User login
Young children with neuromuscular disease are vulnerable to respiratory viruses
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.
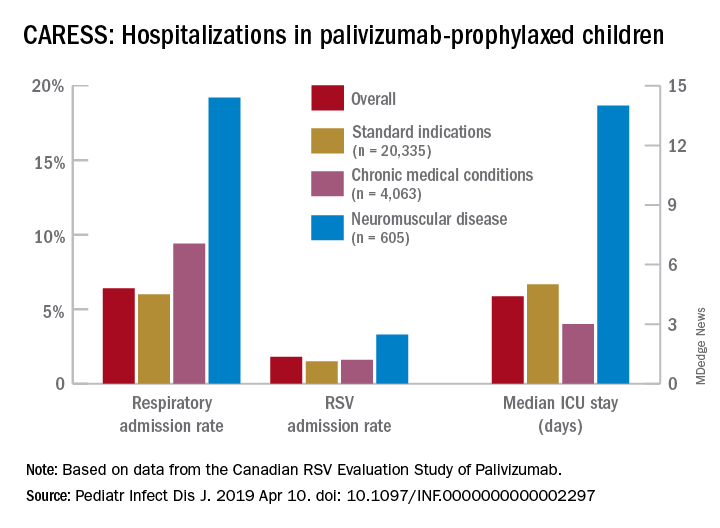
RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
This highlights the need for new vaccines
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Maternal immunization protects against serious RSV infection in infancy
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
REPORTING FROM ESPID 2019
N.Y. hospitals report near-universal CMV screening when newborns fail hearing tests
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
REPORTING FROM PAS 2019
Key clinical point: A metropolitan N.Y. health system provides a model for how to implement cytomegalovirus screening for infants who fail hearing tests.
Major finding: .
Study details: Pre-post quality improvement project.
Disclosures: The lead investigator had no disclosures. No funding source was mentioned.
Source: Chauhan A et al. PAS 2019. Abstract 306.
A gentler approach to gastroschisis improves outcomes
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
BALTIMORE – a condition in which infants are born with their intestines and sometimes other organs protruding through a hole beside the umbilicus.
Neonatologists, maternal-fetal health experts, and pediatric surgeons standardized a literature-based approach that was gentler and less invasive than usual management, emphasizing sutureless closure, sometimes at bedside on the first day of life, and early feeding. Often, it turned out, that’s all that children require.
It’s made a big difference. “We reduced the number of trips to the operating room and exposure to general anesthesia. We reduced the number of babies intubated and days on the ventilator. We reduced opioid days and antibiotic days” without increasing bacteremia, and “there are probably long-term benefits beyond the NICU,” said Kara Calkins, MD, at the Pediatric Academic Societies annual meeting.
I think this is definitely ahead of the curve for NICUs. My hope is that the vast majority of universities adopt a similar approach,” said Dr. Calkins, who is an assistant professor of neonatology at the University of California, Los Angeles.
“When I was a fellow,” she explained, “we took all of these babies and intubated them right away and put them on a drip to paralyze and sedate them. We put their bowels into a silo,” essentially a plastic bag suspended by a string, in the hopes that gravity would pull the bowels back into the abdomen. More often than not, however, “the surgeon would come by every day and slowly push them” back in over a week or so. “The fear was if you did it too quickly, you’d invoke an abdominal compartment syndrome, or respiratory decompensation. You had a baby intubated for a week, sedated and paralyzed.”
Infants were kept on total parenteral nutrition for weeks, sometimes through a Broviac central catheter.
It was overkill, Dr. Calkins said, when only the intestines are out and the abdominal wall defect isn’t too large or too small, which is the case for many infants.
For those children, sutureless closure over 1-3 days is the new goal. The bowel is worked back into the abdomen and the umbilical cord is pulled to the side to approximate the edges of the wound, and tacked down; the defect then heals itself. Antibiotics are discontinued 48 hours after closure. Gastric and rectal decompression helps with reduction.
Also, “we give drops of breast milk in their cheek right away, every couple of hours starting on the first day of life. Once the output from the gastric tube is clear, we start feeds. We still give total parenteral nutrition, but through a [peripherally inserted central catheter] in the arm,” Dr. Calkins said. “Use of breast milk for this population is important” to help establish a healthy microbiome, among other reasons.
Another improvement that had been made, according to Dr. Calkins, is that if only the intestines are out, women carry their baby to term and deliver vaginally. The old practice was to deliver babies preterm by Cesarean section, she explained.
