User login
New deep dive into Paxlovid interactions with CVD meds
Nirmatrelvir/ritonavir (Paxlovid) has been a game changer for high-risk patients with early COVID-19 symptoms but has significant interactions with commonly used cardiovascular medications, a new paper cautions.
COVID-19 patients with cardiovascular disease (CVD) or risk factors such as diabetes, hypertension, and chronic kidney disease are at high risk of severe disease and account for the lion’s share of those receiving Paxlovid. Data from the initial EPIC-HR trial and recent real-world data also suggest they’re among the most likely to benefit from the oral antiviral, regardless of their COVID-19 vaccination status.
“But at the same time, it unfortunately interacts with many very commonly prescribed cardiovascular medications and with many of them in a very clinically meaningful way, which may lead to serious adverse consequences,” senior author Sarju Ganatra, MD, said in an interview. “So, while it’s being prescribed with a good intention to help these people, we may actually end up doing more harm than good.
“We don’t want to deter people from getting their necessary COVID-19 treatment, which is excellent for the most part these days as an outpatient,” he added. “So, we felt the need to make a comprehensive list of cardiac medications and level of interactions with Paxlovid and also to help the clinicians and prescribers at the point of care to make the clinical decision of what modifications they may need to do.”
The paper, published online in the Journal of the American College of Cardiology, details drug-drug interactions with some 80 CV medications including statins, antihypertensive agents, heart failure therapies, and antiplatelet/anticoagulants.
It also includes a color-coded figure denoting whether a drug is safe to coadminister with Paxlovid, may potentially interact and require a dose adjustment or temporary discontinuation, or is contraindicated.
Among the commonly used blood thinners, for example, the paper notes that Paxlovid significantly increases drug levels of the direct oral anticoagulants (DOACs) apixaban, rivaroxaban, edoxaban, and dabigatran and, thus, increases the risk of bleeding.
“It can still be administered, if it’s necessary, but the dose of the DOAC either needs to be reduced or held depending on what they are getting it for, whether they’re getting it for pulmonary embolism or atrial fibrillation, and we adjust for all those things in the table in the paper,” said Dr. Ganatra, from Lahey Hospital and Medical Center, Burlington, Mass.
When the DOAC can’t be interrupted or dose adjusted, however, Paxlovid should not be given, the experts said. The antiviral is safe to use with enoxaparin, a low-molecular-weight heparin, but can increase or decrease levels of warfarin and should be used with close international normalized ratio monitoring.
For patients on antiplatelet agents, clinicians are advised to avoid prescribing nirmatrelvir/ritonavir to those on ticagrelor or clopidogrel unless the agents can be replaced by prasugrel.
Ritonavir – an inhibitor of cytochrome P 450 enzymes, particularly CYP3A4 – poses an increased risk of bleeding when given with ticagrelor, a CYP3A4 substrate, and decreases the active metabolite of clopidogrel, cutting its platelet inhibition by 20%. Although there’s a twofold decrease in the maximum concentration of prasugrel in patients on ritonavir, this does not affect its antiplatelet activity, the paper explains.
Among the lipid-lowering agents, experts suggested temporarily withholding atorvastatin, rosuvastatin, simvastatin, and lovastatin because of an increased risk for myopathy and liver toxicity but say that other statins, fibrates, ezetimibe, and the proprotein convertase subtilisin/kexin type 9 inhibitors evolocumab and alirocumab are safe to coadminister with Paxlovid.
While statins typically leave the body within hours, most of the antiarrhythmic drugs, except for sotalol, are not safe to give with Paxlovid, Dr. Ganatra said. It’s technically not feasible to hold these drugs because most have long half-lives, reaching about 100 days, for example, for amiodarone.
“It’s going to hang around in your system for a long time, so you don’t want to be falsely reassured that you’re holding the drug and it’s going to be fine to go back slowly,” he said. “You need to look for alternative therapies in those scenarios for COVID-19 treatment, which could be other antivirals, or a monoclonal antibody individualized to the patient’s risk.”
Although there’s limited clinical information regarding interaction-related adverse events with Paxlovid, the team used pharmacokinetics and pharmacodynamics data to provide the guidance. Serious adverse events are also well documented for ritonavir, which has been prescribed for years to treat HIV, Dr. Ganatra noted.
The Infectious Disease Society of America also published guidance on the management of potential drug interactions with Paxlovid in May and, earlier in October, the Food and Drug Administration updated its Paxlovid patient eligibility screening checklist.
Still, most prescribers are actually primary care physicians and even pharmacists, who may not be completely attuned, said Dr. Ganatra, who noted that some centers have started programs to help connect primary care physicians with their cardiology colleagues to check on CV drugs in their COVID-19 patients.
“We need to be thinking more broadly and at a system level where the hospital or health care system leverages the electronic health record systems,” he said. “Most of them are sophisticated enough to incorporate simple drug-drug interaction information, so if you try to prescribe someone Paxlovid and it’s a heart transplant patient who is on immunosuppressive therapy or a patient on a blood thinner, then it should give you a warning ... or at least give them a link to our paper or other valuable resources.
“If someone is on a blood thinner and the blood thinner level goes up by ninefold, we can only imagine what we would be dealing with,” Dr. Ganatra said. “So, these interactions should be taken very seriously and I think it’s worth the time and investment.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nirmatrelvir/ritonavir (Paxlovid) has been a game changer for high-risk patients with early COVID-19 symptoms but has significant interactions with commonly used cardiovascular medications, a new paper cautions.
COVID-19 patients with cardiovascular disease (CVD) or risk factors such as diabetes, hypertension, and chronic kidney disease are at high risk of severe disease and account for the lion’s share of those receiving Paxlovid. Data from the initial EPIC-HR trial and recent real-world data also suggest they’re among the most likely to benefit from the oral antiviral, regardless of their COVID-19 vaccination status.
“But at the same time, it unfortunately interacts with many very commonly prescribed cardiovascular medications and with many of them in a very clinically meaningful way, which may lead to serious adverse consequences,” senior author Sarju Ganatra, MD, said in an interview. “So, while it’s being prescribed with a good intention to help these people, we may actually end up doing more harm than good.
“We don’t want to deter people from getting their necessary COVID-19 treatment, which is excellent for the most part these days as an outpatient,” he added. “So, we felt the need to make a comprehensive list of cardiac medications and level of interactions with Paxlovid and also to help the clinicians and prescribers at the point of care to make the clinical decision of what modifications they may need to do.”
The paper, published online in the Journal of the American College of Cardiology, details drug-drug interactions with some 80 CV medications including statins, antihypertensive agents, heart failure therapies, and antiplatelet/anticoagulants.
It also includes a color-coded figure denoting whether a drug is safe to coadminister with Paxlovid, may potentially interact and require a dose adjustment or temporary discontinuation, or is contraindicated.
