User login
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
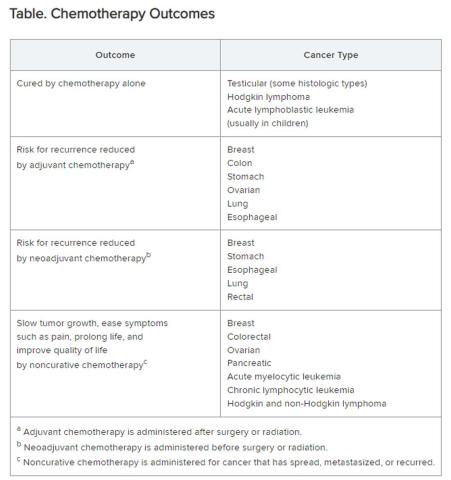
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Can Belzutifan Improve Outcomes in Advanced RCC?
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Liver Transplant Delays Progression in Colorectal Metastasis
TOPLINE:
METHODOLOGY:
- Research has shown promising results for well-selected patients with unresectable colorectal liver metastasis undergoing liver transplant; however, the absence of a suitable comparison group makes it difficult to evaluate the overall effectiveness of this treatment method.
- Researchers evaluated 33 patients with colorectal cancer and unresectable liver metastasis (mean age, 43.5 years; 52% women) who were eligible for liver transplants, according to validated selection criteria.
- Of these, 20 patients (61%) underwent a liver transplant, while 13 (39%) declined transplantation and received alternative therapy.
- Patients who received liver transplants did not undergo regular chemotherapy until recurrence, whereas those in the alternative therapy group continued systemic chemotherapy, with hepatic artery infusion pump placement (n = 5), liver resections (n = 6), and locoregional therapies (n = 6).
- The main outcomes of the study were overall survival and PFS.
TAKEAWAY:
- The median follow-up duration was 986 days in the liver transplant group and 657 days in the alternative therapy group.
- Patients who underwent liver transplant showed higher PFS rates at 1 year (90.0% vs 41.7%), 2 years (72.7% vs 10.4%), and 3 years (36.4% vs 10.4%). The PFS gains were statistically significant (P < .01).
- Overall survival was also higher in the transplant group — 100% vs 83.9% at 1 year, and 90.0% vs 73.4% at both 2 and 3 years. The differences, however, did not reach significance (P = .12).
- Liver transplant was associated with a lower recurrence rate (5% vs 23%), which also did not reach significance (P = .28) possibly because of the small patient population.
IN PRACTICE:
“This study represents the best available data for evaluating alternatives to [liver transplant],” the authors wrote, adding that the patients should be “referred for multidisciplinary evaluation to transplant oncology centers with strict criteria.”
SOURCE:
The study was led by Matthew M. Byrne, MD, Department of Surgery, University of Rochester Medical Center, Rochester, New York, and was published online in JAMA Surgery.
LIMITATIONS:
The patient population was small, making it difficult to interpret statistical significance. The inclusion of patients with financial and social support might limit generalizability. The survival was calculated from the date of transplant or dropout. Additionally, the study did not explore sex-based differences in treatment choice.
DISCLOSURES:
The authors did not disclose any funding information. One author reported holding shares with HistoSonics, not related to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Research has shown promising results for well-selected patients with unresectable colorectal liver metastasis undergoing liver transplant; however, the absence of a suitable comparison group makes it difficult to evaluate the overall effectiveness of this treatment method.
- Researchers evaluated 33 patients with colorectal cancer and unresectable liver metastasis (mean age, 43.5 years; 52% women) who were eligible for liver transplants, according to validated selection criteria.
- Of these, 20 patients (61%) underwent a liver transplant, while 13 (39%) declined transplantation and received alternative therapy.
- Patients who received liver transplants did not undergo regular chemotherapy until recurrence, whereas those in the alternative therapy group continued systemic chemotherapy, with hepatic artery infusion pump placement (n = 5), liver resections (n = 6), and locoregional therapies (n = 6).
- The main outcomes of the study were overall survival and PFS.
TAKEAWAY:
- The median follow-up duration was 986 days in the liver transplant group and 657 days in the alternative therapy group.
- Patients who underwent liver transplant showed higher PFS rates at 1 year (90.0% vs 41.7%), 2 years (72.7% vs 10.4%), and 3 years (36.4% vs 10.4%). The PFS gains were statistically significant (P < .01).
