unintended consequence of the investigation. The study by Desai et al noted that ICD implantations for secondary prevention remained relatively stable during this period, suggesting that appropriate ICD use likely did not decline substantially.
The investigation also clearly showed the power of a large, financially intimidating legal action.
The mere announcement of the investigation appeared to have a large and immediate influence on prompting hospitals to limit inappropriate ICD implantation for primary prevention. As a form of audit and feedback, the Department of Justice investigation appeared to be highly effective in changing practice. Past studies of audit and feedback show relatively modest effects on changing physician behavior, although these studies did not involve allegations of fraud with financial penalties. Clearly, the reward or penalty attached to the feedback influences clinician behavior with penalties likely more effective in promoting change.
Paul A. Heidenreich, MD, professor of cardiovascular medicine at Stanford (Calif.) University, made these comments in an accompanying editorial (JAMA. 2018; 320:40-2).
The federal investigation into inappropriate use of implanted cardioverter defibrillators (ICDs) appeared to be highly effective, both in recovering costs and changing behavior at hospitals.
Even though individuals were not the focus of the investigation, many physicians sensed a new exposure to civil liability, if not criminal penalties, and felt accused of providing substandard care. Did this investigation have the intended effect of improving care?
The numbers suggest it did.
The observed decrease in use raises the question of whether appropriate ICDs were also avoided, a potential unintended consequence of the investigation. The study by Desai et al noted that ICD implantations for secondary prevention remained relatively stable during this period, suggesting that appropriate ICD use likely did not decline substantially.
The investigation also clearly showed the power of a large, financially intimidating legal action.
The mere announcement of the investigation appeared to have a large and immediate influence on prompting hospitals to limit inappropriate ICD implantation for primary prevention. As a form of audit and feedback, the Department of Justice investigation appeared to be highly effective in changing practice. Past studies of audit and feedback show relatively modest effects on changing physician behavior, although these studies did not involve allegations of fraud with financial penalties. Clearly, the reward or penalty attached to the feedback influences clinician behavior with penalties likely more effective in promoting change.
Paul A. Heidenreich, MD, professor of cardiovascular medicine at Stanford (Calif.) University, made these comments in an accompanying editorial (JAMA. 2018; 320:40-2).
A 2005 update in Medicare reimbursement policy had a modest effect on the use of implantable cardioverter defibrillators for primary prevention, but it took a whistle-blower and a federal lawsuit to bring the numbers down substantially.
Usage dropped just slightly from 2007 to 2009, after Medicare updated its appropriate use criteria, Nihar R. Desai, MD, MPH, and his colleagues reported in JAMA. From 2010 to 2011, after the Department of Justice suit became public knowledge, the declines were significantly greater: 7.4% in hospitals that eventually settled for a total of $280 million, and 4.7% in hospitals that weren’t named in the suit.
The government launched the suit in 2010, after a whistle-blower used Medicare data to allege that many hospitals weren’t waiting the appropriate amount of time to implant an implantable cardioverter defibrillator (ICD) after a heart attack or coronary revascularization, wrote Dr. Desai of Yale University, New Haven, Conn., and his team. These procedures would have been against the 2005 Centers for Medicare & Medicaid Services National Coverage Determination (NCD), which required delaying implantation for 40 days after a heart attack and 90 days after a revascularization.
Just a year after the suit was filed, an independent investigator concluded that 22.5% of the ICDs implanted from 2006 to 2009 for primary prevention were not evidence based.
Dr. Desai and his coauthors used CMS data to examine changes in the proportion of primary-prevention ICD implantations at hospitals that eventually settled the suit, and those that did not. The study spanned 2007-2015 and comprised 1,809 U.S. hospitals in the National Cardiovascular Data Registry ICD Registry; of these, 452 hospitals that had done 99,591 procedures reached settlements.
After the steeper drops in 2010 and 2011, the number of procedures leveled off. From July 2011 to 2015, the proportions of ICDs not meeting the NCD criteria were similar and stable in both hospital settlement groups, with an annual change of −0.5% for settlement hospitals and −0.4% for nonsettlement hospitals, the team wrote.
Despite the changes, there was “persistent variation” among hospitals, with more than 14% of the primary-prevention ICDs not meeting NCD criteria at some of the worst-performing hospitals.
The decreases weren’t just in Medicare beneficiaries, though. Hospitals were rethinking this indication for ICD use in everyone, although the investigators found no evidence that the changing clinical landscape endangered the health of patients.