To see how it’s worked out, Dr. Calkins and her colleagues reviewed 70 gastroschisis cases managed under the new guidelines. They were uncomplicated cases, with no intestinal atresia, stricture, or ischemia.
Paralysis was avoided for silo placement in 53 infants (76%) and 32 (46%) avoided intubation. Antibiotics were discontinued in 56 (80%) within 48 hours of abdominal wall closure, and routine narcotics were discontinued in 53 infants (76%). Feeds were initiated in almost all children within 48 hours of non-bilious gastric tube output.
Compared with 168 infants treated before the changes were made, silo placement dropped from 71% to 58% of infants, and total ventilator days from a median of 5 to 2.
There was no difference in length of stay, perhaps because the “intestinal dysmotility intrinsic to gastroschisis remains a rate limiting factor for discharge,” the team concluded.
There was no industry funding, and Dr. Calkins didn’t have any disclosures.
SOURCE: Rottkamp CA et al., PAS 2019. Abstract 51.
REPORTING FROM PAS 2019
Marijuana during prenatal OUD treatment increases premature birth
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
REPORTING FROM PAS 2019
Key clinical point: Warn pregnant women being treated for opioid use disorder to stay away from marijuana.
Major finding: Marijuana use more than doubled the risk of prematurity and low birth weight.
Study details: Review of 2,375 opioid-exposed pregnancies at six hospitals
Disclosures: There was no industry funding for the work, and the lead investigator had no disclosures.
Changing attitudes, perceived norms promote safe sleep in mothers
related to these practices, according to a new study.
In the past, the American Academy of Pediatrics has made safe sleep recommendations regarding infant sleep position and location. According to the new study’s authors, Rachel Y. Moon, MD, and her colleagues, parents had poorly adhered to these recommendations in several studies. However, some improvements with adherence were seen when a mobile health intervention was used in the Social Media and Risk Reduction Training Study (JAMA. 2017;318[4]:351-9). The new study, published in Pediatrics, used the same intervention described in that JAMA paper.
The more recent mobile health project sought to identify which factors, as outlined by a theory of planned behavior, were affected by a mobile health intervention through analysis of survey responses. Of the 1,600 women who provided written consent, 1,263 (78.9%) completed the survey.
According to the results, the intervention did more to affect attitudes (adjusted odds ratio, 2.35; 95% confidence interval, 1.72-3.20) than it did to affect perceived norms (aOR, 1.75; 95% CI, 1.27-2.36) regarding supine sleeping position. It had similar effects on attitudes (aOR, 1.91; 95% CI, 1.54-2.36) versus perceived norms (aOR, 1.37; 95% CI, 1.13-1.66) regarding sleep location as well. The intervention had no significant effect on perceived maternal control regarding either sleeping position or location.
While levels of safe sleep adherence were lower in African Americans and subgroups of low economic status at baseline, the intervention improved the rates of adherence in these groups to levels comparable with other groups included in the study.
“Recognition that these attitudes and social norms may be the main drivers of mothers’ choices regarding infant-sleep practices should inform health messaging strategies, including the use of [mobile heath], to promote [safe sleep],” the researchers concluded.
The study was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the CJ foundation for sudden infant death syndrome. The National Institutes of Health also provided funding.
SOURCE: Moon RY et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2799.
related to these practices, according to a new study.
In the past, the American Academy of Pediatrics has made safe sleep recommendations regarding infant sleep position and location. According to the new study’s authors, Rachel Y. Moon, MD, and her colleagues, parents had poorly adhered to these recommendations in several studies. However, some improvements with adherence were seen when a mobile health intervention was used in the Social Media and Risk Reduction Training Study (JAMA. 2017;318[4]:351-9). The new study, published in Pediatrics, used the same intervention described in that JAMA paper.
The more recent mobile health project sought to identify which factors, as outlined by a theory of planned behavior, were affected by a mobile health intervention through analysis of survey responses. Of the 1,600 women who provided written consent, 1,263 (78.9%) completed the survey.
According to the results, the intervention did more to affect attitudes (adjusted odds ratio, 2.35; 95% confidence interval, 1.72-3.20) than it did to affect perceived norms (aOR, 1.75; 95% CI, 1.27-2.36) regarding supine sleeping position. It had similar effects on attitudes (aOR, 1.91; 95% CI, 1.54-2.36) versus perceived norms (aOR, 1.37; 95% CI, 1.13-1.66) regarding sleep location as well. The intervention had no significant effect on perceived maternal control regarding either sleeping position or location.