Among the commonly used blood thinners, for example, the paper notes that Paxlovid significantly increases drug levels of the direct oral anticoagulants (DOACs) apixaban, rivaroxaban, edoxaban, and dabigatran and, thus, increases the risk of bleeding.
“It can still be administered, if it’s necessary, but the dose of the DOAC either needs to be reduced or held depending on what they are getting it for, whether they’re getting it for pulmonary embolism or atrial fibrillation, and we adjust for all those things in the table in the paper,” said Dr. Ganatra, from Lahey Hospital and Medical Center, Burlington, Mass.
When the DOAC can’t be interrupted or dose adjusted, however, Paxlovid should not be given, the experts said. The antiviral is safe to use with enoxaparin, a low-molecular-weight heparin, but can increase or decrease levels of warfarin and should be used with close international normalized ratio monitoring.
For patients on antiplatelet agents, clinicians are advised to avoid prescribing nirmatrelvir/ritonavir to those on ticagrelor or clopidogrel unless the agents can be replaced by prasugrel.
Ritonavir – an inhibitor of cytochrome P 450 enzymes, particularly CYP3A4 – poses an increased risk of bleeding when given with ticagrelor, a CYP3A4 substrate, and decreases the active metabolite of clopidogrel, cutting its platelet inhibition by 20%. Although there’s a twofold decrease in the maximum concentration of prasugrel in patients on ritonavir, this does not affect its antiplatelet activity, the paper explains.
Among the lipid-lowering agents, experts suggested temporarily withholding atorvastatin, rosuvastatin, simvastatin, and lovastatin because of an increased risk for myopathy and liver toxicity but say that other statins, fibrates, ezetimibe, and the proprotein convertase subtilisin/kexin type 9 inhibitors evolocumab and alirocumab are safe to coadminister with Paxlovid.
While statins typically leave the body within hours, most of the antiarrhythmic drugs, except for sotalol, are not safe to give with Paxlovid, Dr. Ganatra said. It’s technically not feasible to hold these drugs because most have long half-lives, reaching about 100 days, for example, for amiodarone.
“It’s going to hang around in your system for a long time, so you don’t want to be falsely reassured that you’re holding the drug and it’s going to be fine to go back slowly,” he said. “You need to look for alternative therapies in those scenarios for COVID-19 treatment, which could be other antivirals, or a monoclonal antibody individualized to the patient’s risk.”
Although there’s limited clinical information regarding interaction-related adverse events with Paxlovid, the team used pharmacokinetics and pharmacodynamics data to provide the guidance. Serious adverse events are also well documented for ritonavir, which has been prescribed for years to treat HIV, Dr. Ganatra noted.
The Infectious Disease Society of America also published guidance on the management of potential drug interactions with Paxlovid in May and, earlier in October, the Food and Drug Administration updated its Paxlovid patient eligibility screening checklist.
Still, most prescribers are actually primary care physicians and even pharmacists, who may not be completely attuned, said Dr. Ganatra, who noted that some centers have started programs to help connect primary care physicians with their cardiology colleagues to check on CV drugs in their COVID-19 patients.
“We need to be thinking more broadly and at a system level where the hospital or health care system leverages the electronic health record systems,” he said. “Most of them are sophisticated enough to incorporate simple drug-drug interaction information, so if you try to prescribe someone Paxlovid and it’s a heart transplant patient who is on immunosuppressive therapy or a patient on a blood thinner, then it should give you a warning ... or at least give them a link to our paper or other valuable resources.
“If someone is on a blood thinner and the blood thinner level goes up by ninefold, we can only imagine what we would be dealing with,” Dr. Ganatra said. “So, these interactions should be taken very seriously and I think it’s worth the time and investment.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nirmatrelvir/ritonavir (Paxlovid) has been a game changer for high-risk patients with early COVID-19 symptoms but has significant interactions with commonly used cardiovascular medications, a new paper cautions.
COVID-19 patients with cardiovascular disease (CVD) or risk factors such as diabetes, hypertension, and chronic kidney disease are at high risk of severe disease and account for the lion’s share of those receiving Paxlovid. Data from the initial EPIC-HR trial and recent real-world data also suggest they’re among the most likely to benefit from the oral antiviral, regardless of their COVID-19 vaccination status.
“But at the same time, it unfortunately interacts with many very commonly prescribed cardiovascular medications and with many of them in a very clinically meaningful way, which may lead to serious adverse consequences,” senior author Sarju Ganatra, MD, said in an interview. “So, while it’s being prescribed with a good intention to help these people, we may actually end up doing more harm than good.
“We don’t want to deter people from getting their necessary COVID-19 treatment, which is excellent for the most part these days as an outpatient,” he added. “So, we felt the need to make a comprehensive list of cardiac medications and level of interactions with Paxlovid and also to help the clinicians and prescribers at the point of care to make the clinical decision of what modifications they may need to do.”
The paper, published online in the Journal of the American College of Cardiology, details drug-drug interactions with some 80 CV medications including statins, antihypertensive agents, heart failure therapies, and antiplatelet/anticoagulants.
It also includes a color-coded figure denoting whether a drug is safe to coadminister with Paxlovid, may potentially interact and require a dose adjustment or temporary discontinuation, or is contraindicated.
Among the commonly used blood thinners, for example, the paper notes that Paxlovid significantly increases drug levels of the direct oral anticoagulants (DOACs) apixaban, rivaroxaban, edoxaban, and dabigatran and, thus, increases the risk of bleeding.
“It can still be administered, if it’s necessary, but the dose of the DOAC either needs to be reduced or held depending on what they are getting it for, whether they’re getting it for pulmonary embolism or atrial fibrillation, and we adjust for all those things in the table in the paper,” said Dr. Ganatra, from Lahey Hospital and Medical Center, Burlington, Mass.
When the DOAC can’t be interrupted or dose adjusted, however, Paxlovid should not be given, the experts said. The antiviral is safe to use with enoxaparin, a low-molecular-weight heparin, but can increase or decrease levels of warfarin and should be used with close international normalized ratio monitoring.
For patients on antiplatelet agents, clinicians are advised to avoid prescribing nirmatrelvir/ritonavir to those on ticagrelor or clopidogrel unless the agents can be replaced by prasugrel.
Ritonavir – an inhibitor of cytochrome P 450 enzymes, particularly CYP3A4 – poses an increased risk of bleeding when given with ticagrelor, a CYP3A4 substrate, and decreases the active metabolite of clopidogrel, cutting its platelet inhibition by 20%. Although there’s a twofold decrease in the maximum concentration of prasugrel in patients on ritonavir, this does not affect its antiplatelet activity, the paper explains.
Among the lipid-lowering agents, experts suggested temporarily withholding atorvastatin, rosuvastatin, simvastatin, and lovastatin because of an increased risk for myopathy and liver toxicity but say that other statins, fibrates, ezetimibe, and the proprotein convertase subtilisin/kexin type 9 inhibitors evolocumab and alirocumab are safe to coadminister with Paxlovid.