“The analyses of secondary prevention ICDs do not suggest that access to necessary procedures was negatively affected by the investigation. … These analyses offer some reassurance, but further research into hospital responses to the investigation could offer additional insights about possible unintended consequences,” the investigators wrote.
The study was sponsored by the Agency for Healthcare Research and Quality. Dr. Desai had no financial disclosures.
SOURCE: Desai NR et al. JAMA. 2018; 320: 63-71.
A 2005 update in Medicare reimbursement policy had a modest effect on the use of implantable cardioverter defibrillators for primary prevention, but it took a whistle-blower and a federal lawsuit to bring the numbers down substantially.
Usage dropped just slightly from 2007 to 2009, after Medicare updated its appropriate use criteria, Nihar R. Desai, MD, MPH, and his colleagues reported in JAMA. From 2010 to 2011, after the Department of Justice suit became public knowledge, the declines were significantly greater: 7.4% in hospitals that eventually settled for a total of $280 million, and 4.7% in hospitals that weren’t named in the suit.
The government launched the suit in 2010, after a whistle-blower used Medicare data to allege that many hospitals weren’t waiting the appropriate amount of time to implant an implantable cardioverter defibrillator (ICD) after a heart attack or coronary revascularization, wrote Dr. Desai of Yale University, New Haven, Conn., and his team. These procedures would have been against the 2005 Centers for Medicare & Medicaid Services National Coverage Determination (NCD), which required delaying implantation for 40 days after a heart attack and 90 days after a revascularization.
Just a year after the suit was filed, an independent investigator concluded that 22.5% of the ICDs implanted from 2006 to 2009 for primary prevention were not evidence based.
Dr. Desai and his coauthors used CMS data to examine changes in the proportion of primary-prevention ICD implantations at hospitals that eventually settled the suit, and those that did not. The study spanned 2007-2015 and comprised 1,809 U.S. hospitals in the National Cardiovascular Data Registry ICD Registry; of these, 452 hospitals that had done 99,591 procedures reached settlements.
After the steeper drops in 2010 and 2011, the number of procedures leveled off. From July 2011 to 2015, the proportions of ICDs not meeting the NCD criteria were similar and stable in both hospital settlement groups, with an annual change of −0.5% for settlement hospitals and −0.4% for nonsettlement hospitals, the team wrote.
Despite the changes, there was “persistent variation” among hospitals, with more than 14% of the primary-prevention ICDs not meeting NCD criteria at some of the worst-performing hospitals.
The decreases weren’t just in Medicare beneficiaries, though. Hospitals were rethinking this indication for ICD use in everyone, although the investigators found no evidence that the changing clinical landscape endangered the health of patients.
“The analyses of secondary prevention ICDs do not suggest that access to necessary procedures was negatively affected by the investigation. … These analyses offer some reassurance, but further research into hospital responses to the investigation could offer additional insights about possible unintended consequences,” the investigators wrote.
The study was sponsored by the Agency for Healthcare Research and Quality. Dr. Desai had no financial disclosures.
SOURCE: Desai NR et al. JAMA. 2018; 320: 63-71.
FROM JAMA
Key clinical point: A federal lawsuit against hospitals changed the practice of using implantable cardioverter defibrillators for primary prevention.
Major finding: In the year after the Department of Justice lawsuit was announced, ICD for primary prevention use dropped 7.4% in hospitals that settled and dropped 4.7% in hospitals that were nonsettlers.
Study details: An analysis of data from the Centers for Medicare & Medicaid Services.
Disclosures: The Agency for Healthcare Research and Quality sponsored the study. Dr. Desai had no financial disclosures.
Source: Desai NR et al. JAMA. 2018; 320:63-71.