- Overall survival was also higher in the transplant group — 100% vs 83.9% at 1 year, and 90.0% vs 73.4% at both 2 and 3 years. The differences, however, did not reach significance (P = .12).
- Liver transplant was associated with a lower recurrence rate (5% vs 23%), which also did not reach significance (P = .28) possibly because of the small patient population.
IN PRACTICE:
“This study represents the best available data for evaluating alternatives to [liver transplant],” the authors wrote, adding that the patients should be “referred for multidisciplinary evaluation to transplant oncology centers with strict criteria.”
SOURCE:
The study was led by Matthew M. Byrne, MD, Department of Surgery, University of Rochester Medical Center, Rochester, New York, and was published online in JAMA Surgery.
LIMITATIONS:
The patient population was small, making it difficult to interpret statistical significance. The inclusion of patients with financial and social support might limit generalizability. The survival was calculated from the date of transplant or dropout. Additionally, the study did not explore sex-based differences in treatment choice.
DISCLOSURES:
The authors did not disclose any funding information. One author reported holding shares with HistoSonics, not related to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Research has shown promising results for well-selected patients with unresectable colorectal liver metastasis undergoing liver transplant; however, the absence of a suitable comparison group makes it difficult to evaluate the overall effectiveness of this treatment method.
- Researchers evaluated 33 patients with colorectal cancer and unresectable liver metastasis (mean age, 43.5 years; 52% women) who were eligible for liver transplants, according to validated selection criteria.
- Of these, 20 patients (61%) underwent a liver transplant, while 13 (39%) declined transplantation and received alternative therapy.
- Patients who received liver transplants did not undergo regular chemotherapy until recurrence, whereas those in the alternative therapy group continued systemic chemotherapy, with hepatic artery infusion pump placement (n = 5), liver resections (n = 6), and locoregional therapies (n = 6).
- The main outcomes of the study were overall survival and PFS.
TAKEAWAY:
- The median follow-up duration was 986 days in the liver transplant group and 657 days in the alternative therapy group.
- Patients who underwent liver transplant showed higher PFS rates at 1 year (90.0% vs 41.7%), 2 years (72.7% vs 10.4%), and 3 years (36.4% vs 10.4%). The PFS gains were statistically significant (P < .01).
- Overall survival was also higher in the transplant group — 100% vs 83.9% at 1 year, and 90.0% vs 73.4% at both 2 and 3 years. The differences, however, did not reach significance (P = .12).
- Liver transplant was associated with a lower recurrence rate (5% vs 23%), which also did not reach significance (P = .28) possibly because of the small patient population.
IN PRACTICE:
“This study represents the best available data for evaluating alternatives to [liver transplant],” the authors wrote, adding that the patients should be “referred for multidisciplinary evaluation to transplant oncology centers with strict criteria.”
SOURCE:
The study was led by Matthew M. Byrne, MD, Department of Surgery, University of Rochester Medical Center, Rochester, New York, and was published online in JAMA Surgery.
LIMITATIONS:
The patient population was small, making it difficult to interpret statistical significance. The inclusion of patients with financial and social support might limit generalizability. The survival was calculated from the date of transplant or dropout. Additionally, the study did not explore sex-based differences in treatment choice.
DISCLOSURES:
The authors did not disclose any funding information. One author reported holding shares with HistoSonics, not related to the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Traveling To Die: The Latest Form of Medical Tourism
In the 18 months after Francine Milano was diagnosed with a recurrence of the ovarian cancer she thought she’d beaten 20 years ago, she traveled twice from her home in Pennsylvania to Vermont. She went not to ski, hike, or leaf-peep, but to arrange to die.
Dying with medical assistance wasn’t an option when Ms. Milano learned in early 2023 that her disease was incurable. At that point, she would have had to travel to Switzerland — or live in the District of Columbia or one of the 10 states where medical aid in dying was legal.
But Vermont lifted its residency requirement in May 2023, followed by Oregon two months later. (Montana effectively allows aid in dying through a 2009 court decision, but that ruling doesn’t spell out rules around residency. And though New York and California recently considered legislation that would allow out-of-staters to secure aid in dying, neither provision passed.)
Despite the limited options and the challenges — such as finding doctors in a new state, figuring out where to die, and traveling when too sick to walk to the next room, let alone climb into a car — dozens have made the trek to the two states that have opened their doors to terminally ill nonresidents seeking aid in dying.