While levels of safe sleep adherence were lower in African Americans and subgroups of low economic status at baseline, the intervention improved the rates of adherence in these groups to levels comparable with other groups included in the study.
“Recognition that these attitudes and social norms may be the main drivers of mothers’ choices regarding infant-sleep practices should inform health messaging strategies, including the use of [mobile heath], to promote [safe sleep],” the researchers concluded.
The study was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the CJ foundation for sudden infant death syndrome. The National Institutes of Health also provided funding.
SOURCE: Moon RY et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2799.
related to these practices, according to a new study.
In the past, the American Academy of Pediatrics has made safe sleep recommendations regarding infant sleep position and location. According to the new study’s authors, Rachel Y. Moon, MD, and her colleagues, parents had poorly adhered to these recommendations in several studies. However, some improvements with adherence were seen when a mobile health intervention was used in the Social Media and Risk Reduction Training Study (JAMA. 2017;318[4]:351-9). The new study, published in Pediatrics, used the same intervention described in that JAMA paper.
The more recent mobile health project sought to identify which factors, as outlined by a theory of planned behavior, were affected by a mobile health intervention through analysis of survey responses. Of the 1,600 women who provided written consent, 1,263 (78.9%) completed the survey.
According to the results, the intervention did more to affect attitudes (adjusted odds ratio, 2.35; 95% confidence interval, 1.72-3.20) than it did to affect perceived norms (aOR, 1.75; 95% CI, 1.27-2.36) regarding supine sleeping position. It had similar effects on attitudes (aOR, 1.91; 95% CI, 1.54-2.36) versus perceived norms (aOR, 1.37; 95% CI, 1.13-1.66) regarding sleep location as well. The intervention had no significant effect on perceived maternal control regarding either sleeping position or location.
While levels of safe sleep adherence were lower in African Americans and subgroups of low economic status at baseline, the intervention improved the rates of adherence in these groups to levels comparable with other groups included in the study.
“Recognition that these attitudes and social norms may be the main drivers of mothers’ choices regarding infant-sleep practices should inform health messaging strategies, including the use of [mobile heath], to promote [safe sleep],” the researchers concluded.
The study was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the CJ foundation for sudden infant death syndrome. The National Institutes of Health also provided funding.
SOURCE: Moon RY et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2799.
FROM PEDIATRICS
No clear benefit seen for postdischarge oxygen in preemies with BPD
Preterm infants with bronchopulmonary dysplasia (BPD) discharged with supplemental oxygen showed slightly better weight and significantly improved weight-for-length scores, but were more likely to use medical resources and had rates of neurodevelopmental impairment similar to those of infants not discharged with oxygen, according to research published in Pediatrics.
“With this study, we provide important and novel information that may aid the decision of whether to discharge an infant with supplemental oxygen, particularly for those infants who might be weaned off by some clinicians and not by others,” wrote Sara B. DeMauro, MD, MSCE, of University of Pennsylvania, Philadelphia, and Children’s Hospital of Philadelphia, and her colleagues. “This study helps to clarify, both for clinicians and parents, the potential benefits and harms that might be expected from home oxygen therapy among the subset of infants for whom the best course of action is unclear.”
Dr. DeMauro and her colleagues examined 1,039 preterm infants with BPD given supplemental oxygen by nasal cannula between January 2006 and December 2014, who were propensity matched to infants in a control group with a similar severity of BPD who were not discharged with oxygen. The infants were born at less than 27 weeks’ gestation and began receiving oxygen therapy or respiratory support at 36 weeks’ postmenstrual age. These infants were then measured for growth, neurodevelopment, and resource use from discharge to follow-up at 22-26 months corrected age.
At follow-up, infants discharged with oxygen showed marginal weight improvement scores (adjusted mean difference, 0.11) and significantly improved weight-for-length scores (adjusted mean difference, 0.13), but they had rates of neurodevelopmental impairment similar to those of infants with BPD discharged without supplemental oxygen. In addition, infants discharged with oxygen had a greater likelihood of rehospitalization due to respiratory illness (adjusted relative risk, 1.33), use of asthma or BPD medication (adjusted RR, 1.30), and use of medical equipment such as a pulse oximeter (adjusted RR, 2.94).