Heart failure confers poor prognosis in rheumatoid arthritis
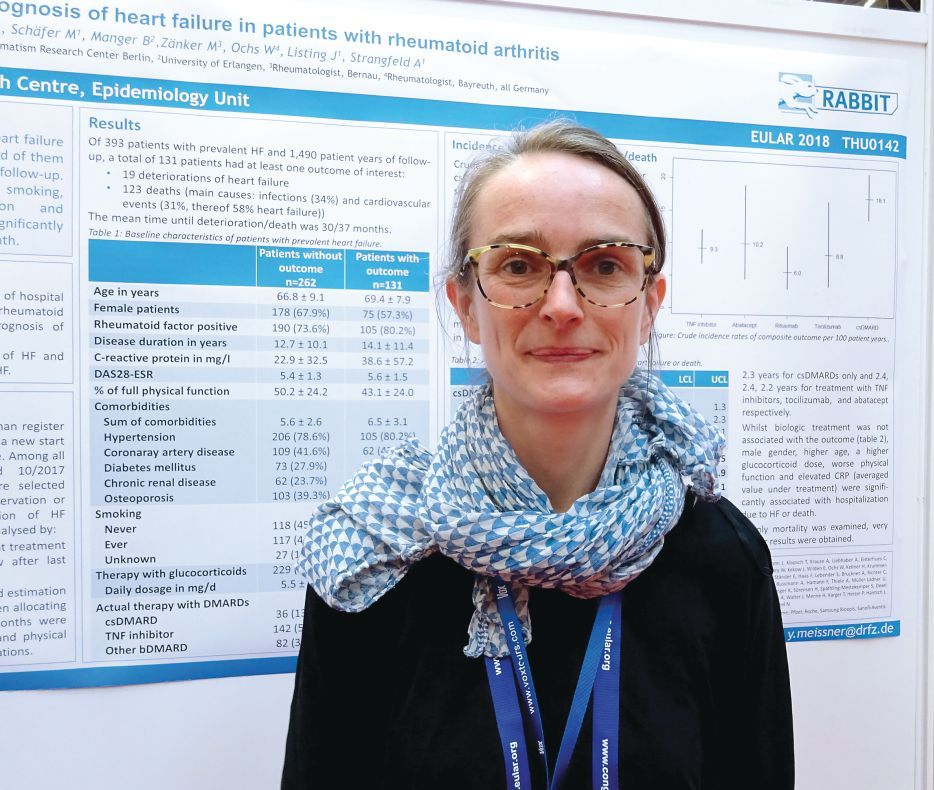
AMSTERDAM – The combination of heart failure and rheumatoid arthritis carries a poor prognosis, according to data from the German biologics register.
Of 393 patients enrolled in RABBIT (Rheumatoide Arthritis: Beobachtung der Biologika-Therapie) who had heart failure in addition to their rheumatoid arthritis, 131 (33%) needed hospital treatment or died over a 10-year period. The mean time to hospitalization or death was 2.5-3.0 years.
“We include patients at the start of their DMARD [disease modifying antirheumatic drug] treatment and follow them up for at least 5 years,” said Dr. Meissner, explaining how the German biologics register works. “For this analysis, we selected all the patients with prevalent heart failure and followed them up until either the database was closed, they dropped out, or had an event.” In this case, an event was defined as a composite of deterioration in heart failure that required hospitalization or death from any cause.
Dr. Meissner, of the German Rheumatism Research Center in Berlin, noted that 19 (14.5%) patients experienced a deterioration in their heart failure and 123 deaths were recorded during the study period that started in May 2001 and ended in October 2017. Around one-third of deaths were attributable to infections (34%), and one-third were attributable to cardiovascular causes (31%). Of the CV deaths, more than half (58%) were attributed to patients’ heart failure.
“What impressed us the most is the number of comorbidities at baseline,” Dr. Meissner said. Not including heart failure or rheumatoid arthritis, patients who experienced an event had an average of 6.5 comorbidities versus 5.6 for those who did not have an event. These additional comorbidities included hypertension, coronary artery disease, diabetes mellitus, chronic renal disease, and osteoporosis.
Crude incidence rates (IRs) for heart failure deterioration or death were calculated according to the rheumatoid arthritis treatment being used and found to be highest in those treated with conventional DMARDs, at 18.1/100 patient-years. IRs with biologic treatments were lower, at 10.2/100 patient-years for abatacept, 9.3 for TNF inhibitors, 8.8 for tocilizumab, and 6.0 for rituximab. What this suggests it that better control of inflammation results in a lower risk for hospitalization and death, Dr. Meissner and her associates reported in their poster presentation.
“If patients are not effectively treated for rheumatoid arthritis, there might be other consequences like the deterioration of heart failure or death,” Dr. Meissner said.
She noted that investigators also looked at identifying risk factors for hospitalization or death and found that there was a greater adjusted relative risk if patients were male (RR = 2.4), older (RR = 1.3 per 5-year increase in age), or if they smoked (RR = 1.7). Using higher doses of glucocorticoids also was a risk factor, with a RR of 1.4 for every 5 mg/day increase in dose.
Better physical function was associated with a lower risk of an event (RR = 0.9) and, along with smoking and adjustment of the steroid dose, is a risk factor that could potentially be influenced, Dr. Meissner proposed.