While statins typically leave the body within hours, most of the antiarrhythmic drugs, except for sotalol, are not safe to give with Paxlovid, Dr. Ganatra said. It’s technically not feasible to hold these drugs because most have long half-lives, reaching about 100 days, for example, for amiodarone.
“It’s going to hang around in your system for a long time, so you don’t want to be falsely reassured that you’re holding the drug and it’s going to be fine to go back slowly,” he said. “You need to look for alternative therapies in those scenarios for COVID-19 treatment, which could be other antivirals, or a monoclonal antibody individualized to the patient’s risk.”
Although there’s limited clinical information regarding interaction-related adverse events with Paxlovid, the team used pharmacokinetics and pharmacodynamics data to provide the guidance. Serious adverse events are also well documented for ritonavir, which has been prescribed for years to treat HIV, Dr. Ganatra noted.
The Infectious Disease Society of America also published guidance on the management of potential drug interactions with Paxlovid in May and, earlier in October, the Food and Drug Administration updated its Paxlovid patient eligibility screening checklist.
Still, most prescribers are actually primary care physicians and even pharmacists, who may not be completely attuned, said Dr. Ganatra, who noted that some centers have started programs to help connect primary care physicians with their cardiology colleagues to check on CV drugs in their COVID-19 patients.
“We need to be thinking more broadly and at a system level where the hospital or health care system leverages the electronic health record systems,” he said. “Most of them are sophisticated enough to incorporate simple drug-drug interaction information, so if you try to prescribe someone Paxlovid and it’s a heart transplant patient who is on immunosuppressive therapy or a patient on a blood thinner, then it should give you a warning ... or at least give them a link to our paper or other valuable resources.
“If someone is on a blood thinner and the blood thinner level goes up by ninefold, we can only imagine what we would be dealing with,” Dr. Ganatra said. “So, these interactions should be taken very seriously and I think it’s worth the time and investment.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
ACC calls for more career flexibility in cardiology
A new statement from the American College of Cardiology is calling for a greater degree of career flexibility in the specialty to promote cardiologists’ personal and professional well-being and preserve excellence in patient care.
The statement recommends that cardiologists, from trainees to those contemplating retirement, be granted more leeway in their careers to allow them to take time for common life events, such as child-rearing, taking care of aged parents, or reducing their workload in case of poor health or physical disabilities, without jeopardizing their careers.
The “2022 ACC Health Policy Statement on Career Flexibility in Cardiology: A Report of the American College of Cardiology Solution Set Oversight Committee” was published online in the Journal of the American College of Cardiology.
‘Hard-driving profession’
The well-being of the cardiovascular workforce is critical to the achievement of the mission of the ACC, which is to transform cardiovascular care and improve heart health, the Health Policy writing committee stated. Career flexibility is an important component of ensuring that well-being, the authors wrote.
“The ACC has critically looked at the factors that contribute to the lack of diversity and inclusion in cardiovascular practice, and one of the issues is the lack of flexibility in our profession,” writing committee chair, Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs, Ascension St. Vincent Heart Center, Indianapolis, Ind., told this news organization.
The notion of work-life balance has become increasingly important but cardiology as a profession has traditionally not been open to the idea of its value, Dr. Walsh said.
“We have a very hard-driving profession. It takes many years to train to do the work we do. The need for on-call services is very significant, and we go along because we have always done it this way, but if you don’t reexamine the way that you are structuring your work, you’ll never change it,” she said.
“For example, the ‘full time, full call, come to work after you’ve been up all night’ work ethic, which is no longer allowed for trainees, is still in effect once you get into university practice or clinical practice. We have interventional cardiologists up all night doing STEMI care for patients and then having a full clinic the next day,” Dr. Walsh said. “The changes that came about for trainees have not trickled up to the faculty or clinical practice level. It’s really a patient safety issue.”
She emphasized that the new policy statement is not focused solely on women. “The need for time away or flexible time around family planning, childbirth, and parental leave is increasingly important to our younger colleagues, both men and women.”
Dr. Walsh pointed out that the writing committee was carefully composed to include representation from all stakeholders.
“We have representation from very young cardiologists, one of whom was in training at the time we began our work. We have two systems CEOs who are cardiologists, we have a chair of medicine, we have two very senior cardiologists, and someone who works in industry,” she said.
The ACC also believes that cardiologists with physically demanding roles should have pathways to transition into other opportunities in patient care, research, or education.
“Right now, there are many cardiology practices that have traditional policies, where you are either all in, or you are all out. They do not allow for what we term a ‘step down’ policy, where you perhaps stop going into the cath lab, but you still do clinic and see patients,” Dr. Walsh noted.
“One of the goals of this policy statement is to allow for such practices to look at their compensation and structure, and to realize that their most senior cardiologists may be willing to stay on for several more years and be contributing members to the practice, but they may no longer wish to stay in the cath lab or be in the night call pool,” she said.
Transparency around compensation is also very important because cardiologists contemplating a reduced work schedule need to know how this will affect the amount of money they will be earning, she added.
“Transparency about policies around compensation are crucial because if an individual cardiologist wishes to pursue a flexible scheduling at any time in their career, it’s clear that they won’t have the same compensation as someone who is a full-time employee. All of this has to be very transparent and clear on both sides, so that the person deciding toward some flexibility understands what the implications are from a financial and compensation standpoint,” Dr. Walsh said.
As an example, a senior career cardiologist who no longer wants to take night calls should know what this may cost financially.
“The practice should set a valuation of night calls, so that the individual who makes the choice to step out of the call pool understands what the impact on their compensation will be. That type of transparency is necessary for all to ensure that individuals who seek flexibility will not be blindsided by the resulting decrease in financial compensation,” she said.
A growing need
“In its new health policy statement, the American College of Cardiology addresses the growing need for career flexibility as an important component of ensuring the well-being of the cardiovascular care workforce,” Harlan M. Krumholz, MD, SM, Harold H. Hines Jr. Professor of Medicine and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn., told this news organization.
“The writing committee reviews opportunities for offering flexibility at all career levels to combat burnout and increase retention in the field, as well as proposes system, policy, and practice solutions to allow both men and women to emphasize and embrace work-life balance,” Dr. Krumholz said.
“The document provides pathways for cardiologists looking to pursue other interests or career transitions while maintaining excellence in clinical care,” he added. “Chief among these recommendations are flexible/part-time hours, leave and reentry policies, changes in job descriptions to support overarching cultural change, and equitable compensation and opportunities. The document is intended to be used as a guide for innovation in the cardiology workforce.”
‘Thoughtful and long overdue’
“This policy statement is thoughtful and long overdue,” Steven E. Nissen, MD, Lewis and Patricia Dickey Chair in Cardiovascular Medicine and professor of medicine at Cleveland Clinic, told this news organization.
“Career flexibility will allow cardiologists to fulfill family responsibilities while continuing to advance their careers. Successfully contributing to patient care and research does not require physicians to isolate themselves from all their other responsibilities,” Dr. Nissen added.