At least 26 people have traveled to Vermont to die, representing nearly 25% of the reported assisted deaths in the state from May 2023 through this June, according to the Vermont Department of Health. In Oregon, 23 out-of-state residents died using medical assistance in 2023, just over 6% of the state total, according to the Oregon Health Authority.
Oncologist Charles Blanke, MD, whose clinic in Portland is devoted to end-of-life care, said he thinks that Oregon’s total is likely an undercount and he expects the numbers to grow. Over the past year, he said, he’s seen two to four out-of-state patients a week — about one-quarter of his practice — and fielded calls from across the U.S., including New York, the Carolinas, Florida, and “tons from Texas.” But just because patients are willing to travel doesn’t mean it’s easy or that they get their desired outcome.
“The law is pretty strict about what has to be done,” Dr. Blanke said.
As in other states that allow what some call physician-assisted death or assisted suicide, Oregon and Vermont require patients to be assessed by two doctors. Patients must have less than six months to live, be mentally and cognitively sound, and be physically able to ingest the drugs to end their lives. Charts and records must be reviewed in the state; neglecting to do so constitutes practicing medicine out of state, which violates medical licensing requirements. For the same reason, the patients must be in the state for the initial exam, when they request the drugs, and when they ingest them.
State legislatures impose those restrictions as safeguards — to balance the rights of patients seeking aid in dying with a legislative imperative not to pass laws that are harmful to anyone, said Peg Sandeen, CEO of the group Death With Dignity. Like many aid-in-dying advocates, however, she said such rules create undue burdens for people who are already suffering.
Diana Barnard, MD, a Vermont palliative care physician, said some patients cannot even come for their appointments. “They end up being sick or not feeling like traveling, so there’s rescheduling involved,” she said. “It’s asking people to use a significant part of their energy to come here when they really deserve to have the option closer to home.”
Those opposed to aid in dying include religious groups that say taking a life is immoral, and medical practitioners who argue their job is to make people more comfortable at the end of life, not to end the life itself.
Anthropologist Anita Hannig, who interviewed dozens of terminally ill patients while researching her 2022 book, “The Day I Die: The Untold Story of Assisted Dying in America,” said she doesn’t expect federal legislation to settle the issue anytime soon. As the Supreme Court did with abortion in 2022, it ruled assisted dying to be a states’ rights issue in 1997.
During the 2023-24 legislative sessions, 19 states (including Ms. Milano’s home state of Pennsylvania) considered aid-in-dying legislation, according to the advocacy group Compassion & Choices. Delaware was the sole state to pass it, but the governor has yet to act on it.
Ms. Sandeen said that many states initially pass restrictive laws — requiring 21-day wait times and psychiatric evaluations, for instance — only to eventually repeal provisions that prove unduly onerous. That makes her optimistic that more states will eventually follow Vermont and Oregon, she said.
Ms. Milano would have preferred to travel to neighboring New Jersey, where aid in dying has been legal since 2019, but its residency requirement made that a nonstarter. And though Oregon has more providers than the largely rural state of Vermont, Ms. Milano opted for the 9-hour car ride to Burlington because it was less physically and financially draining than a cross-country trip.
The logistics were key because Ms. Milano knew she’d have to return. When she traveled to Vermont in May 2023 with her husband and her brother, she wasn’t near death. She figured that the next time she was in Vermont, it would be to request the medication. Then she’d have to wait 15 days to receive it.
The waiting period is standard to ensure that a person has what Dr. Barnard calls “thoughtful time to contemplate the decision,” although she said most have done that long before. Some states have shortened the period or, like Oregon, have a waiver option.
That waiting period can be hard on patients, on top of being away from their health care team, home, and family. Blanke said he has seen as many as 25 relatives attend the death of an Oregon resident, but out-of-staters usually bring only one person. And while finding a place to die can be a problem for Oregonians who are in care homes or hospitals that prohibit aid in dying, it’s especially challenging for nonresidents.
When Oregon lifted its residency requirement, Dr. Blanke advertised on Craigslist and used the results to compile a list of short-term accommodations, including Airbnbs, willing to allow patients to die there. Nonprofits in states with aid-in-dying laws also maintain such lists, Ms. Sandeen said.