No data on how the heart failure was being managed are available for this cohort and more research is needed.
“There is still not enough known about this topic, and we need the research to determine how to best manage these patients,” Dr. Meissner said. “The time to an event was only 3 years from the time of inclusion in the register, so we need better management of those patients with heart failure as a comorbidity.”
RABBIT is supported by a joint, unconditional grant from AbbVie, Bristol-Myers Squibb, Celltrion, Hexal AG, Lilly, MSD Sharp & Dohme, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. Dr. Meissner disclosed being on a speakers bureau for Pfizer.
SOURCE: Meissner Y et al. EULAR 2018 Congress. Abstract THU0142 .
AMSTERDAM – The combination of heart failure and rheumatoid arthritis carries a poor prognosis, according to data from the German biologics register.
Of 393 patients enrolled in RABBIT (Rheumatoide Arthritis: Beobachtung der Biologika-Therapie) who had heart failure in addition to their rheumatoid arthritis, 131 (33%) needed hospital treatment or died over a 10-year period. The mean time to hospitalization or death was 2.5-3.0 years.
“We include patients at the start of their DMARD [disease modifying antirheumatic drug] treatment and follow them up for at least 5 years,” said Dr. Meissner, explaining how the German biologics register works. “For this analysis, we selected all the patients with prevalent heart failure and followed them up until either the database was closed, they dropped out, or had an event.” In this case, an event was defined as a composite of deterioration in heart failure that required hospitalization or death from any cause.
Dr. Meissner, of the German Rheumatism Research Center in Berlin, noted that 19 (14.5%) patients experienced a deterioration in their heart failure and 123 deaths were recorded during the study period that started in May 2001 and ended in October 2017. Around one-third of deaths were attributable to infections (34%), and one-third were attributable to cardiovascular causes (31%). Of the CV deaths, more than half (58%) were attributed to patients’ heart failure.
“What impressed us the most is the number of comorbidities at baseline,” Dr. Meissner said. Not including heart failure or rheumatoid arthritis, patients who experienced an event had an average of 6.5 comorbidities versus 5.6 for those who did not have an event. These additional comorbidities included hypertension, coronary artery disease, diabetes mellitus, chronic renal disease, and osteoporosis.
Crude incidence rates (IRs) for heart failure deterioration or death were calculated according to the rheumatoid arthritis treatment being used and found to be highest in those treated with conventional DMARDs, at 18.1/100 patient-years. IRs with biologic treatments were lower, at 10.2/100 patient-years for abatacept, 9.3 for TNF inhibitors, 8.8 for tocilizumab, and 6.0 for rituximab. What this suggests it that better control of inflammation results in a lower risk for hospitalization and death, Dr. Meissner and her associates reported in their poster presentation.
“If patients are not effectively treated for rheumatoid arthritis, there might be other consequences like the deterioration of heart failure or death,” Dr. Meissner said.
She noted that investigators also looked at identifying risk factors for hospitalization or death and found that there was a greater adjusted relative risk if patients were male (RR = 2.4), older (RR = 1.3 per 5-year increase in age), or if they smoked (RR = 1.7). Using higher doses of glucocorticoids also was a risk factor, with a RR of 1.4 for every 5 mg/day increase in dose.
Better physical function was associated with a lower risk of an event (RR = 0.9) and, along with smoking and adjustment of the steroid dose, is a risk factor that could potentially be influenced, Dr. Meissner proposed.
No data on how the heart failure was being managed are available for this cohort and more research is needed.
“There is still not enough known about this topic, and we need the research to determine how to best manage these patients,” Dr. Meissner said. “The time to an event was only 3 years from the time of inclusion in the register, so we need better management of those patients with heart failure as a comorbidity.”
RABBIT is supported by a joint, unconditional grant from AbbVie, Bristol-Myers Squibb, Celltrion, Hexal AG, Lilly, MSD Sharp & Dohme, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. Dr. Meissner disclosed being on a speakers bureau for Pfizer.
SOURCE: Meissner Y et al. EULAR 2018 Congress. Abstract THU0142 .
AMSTERDAM – The combination of heart failure and rheumatoid arthritis carries a poor prognosis, according to data from the German biologics register.
Of 393 patients enrolled in RABBIT (Rheumatoide Arthritis: Beobachtung der Biologika-Therapie) who had heart failure in addition to their rheumatoid arthritis, 131 (33%) needed hospital treatment or died over a 10-year period. The mean time to hospitalization or death was 2.5-3.0 years.