“I am pleased that the ACC has articulated the value of a balanced approach to career and family.”
Dr. Walsh, Dr. Krumholz, and Dr. Nissen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new statement from the American College of Cardiology is calling for a greater degree of career flexibility in the specialty to promote cardiologists’ personal and professional well-being and preserve excellence in patient care.
The statement recommends that cardiologists, from trainees to those contemplating retirement, be granted more leeway in their careers to allow them to take time for common life events, such as child-rearing, taking care of aged parents, or reducing their workload in case of poor health or physical disabilities, without jeopardizing their careers.
The “2022 ACC Health Policy Statement on Career Flexibility in Cardiology: A Report of the American College of Cardiology Solution Set Oversight Committee” was published online in the Journal of the American College of Cardiology.
‘Hard-driving profession’
The well-being of the cardiovascular workforce is critical to the achievement of the mission of the ACC, which is to transform cardiovascular care and improve heart health, the Health Policy writing committee stated. Career flexibility is an important component of ensuring that well-being, the authors wrote.
“The ACC has critically looked at the factors that contribute to the lack of diversity and inclusion in cardiovascular practice, and one of the issues is the lack of flexibility in our profession,” writing committee chair, Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs, Ascension St. Vincent Heart Center, Indianapolis, Ind., told this news organization.
The notion of work-life balance has become increasingly important but cardiology as a profession has traditionally not been open to the idea of its value, Dr. Walsh said.
“We have a very hard-driving profession. It takes many years to train to do the work we do. The need for on-call services is very significant, and we go along because we have always done it this way, but if you don’t reexamine the way that you are structuring your work, you’ll never change it,” she said.
“For example, the ‘full time, full call, come to work after you’ve been up all night’ work ethic, which is no longer allowed for trainees, is still in effect once you get into university practice or clinical practice. We have interventional cardiologists up all night doing STEMI care for patients and then having a full clinic the next day,” Dr. Walsh said. “The changes that came about for trainees have not trickled up to the faculty or clinical practice level. It’s really a patient safety issue.”
She emphasized that the new policy statement is not focused solely on women. “The need for time away or flexible time around family planning, childbirth, and parental leave is increasingly important to our younger colleagues, both men and women.”
Dr. Walsh pointed out that the writing committee was carefully composed to include representation from all stakeholders.
“We have representation from very young cardiologists, one of whom was in training at the time we began our work. We have two systems CEOs who are cardiologists, we have a chair of medicine, we have two very senior cardiologists, and someone who works in industry,” she said.
The ACC also believes that cardiologists with physically demanding roles should have pathways to transition into other opportunities in patient care, research, or education.
“Right now, there are many cardiology practices that have traditional policies, where you are either all in, or you are all out. They do not allow for what we term a ‘step down’ policy, where you perhaps stop going into the cath lab, but you still do clinic and see patients,” Dr. Walsh noted.
“One of the goals of this policy statement is to allow for such practices to look at their compensation and structure, and to realize that their most senior cardiologists may be willing to stay on for several more years and be contributing members to the practice, but they may no longer wish to stay in the cath lab or be in the night call pool,” she said.
Transparency around compensation is also very important because cardiologists contemplating a reduced work schedule need to know how this will affect the amount of money they will be earning, she added.
“Transparency about policies around compensation are crucial because if an individual cardiologist wishes to pursue a flexible scheduling at any time in their career, it’s clear that they won’t have the same compensation as someone who is a full-time employee. All of this has to be very transparent and clear on both sides, so that the person deciding toward some flexibility understands what the implications are from a financial and compensation standpoint,” Dr. Walsh said.
As an example, a senior career cardiologist who no longer wants to take night calls should know what this may cost financially.
“The practice should set a valuation of night calls, so that the individual who makes the choice to step out of the call pool understands what the impact on their compensation will be. That type of transparency is necessary for all to ensure that individuals who seek flexibility will not be blindsided by the resulting decrease in financial compensation,” she said.
A growing need
“In its new health policy statement, the American College of Cardiology addresses the growing need for career flexibility as an important component of ensuring the well-being of the cardiovascular care workforce,” Harlan M. Krumholz, MD, SM, Harold H. Hines Jr. Professor of Medicine and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn., told this news organization.
“The writing committee reviews opportunities for offering flexibility at all career levels to combat burnout and increase retention in the field, as well as proposes system, policy, and practice solutions to allow both men and women to emphasize and embrace work-life balance,” Dr. Krumholz said.
“The document provides pathways for cardiologists looking to pursue other interests or career transitions while maintaining excellence in clinical care,” he added. “Chief among these recommendations are flexible/part-time hours, leave and reentry policies, changes in job descriptions to support overarching cultural change, and equitable compensation and opportunities. The document is intended to be used as a guide for innovation in the cardiology workforce.”
‘Thoughtful and long overdue’
“This policy statement is thoughtful and long overdue,” Steven E. Nissen, MD, Lewis and Patricia Dickey Chair in Cardiovascular Medicine and professor of medicine at Cleveland Clinic, told this news organization.
“Career flexibility will allow cardiologists to fulfill family responsibilities while continuing to advance their careers. Successfully contributing to patient care and research does not require physicians to isolate themselves from all their other responsibilities,” Dr. Nissen added.
“I am pleased that the ACC has articulated the value of a balanced approach to career and family.”
Dr. Walsh, Dr. Krumholz, and Dr. Nissen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new statement from the American College of Cardiology is calling for a greater degree of career flexibility in the specialty to promote cardiologists’ personal and professional well-being and preserve excellence in patient care.
The statement recommends that cardiologists, from trainees to those contemplating retirement, be granted more leeway in their careers to allow them to take time for common life events, such as child-rearing, taking care of aged parents, or reducing their workload in case of poor health or physical disabilities, without jeopardizing their careers.
The “2022 ACC Health Policy Statement on Career Flexibility in Cardiology: A Report of the American College of Cardiology Solution Set Oversight Committee” was published online in the Journal of the American College of Cardiology.
‘Hard-driving profession’
The well-being of the cardiovascular workforce is critical to the achievement of the mission of the ACC, which is to transform cardiovascular care and improve heart health, the Health Policy writing committee stated. Career flexibility is an important component of ensuring that well-being, the authors wrote.
“The ACC has critically looked at the factors that contribute to the lack of diversity and inclusion in cardiovascular practice, and one of the issues is the lack of flexibility in our profession,” writing committee chair, Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs, Ascension St. Vincent Heart Center, Indianapolis, Ind., told this news organization.
The notion of work-life balance has become increasingly important but cardiology as a profession has traditionally not been open to the idea of its value, Dr. Walsh said.
“We have a very hard-driving profession. It takes many years to train to do the work we do. The need for on-call services is very significant, and we go along because we have always done it this way, but if you don’t reexamine the way that you are structuring your work, you’ll never change it,” she said.