The researchers noted that their study’s design prevented them from examining all infants with BPD discharged with supplemental oxygen and what factors influenced discharge of infants with supplemental oxygen, as well as the effects of various durations of supplemental oxygen exposure.
“Definitive evaluation of the risk/benefit ratio of this therapy will require prospective controlled trials,” Dr. DeMauro and her colleagues wrote. “Such research will facilitate a more evidence-based approach to clinical decisions about postdischarge care of infants with BPD.”
This study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network and the National Institutes of Health. The authors reported no relevant financial disclosures.
SOURCE: DeMauro SB et al. Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2018-2956.
While oxygen use recommendations for preterm infants in the delivery room and neonatal ICU have changed, postdischarge oxygen instructions have largely not, with variations among practices and evidence for its use not well established.
The results from DeMauro et al., while not establishing causality, can instead be used to design a prospective trial to identify which preterm infants with BPD require oxygen post discharge, Reese H. Clark, MD, and Veeral N. Tolia, MD, wrote in a related editorial.
Supplemental oxygen also was associated with greater resource use among infants in the study, and they were more likely to require medications for asthma and BPD, procedures such as tracheotomy, and rehospitalization, which is in line with previous clinical studies analyzing oxygen use in the NICU, they noted.
The findings by DeMauro et al. could be used to improve the design and safety of a prospective study. For example, “it may not be feasible or ethical to include some infants with more severe BPD in future trials,” they noted. “Once again, we are challenged to reevaluate our clinical beliefs and biases about the use of oxygen,” said Dr. Clark and Dr. Tolia. “Now we must collaborate to design and implement a trial to help us determine which infants should receive oxygen after discharge. We look forward to seeing those results.”
Dr. Clark is from the Center for Research and Education at MEDNAX in Sunrise, Fla., and Dr. Tolia is at Baylor University Medical Center and Pediatrix Medical Group in Dallas. This is a summary of the editorial accompanying the report by DeMauro et al. (Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2019-0372). They reported no relevant financial disclosures or external funding.
While oxygen use recommendations for preterm infants in the delivery room and neonatal ICU have changed, postdischarge oxygen instructions have largely not, with variations among practices and evidence for its use not well established.
The results from DeMauro et al., while not establishing causality, can instead be used to design a prospective trial to identify which preterm infants with BPD require oxygen post discharge, Reese H. Clark, MD, and Veeral N. Tolia, MD, wrote in a related editorial.
Supplemental oxygen also was associated with greater resource use among infants in the study, and they were more likely to require medications for asthma and BPD, procedures such as tracheotomy, and rehospitalization, which is in line with previous clinical studies analyzing oxygen use in the NICU, they noted.
The findings by DeMauro et al. could be used to improve the design and safety of a prospective study. For example, “it may not be feasible or ethical to include some infants with more severe BPD in future trials,” they noted. “Once again, we are challenged to reevaluate our clinical beliefs and biases about the use of oxygen,” said Dr. Clark and Dr. Tolia. “Now we must collaborate to design and implement a trial to help us determine which infants should receive oxygen after discharge. We look forward to seeing those results.”
Dr. Clark is from the Center for Research and Education at MEDNAX in Sunrise, Fla., and Dr. Tolia is at Baylor University Medical Center and Pediatrix Medical Group in Dallas. This is a summary of the editorial accompanying the report by DeMauro et al. (Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2019-0372). They reported no relevant financial disclosures or external funding.
While oxygen use recommendations for preterm infants in the delivery room and neonatal ICU have changed, postdischarge oxygen instructions have largely not, with variations among practices and evidence for its use not well established.
The results from DeMauro et al., while not establishing causality, can instead be used to design a prospective trial to identify which preterm infants with BPD require oxygen post discharge, Reese H. Clark, MD, and Veeral N. Tolia, MD, wrote in a related editorial.
Supplemental oxygen also was associated with greater resource use among infants in the study, and they were more likely to require medications for asthma and BPD, procedures such as tracheotomy, and rehospitalization, which is in line with previous clinical studies analyzing oxygen use in the NICU, they noted.
The findings by DeMauro et al. could be used to improve the design and safety of a prospective study. For example, “it may not be feasible or ethical to include some infants with more severe BPD in future trials,” they noted. “Once again, we are challenged to reevaluate our clinical beliefs and biases about the use of oxygen,” said Dr. Clark and Dr. Tolia. “Now we must collaborate to design and implement a trial to help us determine which infants should receive oxygen after discharge. We look forward to seeing those results.”