“We include patients at the start of their DMARD [disease modifying antirheumatic drug] treatment and follow them up for at least 5 years,” said Dr. Meissner, explaining how the German biologics register works. “For this analysis, we selected all the patients with prevalent heart failure and followed them up until either the database was closed, they dropped out, or had an event.” In this case, an event was defined as a composite of deterioration in heart failure that required hospitalization or death from any cause.
Dr. Meissner, of the German Rheumatism Research Center in Berlin, noted that 19 (14.5%) patients experienced a deterioration in their heart failure and 123 deaths were recorded during the study period that started in May 2001 and ended in October 2017. Around one-third of deaths were attributable to infections (34%), and one-third were attributable to cardiovascular causes (31%). Of the CV deaths, more than half (58%) were attributed to patients’ heart failure.
“What impressed us the most is the number of comorbidities at baseline,” Dr. Meissner said. Not including heart failure or rheumatoid arthritis, patients who experienced an event had an average of 6.5 comorbidities versus 5.6 for those who did not have an event. These additional comorbidities included hypertension, coronary artery disease, diabetes mellitus, chronic renal disease, and osteoporosis.
Crude incidence rates (IRs) for heart failure deterioration or death were calculated according to the rheumatoid arthritis treatment being used and found to be highest in those treated with conventional DMARDs, at 18.1/100 patient-years. IRs with biologic treatments were lower, at 10.2/100 patient-years for abatacept, 9.3 for TNF inhibitors, 8.8 for tocilizumab, and 6.0 for rituximab. What this suggests it that better control of inflammation results in a lower risk for hospitalization and death, Dr. Meissner and her associates reported in their poster presentation.
“If patients are not effectively treated for rheumatoid arthritis, there might be other consequences like the deterioration of heart failure or death,” Dr. Meissner said.
She noted that investigators also looked at identifying risk factors for hospitalization or death and found that there was a greater adjusted relative risk if patients were male (RR = 2.4), older (RR = 1.3 per 5-year increase in age), or if they smoked (RR = 1.7). Using higher doses of glucocorticoids also was a risk factor, with a RR of 1.4 for every 5 mg/day increase in dose.
Better physical function was associated with a lower risk of an event (RR = 0.9) and, along with smoking and adjustment of the steroid dose, is a risk factor that could potentially be influenced, Dr. Meissner proposed.
No data on how the heart failure was being managed are available for this cohort and more research is needed.
“There is still not enough known about this topic, and we need the research to determine how to best manage these patients,” Dr. Meissner said. “The time to an event was only 3 years from the time of inclusion in the register, so we need better management of those patients with heart failure as a comorbidity.”
RABBIT is supported by a joint, unconditional grant from AbbVie, Bristol-Myers Squibb, Celltrion, Hexal AG, Lilly, MSD Sharp & Dohme, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. Dr. Meissner disclosed being on a speakers bureau for Pfizer.
SOURCE: Meissner Y et al. EULAR 2018 Congress. Abstract THU0142 .
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Comorbid heart failure and rheumatoid arthritis carry an unfavorable prognosis, but risk factors have been identified.
Major finding: One-third of patients were hospitalized or died, with a mean time to deterioration or death of 2.5-3 years.
Study details: 393 patients with both heart failure and rheumatoid arthritis enrolled in the German biologics register RABBIT.
Disclosures: RABBIT is supported by a joint, unconditional grant from AbbVie, Bristol-Myers Squibb, Celltrion, Hexal AG, Lilly, MSD Sharp & Dohme, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB. Dr. Meissner disclosed being part of a speakers bureau for Pfizer.
Source: Meissner Y et al. EULAR 2018 Congress, Abstract THU0142.
Galectin-3: A new post-MI prognostic biomarker?
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
REPORTING FROM ACC 18
Key clinical point: Galectin-3 level post-MI is a potent long-term predictor of both heart failure and mortality independent of known prognostic markers.
Major finding: Post-MI patients in the top tertile of circulating galectin-3 were at an adjusted 2.4-fold increased mortality risk and a 2.05-fold greater risk of developing heart failure compared with those in the lowest tertile.
Study details: This prospective population-based cohort study included 1,401 MI patients followed for a mean of 5.3 years.
Disclosures: The National Institutes of Health supported the study. The presenter reported having no financial conflicts of interest.