“For example, the ‘full time, full call, come to work after you’ve been up all night’ work ethic, which is no longer allowed for trainees, is still in effect once you get into university practice or clinical practice. We have interventional cardiologists up all night doing STEMI care for patients and then having a full clinic the next day,” Dr. Walsh said. “The changes that came about for trainees have not trickled up to the faculty or clinical practice level. It’s really a patient safety issue.”
She emphasized that the new policy statement is not focused solely on women. “The need for time away or flexible time around family planning, childbirth, and parental leave is increasingly important to our younger colleagues, both men and women.”
Dr. Walsh pointed out that the writing committee was carefully composed to include representation from all stakeholders.
“We have representation from very young cardiologists, one of whom was in training at the time we began our work. We have two systems CEOs who are cardiologists, we have a chair of medicine, we have two very senior cardiologists, and someone who works in industry,” she said.
The ACC also believes that cardiologists with physically demanding roles should have pathways to transition into other opportunities in patient care, research, or education.
“Right now, there are many cardiology practices that have traditional policies, where you are either all in, or you are all out. They do not allow for what we term a ‘step down’ policy, where you perhaps stop going into the cath lab, but you still do clinic and see patients,” Dr. Walsh noted.
“One of the goals of this policy statement is to allow for such practices to look at their compensation and structure, and to realize that their most senior cardiologists may be willing to stay on for several more years and be contributing members to the practice, but they may no longer wish to stay in the cath lab or be in the night call pool,” she said.
Transparency around compensation is also very important because cardiologists contemplating a reduced work schedule need to know how this will affect the amount of money they will be earning, she added.
“Transparency about policies around compensation are crucial because if an individual cardiologist wishes to pursue a flexible scheduling at any time in their career, it’s clear that they won’t have the same compensation as someone who is a full-time employee. All of this has to be very transparent and clear on both sides, so that the person deciding toward some flexibility understands what the implications are from a financial and compensation standpoint,” Dr. Walsh said.
As an example, a senior career cardiologist who no longer wants to take night calls should know what this may cost financially.
“The practice should set a valuation of night calls, so that the individual who makes the choice to step out of the call pool understands what the impact on their compensation will be. That type of transparency is necessary for all to ensure that individuals who seek flexibility will not be blindsided by the resulting decrease in financial compensation,” she said.
A growing need
“In its new health policy statement, the American College of Cardiology addresses the growing need for career flexibility as an important component of ensuring the well-being of the cardiovascular care workforce,” Harlan M. Krumholz, MD, SM, Harold H. Hines Jr. Professor of Medicine and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn., told this news organization.
“The writing committee reviews opportunities for offering flexibility at all career levels to combat burnout and increase retention in the field, as well as proposes system, policy, and practice solutions to allow both men and women to emphasize and embrace work-life balance,” Dr. Krumholz said.
“The document provides pathways for cardiologists looking to pursue other interests or career transitions while maintaining excellence in clinical care,” he added. “Chief among these recommendations are flexible/part-time hours, leave and reentry policies, changes in job descriptions to support overarching cultural change, and equitable compensation and opportunities. The document is intended to be used as a guide for innovation in the cardiology workforce.”
‘Thoughtful and long overdue’
“This policy statement is thoughtful and long overdue,” Steven E. Nissen, MD, Lewis and Patricia Dickey Chair in Cardiovascular Medicine and professor of medicine at Cleveland Clinic, told this news organization.
“Career flexibility will allow cardiologists to fulfill family responsibilities while continuing to advance their careers. Successfully contributing to patient care and research does not require physicians to isolate themselves from all their other responsibilities,” Dr. Nissen added.
“I am pleased that the ACC has articulated the value of a balanced approach to career and family.”
Dr. Walsh, Dr. Krumholz, and Dr. Nissen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA approves self-administered, SubQ furosemide preparation
The Food and Drug Administration has approved a furosemide preparation (Furoscix, scPharmaceuticals) intended for subcutaneous self-administration by outpatients with chronic heart failure and volume overload, the company has announced.
The product is indicated for use with a SmartDose On-Body Infuser (West Pharmaceutical Services) single-use subcutaneous administration device, which affixes to the abdomen.
The infuser is loaded by the patient or caregiver with a prefilled cartridge and is programmed to deliver Furoscix 30 mg over 1 hour followed by a 4-hour infusion at 12.5 mg/h, for a total fixed dose of 80 mg, scPharmaceuticals said in a press release on the drug approval.
Furosemide, a loop diuretic and one of the world’s most frequently used drugs, is conventionally given intravenously in the hospital or orally on an outpatient basis.
The company describes its furosemide preparation, used with the infuser, as “the first and only FDA-approved subcutaneous loop diuretic that delivers [intravenous]-equivalent diuresis at home.” It has been shown to “produce similar diuresis and natriuresis compared to intravenous furosemide.”
“This marks a tremendous opportunity to improve the at-home management of worsening congestion in patients with heart failure who display reduced responsiveness to oral diuretics and require administration of [intravenous] diuretics, which typically requires admission to the hospital,” William T. Abraham, MD, said in the press release.
The FDA approval “is significant and will allow patients to be treated outside of the hospital setting,” said Dr. Abraham, of Ohio State University, Columbus, and an scPharmaceuticals board member.
The Furoscix indication doesn’t cover emergent use or use in acute pulmonary edema, nor is it meant to be used chronically “and should be replaced with oral diuretics as soon as practical,” the company states.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a furosemide preparation (Furoscix, scPharmaceuticals) intended for subcutaneous self-administration by outpatients with chronic heart failure and volume overload, the company has announced.
The product is indicated for use with a SmartDose On-Body Infuser (West Pharmaceutical Services) single-use subcutaneous administration device, which affixes to the abdomen.
The infuser is loaded by the patient or caregiver with a prefilled cartridge and is programmed to deliver Furoscix 30 mg over 1 hour followed by a 4-hour infusion at 12.5 mg/h, for a total fixed dose of 80 mg, scPharmaceuticals said in a press release on the drug approval.
Furosemide, a loop diuretic and one of the world’s most frequently used drugs, is conventionally given intravenously in the hospital or orally on an outpatient basis.
The company describes its furosemide preparation, used with the infuser, as “the first and only FDA-approved subcutaneous loop diuretic that delivers [intravenous]-equivalent diuresis at home.” It has been shown to “produce similar diuresis and natriuresis compared to intravenous furosemide.”
“This marks a tremendous opportunity to improve the at-home management of worsening congestion in patients with heart failure who display reduced responsiveness to oral diuretics and require administration of [intravenous] diuretics, which typically requires admission to the hospital,” William T. Abraham, MD, said in the press release.
The FDA approval “is significant and will allow patients to be treated outside of the hospital setting,” said Dr. Abraham, of Ohio State University, Columbus, and an scPharmaceuticals board member.
The Furoscix indication doesn’t cover emergent use or use in acute pulmonary edema, nor is it meant to be used chronically “and should be replaced with oral diuretics as soon as practical,” the company states.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a furosemide preparation (Furoscix, scPharmaceuticals) intended for subcutaneous self-administration by outpatients with chronic heart failure and volume overload, the company has announced.