Dr. Clark is from the Center for Research and Education at MEDNAX in Sunrise, Fla., and Dr. Tolia is at Baylor University Medical Center and Pediatrix Medical Group in Dallas. This is a summary of the editorial accompanying the report by DeMauro et al. (Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2019-0372). They reported no relevant financial disclosures or external funding.
Preterm infants with bronchopulmonary dysplasia (BPD) discharged with supplemental oxygen showed slightly better weight and significantly improved weight-for-length scores, but were more likely to use medical resources and had rates of neurodevelopmental impairment similar to those of infants not discharged with oxygen, according to research published in Pediatrics.
“With this study, we provide important and novel information that may aid the decision of whether to discharge an infant with supplemental oxygen, particularly for those infants who might be weaned off by some clinicians and not by others,” wrote Sara B. DeMauro, MD, MSCE, of University of Pennsylvania, Philadelphia, and Children’s Hospital of Philadelphia, and her colleagues. “This study helps to clarify, both for clinicians and parents, the potential benefits and harms that might be expected from home oxygen therapy among the subset of infants for whom the best course of action is unclear.”
Dr. DeMauro and her colleagues examined 1,039 preterm infants with BPD given supplemental oxygen by nasal cannula between January 2006 and December 2014, who were propensity matched to infants in a control group with a similar severity of BPD who were not discharged with oxygen. The infants were born at less than 27 weeks’ gestation and began receiving oxygen therapy or respiratory support at 36 weeks’ postmenstrual age. These infants were then measured for growth, neurodevelopment, and resource use from discharge to follow-up at 22-26 months corrected age.
At follow-up, infants discharged with oxygen showed marginal weight improvement scores (adjusted mean difference, 0.11) and significantly improved weight-for-length scores (adjusted mean difference, 0.13), but they had rates of neurodevelopmental impairment similar to those of infants with BPD discharged without supplemental oxygen. In addition, infants discharged with oxygen had a greater likelihood of rehospitalization due to respiratory illness (adjusted relative risk, 1.33), use of asthma or BPD medication (adjusted RR, 1.30), and use of medical equipment such as a pulse oximeter (adjusted RR, 2.94).
The researchers noted that their study’s design prevented them from examining all infants with BPD discharged with supplemental oxygen and what factors influenced discharge of infants with supplemental oxygen, as well as the effects of various durations of supplemental oxygen exposure.
“Definitive evaluation of the risk/benefit ratio of this therapy will require prospective controlled trials,” Dr. DeMauro and her colleagues wrote. “Such research will facilitate a more evidence-based approach to clinical decisions about postdischarge care of infants with BPD.”
This study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network and the National Institutes of Health. The authors reported no relevant financial disclosures.
SOURCE: DeMauro SB et al. Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2018-2956.
Preterm infants with bronchopulmonary dysplasia (BPD) discharged with supplemental oxygen showed slightly better weight and significantly improved weight-for-length scores, but were more likely to use medical resources and had rates of neurodevelopmental impairment similar to those of infants not discharged with oxygen, according to research published in Pediatrics.
“With this study, we provide important and novel information that may aid the decision of whether to discharge an infant with supplemental oxygen, particularly for those infants who might be weaned off by some clinicians and not by others,” wrote Sara B. DeMauro, MD, MSCE, of University of Pennsylvania, Philadelphia, and Children’s Hospital of Philadelphia, and her colleagues. “This study helps to clarify, both for clinicians and parents, the potential benefits and harms that might be expected from home oxygen therapy among the subset of infants for whom the best course of action is unclear.”
Dr. DeMauro and her colleagues examined 1,039 preterm infants with BPD given supplemental oxygen by nasal cannula between January 2006 and December 2014, who were propensity matched to infants in a control group with a similar severity of BPD who were not discharged with oxygen. The infants were born at less than 27 weeks’ gestation and began receiving oxygen therapy or respiratory support at 36 weeks’ postmenstrual age. These infants were then measured for growth, neurodevelopment, and resource use from discharge to follow-up at 22-26 months corrected age.
At follow-up, infants discharged with oxygen showed marginal weight improvement scores (adjusted mean difference, 0.11) and significantly improved weight-for-length scores (adjusted mean difference, 0.13), but they had rates of neurodevelopmental impairment similar to those of infants with BPD discharged without supplemental oxygen. In addition, infants discharged with oxygen had a greater likelihood of rehospitalization due to respiratory illness (adjusted relative risk, 1.33), use of asthma or BPD medication (adjusted RR, 1.30), and use of medical equipment such as a pulse oximeter (adjusted RR, 2.94).