TAVR for low-risk patients shines at 6 years in NOTION
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
PARIS – Follow-up data from a randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with symptomatic severe aortic stenosis showed sustained superior hemodynamic valve performance and less structural valve deterioration in the transcatheter group through 6 years, Lars Sondergaard, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, the rate of bioprosthetic valve failure as formally defined in a recent European consensus statement (Eur Heart J. 2017 Dec 1;38[45]:3382-90) was similarly low in the transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) arms at about 7%, in the randomized study known as NOTION (Nordic Aortic Valve Intervention), added Dr. Sondergaard, professor of cardiology at the University of Copenhagen, who was a coauthor of the consensus statement.
“As we look to expand TAVR to younger patients with longer life expectancy, durability, of course, becomes much more important,” the cardiologist observed.
NOTION was a pioneering prospective, multicenter, nonblinded, randomized trial of first-generation TAVR technology. The 280 participants, average age 79 years, were truly a low-surgical-risk population, with a mean Society of Thoracic Surgeons risk score of 3%.
NOTION is a small trial, but the results at 6 years of a planned 10-year follow-up augur well for TAVR success in the large, ongoing, definitive, randomized trials of TAVR versus SAVR in low-risk patients. That’s because NOTION participants were treated in 2009-2013, when the self-expanding TAVR CoreValve was implanted on the basis of aortic annulus measurements obtained via echocardiography, which is considerably less accurate than CT, the standard practice today. For this reason, it’s highly unlikely that the larger, ongoing trials, including PARTNER 3 and the Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients trial, will experience moderate paravalvular leak rates anything like the 20.9% rate seen in the TAVR group in NOTION, where the SAVR group’s rate was just 1.5%.
“I’m sure quite a few of the NOTION patients would have a larger TAVR valve prosthesis if they were treated today,” according to Dr. Sondergaard.
Valve function
The rate of moderate hemodynamic structural valve deterioration through 6 years of follow-up was 3.6% in the TAVR arm, compared with 23.7% in the SAVR group. The rate of nonstructural valve deterioration was 54.0% with TAVR and 57.8% with SAVR, and there were no cases of moderate or severe aortic regurgitation in either group.
The mean aortic valve gradient in the TAVR group went from 44.9 mm Hg at baseline to 12.2 mm Hg at 3 months and to 14.7 mm Hg at 6 years. In the SAVR group, the figures were 44.9 mm Hg, 8.3 mm Hg, and 9.9 mm Hg, respectively.
The effective orifice area in the TAVR group improved from 0.74 cm2 at baseline to 1.37 cm2 at 3 months and to 1.16 cm2 at 6 years. With SAVR, the effective orifice area was 0.74 cm2 at baseline, 1.66 cm2 at 3 months, and 1.53 cm2 at 6 years.
Clinical outcomes
Through 6 years of follow-up, there were no cases of thrombosis in either study arm, and the rate of endocarditis was just under 6% in each group through 6 years. All-cause mortality through 6 years was 42.5% in the TAVR group, not significantly different from the 37.7% rate in the SAVR arm.
The valve-related death rate was 5.0% in the TAVR arm and similar at 3.7% with SAVR. Reintervention occurred in 2.2% of TAVR patients and in 0.7% of SAVR patients. Severe hemodynamic structural valve deterioration was documented in 0.7% of TAVR patients and 3.0% of SAVR patients. The overall rate of bioprosthetic valve failure – a composite of these three endpoints – was 7.5% with TAVR and similar at 6.7% with SAVR.
Session cochair Alain G. Cribier, MD, a TAVR pioneer who is professor of medicine and director of cardiology at Charles Nicolle Hospital, University of Rouen (France), declared, “This is extremely encouraging.”
However, discussant Corrado Tamburino, MD, was more circumspect.
“If you look, there’s a trend for increased all-cause mortality in the TAVR versus SAVR group at 6 years. This could be related to the big difference in paravalvular leak. Do you think paravalvular leak could have a real impact on mortality?” asked Dr. Tamburino, professor of cardiology at the University of Catania, Italy.
Not in the NOTION study, where they looked for but didn’t find any such association, according to Dr. Sondergaard.
“If you look at the survival curves from the beginning out to 6 years, you’ll see that the lines cross each other several times, so this small difference right now could just be random. I don’t think we can say there’s a higher mortality rate with TAVR for the time being,” he added.
The NOTION trial was funded by the Danish Heart Foundation. Dr. Sondergaard reported receiving research grants from and/or serving as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis.