The product is indicated for use with a SmartDose On-Body Infuser (West Pharmaceutical Services) single-use subcutaneous administration device, which affixes to the abdomen.
The infuser is loaded by the patient or caregiver with a prefilled cartridge and is programmed to deliver Furoscix 30 mg over 1 hour followed by a 4-hour infusion at 12.5 mg/h, for a total fixed dose of 80 mg, scPharmaceuticals said in a press release on the drug approval.
Furosemide, a loop diuretic and one of the world’s most frequently used drugs, is conventionally given intravenously in the hospital or orally on an outpatient basis.
The company describes its furosemide preparation, used with the infuser, as “the first and only FDA-approved subcutaneous loop diuretic that delivers [intravenous]-equivalent diuresis at home.” It has been shown to “produce similar diuresis and natriuresis compared to intravenous furosemide.”
“This marks a tremendous opportunity to improve the at-home management of worsening congestion in patients with heart failure who display reduced responsiveness to oral diuretics and require administration of [intravenous] diuretics, which typically requires admission to the hospital,” William T. Abraham, MD, said in the press release.
The FDA approval “is significant and will allow patients to be treated outside of the hospital setting,” said Dr. Abraham, of Ohio State University, Columbus, and an scPharmaceuticals board member.
The Furoscix indication doesn’t cover emergent use or use in acute pulmonary edema, nor is it meant to be used chronically “and should be replaced with oral diuretics as soon as practical,” the company states.
A version of this article first appeared on Medscape.com.
Similar transplant outcomes with hearts donated after circulatory death
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
SMART-CHOICE 3-year results support dropping aspirin after PCI
Shortening the duration of dual-antiplatelet therapy (DAPT) and continuing with a P2Y12 inhibitor alone after percutaneous coronary intervention (PCI) was associated with a similar rate of ischemic events but with less bleeding than prolonged DAPT after 3 years of follow-up in the SMART-CHOICE trial.
“The current the investigators, with lead author Ki Hong Choi, MD, division of cardiology, department of medicine, Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, conclude.
The 3-year results from the study were published online in JAMA Cardiology.
The authors explain that although dual therapy with aspirin and a P2Y12 inhibitor after PCI with a drug-eluting stent (DES) is crucial to reduce the risk of ischemic events, it raises concerns about increased risk of bleeding, and the antiplatelet strategy after PCI is currently shifting to reduce the duration of DAPT.
Several recent randomized studies have consistently shown that a short duration of DAPT (1-3 months) followed by P2Y12 inhibitor monotherapy had ischemia protection effects comparable with that of DAPT of longer duration, and it was associated with a significantly reduced risk of bleeding events in patients who underwent PCI, they note. However, these studies have so far reported only 1-year outcomes, and long-term results are not yet available.
The SMART-CHOICE trial compared two antiplatelet strategies – 3 months of DAPT followed by long-term P2Y12 inhibitor monotherapy (mainly with clopidogrel) or prolonged DAPT for 12 months or longer – in 2,993 patients who had undergone PCI with a drug-eluting stent. Results at 12 months showed a similar rate of ischemic events with both strategies but a lower rate of bleeding in the group that received shortened DAPT.
The SMART-CHOICE investigators now report the 3-year results showing similar outcomes.
At 3 years, the primary endpoint, a composite of all-cause death, myocardial infarction, or stroke, had occurred in 6.3% of the shortened DAPT group and 6.1% in the prolonged DAPT group, giving a hazard ratio of 1.06 (95% confidence interval, 0.79-1.44).
But in the shortened DAPT group, the risk of bleeding was reduced. Bleeding Academic Research Consortium (BARC) types 2-5 bleeding had occurred in 3.2% of the shortened DAPT group and in 8.2% of the prolonged DAPT group (hazard ratio, 0.39; 95% CI, 0.28-0.55). Major bleeding, BARC types 3-5, occurred in 1.2% of the shortened DAPT group and in 2.4% of the prolonged DAPT group (HR, 0.56; 95% CI 0.31-0.99).
The landmark analyses between 3 months and 3 years and per-protocol analyses showed consistent results.
The researchers point out that this is the first trial to report on the long-term safety and efficacy of P2Y12-inhibitor monotherapy as long-term maintenance therapy for stable patients treated with PCI.
“Especially considering that extended DAPT significantly reduced the risks of ischemic events compared with aspirin monotherapy in a couple of trials, comparison between P2Y12-inhibitor monotherapy and prolonged DAPT for recurrent ischemic events over a longer period beyond 1 year is of great importance,” they say.
They cite two other trials – HOST-EXAM and GLOBAL LEADERS – which have shown P2Y12-inhibitor monotherapy to be superior to aspirin monotherapy in preventing both ischemic and bleeding events during the long-term maintenance period after PCI.
“Combining the results of the current study, HOST-EXAM trial, and landmark analysis of the GLOBAL LEADERS trial, long-term P2Y12-inhibitor monotherapy after a minimum period of DAPT might be the most reliable option from among aspirin monotherapy, P2Y12 monotherapy, and extended DAPT for maintenance therapy after stabilizing patients who have undergone PCI with a current-generation DES,” they conclude.
They note that the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularization newly recommends a shorter course of DAPT followed by P2Y12 monotherapy as a class IIa indication. The recommendation is based on results of five large, randomized clinical trials, including SMART-CHOICE, TWILIGHT, STOPDAPT-2, TICO, and GLOBAL LEADERS.
“The current results of extended follow-up from the SMART-CHOICE trial support evidence of aspirin-dropping strategy with indefinite use of P2Y12 inhibitor after minimum use of DAPT in patients who underwent PCI,” they say.
They point out that two further trials, A-CLOSE in high-risk patients and SMART-CHOICE III, will be helpful to confirm these findings.
P2Y12-inhibitor monotherapy ‘attractive concept’
In an accompanying editor’s note, Ajay Kirtane, MD, Columbia University Irving Medical Center/New York–Presbyterian Hospital, New York, and Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai and the Cardiovascular Research Foundation, New York, note that current guidelines recommend 3-6 months of DAPT following PCI with current-generation drug-eluting stents in stable patients and 6-12 months or longer for those with acute coronary syndromes. For patients at higher risk of bleeding, even shorter DAPT durations can be considered on a case-by-case basis.
Historically, the component of DAPT subject to discontinuation decisions was the P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), but more recent trials have further explored whether discontinuation of the aspirin component of DAPT can mitigate bleeding while preserving anti-ischemic efficacy.
The editorialists explain that the concept of P2Y1-inhibitor monotherapy is attractive because it may optimize antiplatelet effects through a single agent that can avoid the gastrointestinal toxicity of aspirin as well as the increased bleeding that comes with combing multiple antithrombotic agents.