The researchers noted that their study’s design prevented them from examining all infants with BPD discharged with supplemental oxygen and what factors influenced discharge of infants with supplemental oxygen, as well as the effects of various durations of supplemental oxygen exposure.
“Definitive evaluation of the risk/benefit ratio of this therapy will require prospective controlled trials,” Dr. DeMauro and her colleagues wrote. “Such research will facilitate a more evidence-based approach to clinical decisions about postdischarge care of infants with BPD.”
This study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network and the National Institutes of Health. The authors reported no relevant financial disclosures.
SOURCE: DeMauro SB et al. Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2018-2956.
FROM PEDIATRICS
Key clinical point: compared with controls.
Major finding: At 22-26 months of age, infants discharged with oxygen showed marginal improvement in weight z scores (adjusted mean difference, 0.11) and significantly improved weight-for-length z scores (adjusted mean difference, 0.13), but similar rates of neurodevelopmental impairment.
Study details: A retrospective propensity-matched cohort study of 1,039 preterm infants given supplemental oxygen by nasal cannula between January 2006 and December 2014 and analyzed over 2 years of life.
Disclosures: This study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network and the National Institutes of Health. The authors reported no relevant financial disclosures.
Source: DeMauro SB et al. Pediatrics. 2019 Apr 11. doi: 10.1542/peds.2018-2956.
Oscillatory ventilation reduced reintubation risk for preterm infants
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
Nasal high-frequency oscillatory ventilation, in a randomized trial of 206 preterm infants with respiratory failure.
Previous studies have supported the use of NHFOV as more effective for reducing CO2 and for lowering the risk of reintubation compared with NCPAP. But no randomized, controlled trials had compared the outcomes for preterm infants in particular, wrote Long Chen, MD, PhD, of Children’s Hospital of Chongqing Medical University, Chongqing, China, and colleagues.
Their study, published in Chest, was conducted at a single tertiary NICU in China between May 2017 and May 2018, and randomized infants with a gestational age less than 37 weeks to NHFOV (103 infants) or NCPAP (103 infants). Infants with major congenital abnormalities were excluded. The infants included 127 (61.7%) diagnosed with respiratory distress syndrome (RDS), 53 (25.7%) diagnosed with acute RDS (ARDS), and 26 (12.6%) diagnosed with both RDS and ARDS.
Overall, the reintubation rate within 6 hours was significantly lower among infants treated with NHFOV compared with those treated with NCPAP (15.5% vs. 34%, P = .002), and in the subset of infants with ARDS (23.5% vs. 52.6%, P = .032). Among infants with a gestational age of 32 weeks or less, reintuibation rates were also significantly lower among those treated with NHFOV (26.1% vs. 55.6%, P = .004).
In addition, PCO2 levels, 6 hours after extubation, were significantly lower among infants on NHFOV, compared with those on NCPAP (49.6 vs. 56.9 P = .00). The hospital stay, a secondary outcome, was significantly shorter among the infants treated with NHFOV, than those treated with NCPAP (22 days, vs. 27.6 days, P =.011).
Although the researchers observed some nasal trauma in NHFOV-treated patients, and intestinal dilation in both groups similar to side effects seen in previous studies, no feeding intolerance or skin lesions were associated with NHFOV. The study findings were consistent with those from previous studies, and suggested that the causes of respiratory failure might account for the differences between the treatment groups, they noted.
“RDS is primarily restrictive in the acute phase, and the high frequency oscillation over CPAP does not therefore bring any benefit. However, ARDS is both restrictive and obstructive in the acute phase due to the nature of ARDS,” and NHFOV is “able to improve oxygenation,” they added.
The study findings were limited by several factors including the use of data from a single center and the small number of infants younger than 28 weeks’ gestation, the researchers noted. However, they added, two international, multicenter, randomized controlled trials are in the works.
The study was supported by Social Livelihood Program of 38 Chongqing Science and Technology Commission, China. The researchers had no financial conflicts to disclose.
SOURCE: Long C et al. Chest. 2019; 155(4): 740-8.
FROM CHEST
Earlier diagnosis, treatment needed to curb dramatic rise in neonatal HSV
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
FROM PEDIATRICS
Swedish strategies improve survival for premature infants
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
FROM JAMA