REPORTING FROM EUROPCR 2018
Key clinical point: TAVR provided superior hemodynamic valve performance and less structural valve deterioration than SAVR in low-surgical-risk patients through 6 years of follow-up.
Major finding: The rate of moderate hemodynamic structural valve deterioration through 6 years was 3.6% with TAVR and 23.7% with SAVR.
Study details: An analysis of 6-year follow-up data from NOTION, a prospective, multicenter, randomized trial in 280 low-surgical-risk patients.
Disclosures: NOTION was funded by the Danish Heart Foundation. The presenter reported receiving research grants from and/or serving as a consultant to several medical device companies.
Myocarditis shows causal role in frequent PVCs
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: Immunosuppressive therapy resolved myocarditis in two-thirds of 51% of patients with new-onset, frequents PVCs.
Study details: Single-center series with 107 patients.
Disclosures: Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
Source: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
A fib ablation in HFrEF patients gains momentum
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Results from two recent trials suggest that cardiologists may have a new way to improve outcomes in patients with heart failure with reduced ejection fraction if they also have atrial fibrillation: Cut the patient’s atrial fibrillation burden with catheter ablation.
This seemingly off-target approach to improving survival, avoiding heart failure hospitalizations, and possibly reducing other adverse events first gained attention with results from the CASTLE-AF (Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF) randomized trial, first reported in 2017. The study showed in 363 patients that atrial fibrillation (AF) ablation in patients with heart failure with reduced ejection fraction (HFrEF) led to a statistically significant 38% relative reduction in the primary endpoint of mortality or heart failure hospitalization during a median 38 months of follow-up (N Engl J Med. 2018 Feb 1;378[5]:417-27).
This groundbreaking finding then received some degree of confirmation when Douglas L. Packer, MD, reported primary results from CABANA (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial) at the annual scientific sessions of the Heart Rhythm Society. CABANA compared upfront ablation against first-line medical management of AF in 2,203 patients. While the primary endpoint of the cumulative rate of all-cause death, disabling stroke, serious bleeding, or cardiac arrest over a median follow-up of just over 4 years was neutral, with no statistically significant difference between the two treatment arms, a subgroup analysis showed a tantalizing suggestion of benefit in the 337 enrolled patients with a history of congestive heart failure (15% of the total study group).
In this subgroup, treatment with ablation cut the primary endpoint by 39% relative to those treated upfront with medical management, an effect that came close to statistical significance. In addition, Dr. Packer took special note of the per-protocol analysis, which censored out the crossover patients who constituted roughly a fifth of all enrolled patients. In the subgroup analysis using the per-protocol data, ablation was linked with a statistically significant 49% relative reduction in the primary endpoint among patients with a history of heart failure.
The patients for whom there may be the quickest shift to upfront ablation to treat AF based on the CABANA results will be those with heart failure and others with high underlying risk, Dr. Packer predicted at the meeting.
“The CASTLE-AF results were interesting, but in fewer than 400 patients. Now we’ve basically seen the same thing” in CABANA, said Dr. Packer, professor and a cardiac electrophysiologist at the Mayo Clinic in Rochester, Minn.
Notably however, the results Dr. Packer reported on the heart failure subgroup did not include any information on how many of these were patients who had HFrEF or heart failure with preserved ejection fraction and how the apparent benefit from AF ablation affected each of these two heart failure types. In addition, the reported CABANA results did not have an endpoint result that completely matched the mortality and heart failure hospitalization composite endpoint used in CASTLE-AF. The closest endpoint that Dr. Packer reported from CABANA was a composite of mortality and cardiovascular hospitalization that showed, for the entire CABANA cohort, a statistically significant 17% relative reduction with ablation in the intention-to-treat analysis. Dr. Packer gave no data on how this outcome shook out in the subgroup of heart failure patients.
Despite these limitations, in trying to synthesize the CABANA and CASTLE-AF results, several electrophysiologists who heard the results agreed with Dr. Packer that the CABANA results confirmed the CASTLE-AF findings and helped strengthen the case for strongly considering AF ablation as first-line treatment in patients with heart failure.
“It’s clear that sinus rhythm is important in patients with heart failure. CASTLE-AF and now these results; that’s very strong to me,” said Eric N. Prystowsky, MD, a cardiac electrophysiologist with the St. Vincent Medical Group in Indianapolis and designated discussant for CABANA at the meeting.
“It’s confirmatory,” said Nassir F. Marrouche, MD, lead investigator for CASTLE-AF, and professor and director of the electrophysiology laboratory at the University of Utah in Salt Lake City.