They suggest that the long-term results from the SMART-CHOICE trial “should lead clinicians to consider a strategy of monotherapy after a short period of DAPT as a viable one to mitigate bleeding risk,” although they also point out that SMART-CHOICE was underpowered to rigorously assess ischemic differences, so caution is warranted.
“For patients at greatest risk for recurrent ischemic events, the role of continued DAPT is always an option, but these data (and other consistent trials) give clinicians more options to pursue individualized treatment decisions,” they write.
“To some, the continually moving field of post-PCI antiplatelet therapy has provided too many choices, which can at times be dizzying. To us, every patient is different, and thoughtful evidence-based consideration is increasingly possible for many of our treatment decisions,” they conclude.
The SMART-CHOICE study was supported by unrestricted grants from the Korean Society of Interventional Cardiology, Abbott Vascular, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
Shortening the duration of dual-antiplatelet therapy (DAPT) and continuing with a P2Y12 inhibitor alone after percutaneous coronary intervention (PCI) was associated with a similar rate of ischemic events but with less bleeding than prolonged DAPT after 3 years of follow-up in the SMART-CHOICE trial.
“The current the investigators, with lead author Ki Hong Choi, MD, division of cardiology, department of medicine, Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, conclude.
The 3-year results from the study were published online in JAMA Cardiology.
The authors explain that although dual therapy with aspirin and a P2Y12 inhibitor after PCI with a drug-eluting stent (DES) is crucial to reduce the risk of ischemic events, it raises concerns about increased risk of bleeding, and the antiplatelet strategy after PCI is currently shifting to reduce the duration of DAPT.
Several recent randomized studies have consistently shown that a short duration of DAPT (1-3 months) followed by P2Y12 inhibitor monotherapy had ischemia protection effects comparable with that of DAPT of longer duration, and it was associated with a significantly reduced risk of bleeding events in patients who underwent PCI, they note. However, these studies have so far reported only 1-year outcomes, and long-term results are not yet available.
The SMART-CHOICE trial compared two antiplatelet strategies – 3 months of DAPT followed by long-term P2Y12 inhibitor monotherapy (mainly with clopidogrel) or prolonged DAPT for 12 months or longer – in 2,993 patients who had undergone PCI with a drug-eluting stent. Results at 12 months showed a similar rate of ischemic events with both strategies but a lower rate of bleeding in the group that received shortened DAPT.
The SMART-CHOICE investigators now report the 3-year results showing similar outcomes.
At 3 years, the primary endpoint, a composite of all-cause death, myocardial infarction, or stroke, had occurred in 6.3% of the shortened DAPT group and 6.1% in the prolonged DAPT group, giving a hazard ratio of 1.06 (95% confidence interval, 0.79-1.44).
But in the shortened DAPT group, the risk of bleeding was reduced. Bleeding Academic Research Consortium (BARC) types 2-5 bleeding had occurred in 3.2% of the shortened DAPT group and in 8.2% of the prolonged DAPT group (hazard ratio, 0.39; 95% CI, 0.28-0.55). Major bleeding, BARC types 3-5, occurred in 1.2% of the shortened DAPT group and in 2.4% of the prolonged DAPT group (HR, 0.56; 95% CI 0.31-0.99).
The landmark analyses between 3 months and 3 years and per-protocol analyses showed consistent results.
The researchers point out that this is the first trial to report on the long-term safety and efficacy of P2Y12-inhibitor monotherapy as long-term maintenance therapy for stable patients treated with PCI.
“Especially considering that extended DAPT significantly reduced the risks of ischemic events compared with aspirin monotherapy in a couple of trials, comparison between P2Y12-inhibitor monotherapy and prolonged DAPT for recurrent ischemic events over a longer period beyond 1 year is of great importance,” they say.
They cite two other trials – HOST-EXAM and GLOBAL LEADERS – which have shown P2Y12-inhibitor monotherapy to be superior to aspirin monotherapy in preventing both ischemic and bleeding events during the long-term maintenance period after PCI.
“Combining the results of the current study, HOST-EXAM trial, and landmark analysis of the GLOBAL LEADERS trial, long-term P2Y12-inhibitor monotherapy after a minimum period of DAPT might be the most reliable option from among aspirin monotherapy, P2Y12 monotherapy, and extended DAPT for maintenance therapy after stabilizing patients who have undergone PCI with a current-generation DES,” they conclude.
They note that the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularization newly recommends a shorter course of DAPT followed by P2Y12 monotherapy as a class IIa indication. The recommendation is based on results of five large, randomized clinical trials, including SMART-CHOICE, TWILIGHT, STOPDAPT-2, TICO, and GLOBAL LEADERS.
“The current results of extended follow-up from the SMART-CHOICE trial support evidence of aspirin-dropping strategy with indefinite use of P2Y12 inhibitor after minimum use of DAPT in patients who underwent PCI,” they say.
They point out that two further trials, A-CLOSE in high-risk patients and SMART-CHOICE III, will be helpful to confirm these findings.
P2Y12-inhibitor monotherapy ‘attractive concept’
In an accompanying editor’s note, Ajay Kirtane, MD, Columbia University Irving Medical Center/New York–Presbyterian Hospital, New York, and Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai and the Cardiovascular Research Foundation, New York, note that current guidelines recommend 3-6 months of DAPT following PCI with current-generation drug-eluting stents in stable patients and 6-12 months or longer for those with acute coronary syndromes. For patients at higher risk of bleeding, even shorter DAPT durations can be considered on a case-by-case basis.
Historically, the component of DAPT subject to discontinuation decisions was the P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), but more recent trials have further explored whether discontinuation of the aspirin component of DAPT can mitigate bleeding while preserving anti-ischemic efficacy.
The editorialists explain that the concept of P2Y1-inhibitor monotherapy is attractive because it may optimize antiplatelet effects through a single agent that can avoid the gastrointestinal toxicity of aspirin as well as the increased bleeding that comes with combing multiple antithrombotic agents.
They suggest that the long-term results from the SMART-CHOICE trial “should lead clinicians to consider a strategy of monotherapy after a short period of DAPT as a viable one to mitigate bleeding risk,” although they also point out that SMART-CHOICE was underpowered to rigorously assess ischemic differences, so caution is warranted.
“For patients at greatest risk for recurrent ischemic events, the role of continued DAPT is always an option, but these data (and other consistent trials) give clinicians more options to pursue individualized treatment decisions,” they write.
“To some, the continually moving field of post-PCI antiplatelet therapy has provided too many choices, which can at times be dizzying. To us, every patient is different, and thoughtful evidence-based consideration is increasingly possible for many of our treatment decisions,” they conclude.
The SMART-CHOICE study was supported by unrestricted grants from the Korean Society of Interventional Cardiology, Abbott Vascular, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
Shortening the duration of dual-antiplatelet therapy (DAPT) and continuing with a P2Y12 inhibitor alone after percutaneous coronary intervention (PCI) was associated with a similar rate of ischemic events but with less bleeding than prolonged DAPT after 3 years of follow-up in the SMART-CHOICE trial.