The “signal” of benefit from AF ablation in heart failure patients in CABANA “replicates what was seen in CASTLE-AF. The results are highly consistent and very important regarding how to treat patients with AF and heart failure,” said Jeremy N. Ruskin, MD, professor of medicine at Harvard Medical School and director of the cardiac arrhythmia service at Massachusetts General Hospital, both in Boston. “The data strongly suggest that catheter ablation is helpful for restoring and preserving [heart] muscle function,” Dr. Ruskin said in a video interview. He noted that AF occurs in at least about a quarter of heart failure patients.
Other cardiologists at the meeting noted that, on the basis of the CASTLE-AF results alone, they have already become more aggressive about treating AF with ablation in patients with heart failure in routine practice.
“It adds to the armamentarium for treatment of patients with heart failure,” said Johannes Brachmann, MD, professor and chief of cardiology at the Coburg (Germany) Clinic and a senior coinvestigator for CASTLE-AF.
William T. Abraham, MD, a heart failure specialist at The Ohio State University in Columbus, offered a broader perspective on where AF diagnosis, treatment, and ablation currently stand in U.S. heart failure practice.
“There is a very tight link between AF burden and worse outcomes in heart failure, so there is something intuitively appealing about restoring sinus rhythm in heart failure patients. I think most heart failure clinicians believe, like me, that heart failure patients with AF benefit from restoration of normal sinus rhythm. But I don’t believe that the CASTLE-AF results have so far had much impact on practice, in part because it was a relatively small study. The heart failure community is looking for some confirmation,” said Dr. Abraham, professor and director of cardiovascular medicine at Ohio State.
“I think the CABANA results are encouraging, but they came from only 15% of the enrolled patients who also had heart failure. CABANA adds to our knowledge, but I’m not sure it’s definitive for the heart failure population. I’m not sure it tells us if you treat patients with heart failure with anti-arrhythmia drugs and successfully maintain sinus rhythm do those patients do just as well as those who get ablated,” he said in an interview. “I’d love to see a study of heart failure patients maintained in sinus rhythm with drugs compared with those treated with ablation.”
For most patients with heart failure, the coexistence of AF is identified because of AF symptoms, or when asymptomatic AF is found in recordings made by an implanted cardiac device. “I’m more aggressive about addressing asymptomatic AF in my heart failure patients, and I believe the heart failure community is moving rapidly in that direction because of the association between higher AF burden and worse heart failure outcomes,” Dr. Abraham said.
A more cautious view came from another heart failure specialist, Clyde Yancy, MD, professor and chief of cardiology at Northwestern University in Chicago. “It’s pretty evident that in certain patients with heart failure AF ablation might be the right treatment, but is it every HFrEF patient with AF?” he wondered. “It’s nice to have more evidence so we can be more comfortable sending heart failure patients for ablation, but I want to see more information about the risk” from ablation in heart failure patients, “the sustainability of the effect, and the consequences of ablation.”
But the reservations expressed by cardiologists like Dr. Yancy contrasted with the views of colleagues who consider the current evidence much more convincing.
“It seems logical to look harder for AF” in heart failure patients, based on the accumulated evidence from CASTLE-AF and CABANA, said Dr. Ruskin. “I don’t think we can offer advice to heart failure physicians to screen their heart failure patients for AF, but if it’s seen I think we have some useful information on how to address it.”
CASTLE-AF was funded by Biotronik. CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to and has received research funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude, and also from several other companies. Dr. Prystowsky as been a consultant to CardioNet and Medtronic, he has an equity interest in Stereotaxis, and he receives fellowship support from Medtronic and St. Jude. Dr. Marrouche has been a consultant to Biosense Webster, Biotronik, Boston Scientific, and St. Jude. He has received research support from Medtronic, and he has had financial relationships with several other companies. Dr. Ruskin has been a consultant to Biosense Webster and Medtronic and several other companies, has an ownership interest in Amgen, Cameron Health, InfoBionic, Newpace, Portola, and Regeneron, and has a fiduciary role in Pharmaco-Kinesis. Dr. Russo and Dr. Yancy had no disclosures. Dr. Brachmann has been a consultant to and has received research funding from Biotronik, Boston Scientific, St. Jude, and several other companies. Dr. Abraham has been a consultant to Abbott Vascular, Medtronic, Novartis, and St. Jude.
EXPERT ANALYSIS FROM HEART RHYTHM 2018
FDA recalls HeartMate 3 LV assist device
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).