“The current the investigators, with lead author Ki Hong Choi, MD, division of cardiology, department of medicine, Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, conclude.
The 3-year results from the study were published online in JAMA Cardiology.
The authors explain that although dual therapy with aspirin and a P2Y12 inhibitor after PCI with a drug-eluting stent (DES) is crucial to reduce the risk of ischemic events, it raises concerns about increased risk of bleeding, and the antiplatelet strategy after PCI is currently shifting to reduce the duration of DAPT.
Several recent randomized studies have consistently shown that a short duration of DAPT (1-3 months) followed by P2Y12 inhibitor monotherapy had ischemia protection effects comparable with that of DAPT of longer duration, and it was associated with a significantly reduced risk of bleeding events in patients who underwent PCI, they note. However, these studies have so far reported only 1-year outcomes, and long-term results are not yet available.
The SMART-CHOICE trial compared two antiplatelet strategies – 3 months of DAPT followed by long-term P2Y12 inhibitor monotherapy (mainly with clopidogrel) or prolonged DAPT for 12 months or longer – in 2,993 patients who had undergone PCI with a drug-eluting stent. Results at 12 months showed a similar rate of ischemic events with both strategies but a lower rate of bleeding in the group that received shortened DAPT.
The SMART-CHOICE investigators now report the 3-year results showing similar outcomes.
At 3 years, the primary endpoint, a composite of all-cause death, myocardial infarction, or stroke, had occurred in 6.3% of the shortened DAPT group and 6.1% in the prolonged DAPT group, giving a hazard ratio of 1.06 (95% confidence interval, 0.79-1.44).
But in the shortened DAPT group, the risk of bleeding was reduced. Bleeding Academic Research Consortium (BARC) types 2-5 bleeding had occurred in 3.2% of the shortened DAPT group and in 8.2% of the prolonged DAPT group (hazard ratio, 0.39; 95% CI, 0.28-0.55). Major bleeding, BARC types 3-5, occurred in 1.2% of the shortened DAPT group and in 2.4% of the prolonged DAPT group (HR, 0.56; 95% CI 0.31-0.99).
The landmark analyses between 3 months and 3 years and per-protocol analyses showed consistent results.
The researchers point out that this is the first trial to report on the long-term safety and efficacy of P2Y12-inhibitor monotherapy as long-term maintenance therapy for stable patients treated with PCI.
“Especially considering that extended DAPT significantly reduced the risks of ischemic events compared with aspirin monotherapy in a couple of trials, comparison between P2Y12-inhibitor monotherapy and prolonged DAPT for recurrent ischemic events over a longer period beyond 1 year is of great importance,” they say.
They cite two other trials – HOST-EXAM and GLOBAL LEADERS – which have shown P2Y12-inhibitor monotherapy to be superior to aspirin monotherapy in preventing both ischemic and bleeding events during the long-term maintenance period after PCI.
“Combining the results of the current study, HOST-EXAM trial, and landmark analysis of the GLOBAL LEADERS trial, long-term P2Y12-inhibitor monotherapy after a minimum period of DAPT might be the most reliable option from among aspirin monotherapy, P2Y12 monotherapy, and extended DAPT for maintenance therapy after stabilizing patients who have undergone PCI with a current-generation DES,” they conclude.
They note that the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularization newly recommends a shorter course of DAPT followed by P2Y12 monotherapy as a class IIa indication. The recommendation is based on results of five large, randomized clinical trials, including SMART-CHOICE, TWILIGHT, STOPDAPT-2, TICO, and GLOBAL LEADERS.
“The current results of extended follow-up from the SMART-CHOICE trial support evidence of aspirin-dropping strategy with indefinite use of P2Y12 inhibitor after minimum use of DAPT in patients who underwent PCI,” they say.
They point out that two further trials, A-CLOSE in high-risk patients and SMART-CHOICE III, will be helpful to confirm these findings.
P2Y12-inhibitor monotherapy ‘attractive concept’
In an accompanying editor’s note, Ajay Kirtane, MD, Columbia University Irving Medical Center/New York–Presbyterian Hospital, New York, and Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai and the Cardiovascular Research Foundation, New York, note that current guidelines recommend 3-6 months of DAPT following PCI with current-generation drug-eluting stents in stable patients and 6-12 months or longer for those with acute coronary syndromes. For patients at higher risk of bleeding, even shorter DAPT durations can be considered on a case-by-case basis.
Historically, the component of DAPT subject to discontinuation decisions was the P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), but more recent trials have further explored whether discontinuation of the aspirin component of DAPT can mitigate bleeding while preserving anti-ischemic efficacy.
The editorialists explain that the concept of P2Y1-inhibitor monotherapy is attractive because it may optimize antiplatelet effects through a single agent that can avoid the gastrointestinal toxicity of aspirin as well as the increased bleeding that comes with combing multiple antithrombotic agents.
They suggest that the long-term results from the SMART-CHOICE trial “should lead clinicians to consider a strategy of monotherapy after a short period of DAPT as a viable one to mitigate bleeding risk,” although they also point out that SMART-CHOICE was underpowered to rigorously assess ischemic differences, so caution is warranted.
“For patients at greatest risk for recurrent ischemic events, the role of continued DAPT is always an option, but these data (and other consistent trials) give clinicians more options to pursue individualized treatment decisions,” they write.
“To some, the continually moving field of post-PCI antiplatelet therapy has provided too many choices, which can at times be dizzying. To us, every patient is different, and thoughtful evidence-based consideration is increasingly possible for many of our treatment decisions,” they conclude.
The SMART-CHOICE study was supported by unrestricted grants from the Korean Society of Interventional Cardiology, Abbott Vascular, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Cre8 EVO stent loses sweet spot in diabetes at 2 years: SUGAR
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Add PCSK9 inhibitor to high-intensity statin at primary PCI, proposes sham-controlled EPIC-STEMI
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.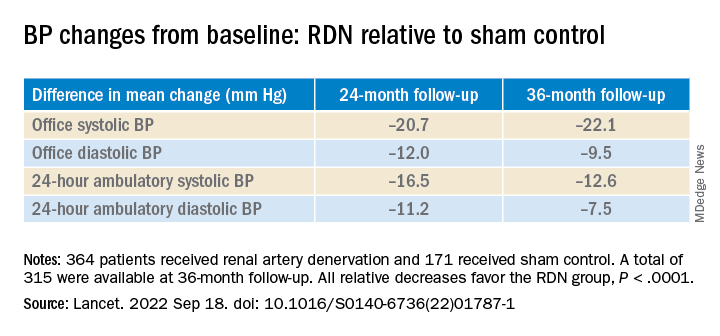
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Post-PCI FFR in multivessel disease predicts target vessel failure: FAME 3 analysis
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
Risk by FFR is continuous variable
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
FROM TCT 2022
Amulet, Watchman 2.5 LAAO outcomes neck and neck at 3 years
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM TCT 2022