Ms. Milano hasn’t gotten to the point where she needs to find a place to take the meds and end her life. In fact, because she had a relatively healthy year after her first trip to Vermont, she let her 6-month approval period lapse.
In June, though, she headed back to open another 6-month window. This time, she went with a girlfriend who has a camper van. They drove 6 hours to cross the state border, stopping at a playground and gift shop before sitting in a parking lot where Ms. Milano had a Zoom appointment with her doctors rather than driving 3 more hours to Burlington to meet in person.
“I don’t know if they do GPS tracking or IP address kind of stuff, but I would have been afraid not to be honest,” she said.
That’s not all that scares her. She worries she’ll be too sick to return to Vermont when she is ready to die. And, even if she can get there, she wonders whether she’ll have the courage to take the medication. About one-third of people approved for assisted death don’t follow through, Dr. Blanke said. For them, it’s often enough to know they have the meds — the control — to end their lives when they want.
Ms. Milano said she is grateful she has that power now while she’s still healthy enough to travel and enjoy life. “I just wish more people had the option,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
In the 18 months after Francine Milano was diagnosed with a recurrence of the ovarian cancer she thought she’d beaten 20 years ago, she traveled twice from her home in Pennsylvania to Vermont. She went not to ski, hike, or leaf-peep, but to arrange to die.
Dying with medical assistance wasn’t an option when Ms. Milano learned in early 2023 that her disease was incurable. At that point, she would have had to travel to Switzerland — or live in the District of Columbia or one of the 10 states where medical aid in dying was legal.
But Vermont lifted its residency requirement in May 2023, followed by Oregon two months later. (Montana effectively allows aid in dying through a 2009 court decision, but that ruling doesn’t spell out rules around residency. And though New York and California recently considered legislation that would allow out-of-staters to secure aid in dying, neither provision passed.)
Despite the limited options and the challenges — such as finding doctors in a new state, figuring out where to die, and traveling when too sick to walk to the next room, let alone climb into a car — dozens have made the trek to the two states that have opened their doors to terminally ill nonresidents seeking aid in dying.
At least 26 people have traveled to Vermont to die, representing nearly 25% of the reported assisted deaths in the state from May 2023 through this June, according to the Vermont Department of Health. In Oregon, 23 out-of-state residents died using medical assistance in 2023, just over 6% of the state total, according to the Oregon Health Authority.
Oncologist Charles Blanke, MD, whose clinic in Portland is devoted to end-of-life care, said he thinks that Oregon’s total is likely an undercount and he expects the numbers to grow. Over the past year, he said, he’s seen two to four out-of-state patients a week — about one-quarter of his practice — and fielded calls from across the U.S., including New York, the Carolinas, Florida, and “tons from Texas.” But just because patients are willing to travel doesn’t mean it’s easy or that they get their desired outcome.
“The law is pretty strict about what has to be done,” Dr. Blanke said.
As in other states that allow what some call physician-assisted death or assisted suicide, Oregon and Vermont require patients to be assessed by two doctors. Patients must have less than six months to live, be mentally and cognitively sound, and be physically able to ingest the drugs to end their lives. Charts and records must be reviewed in the state; neglecting to do so constitutes practicing medicine out of state, which violates medical licensing requirements. For the same reason, the patients must be in the state for the initial exam, when they request the drugs, and when they ingest them.
State legislatures impose those restrictions as safeguards — to balance the rights of patients seeking aid in dying with a legislative imperative not to pass laws that are harmful to anyone, said Peg Sandeen, CEO of the group Death With Dignity. Like many aid-in-dying advocates, however, she said such rules create undue burdens for people who are already suffering.
Diana Barnard, MD, a Vermont palliative care physician, said some patients cannot even come for their appointments. “They end up being sick or not feeling like traveling, so there’s rescheduling involved,” she said. “It’s asking people to use a significant part of their energy to come here when they really deserve to have the option closer to home.”
Those opposed to aid in dying include religious groups that say taking a life is immoral, and medical practitioners who argue their job is to make people more comfortable at the end of life, not to end the life itself.
Anthropologist Anita Hannig, who interviewed dozens of terminally ill patients while researching her 2022 book, “The Day I Die: The Untold Story of Assisted Dying in America,” said she doesn’t expect federal legislation to settle the issue anytime soon. As the Supreme Court did with abortion in 2022, it ruled assisted dying to be a states’ rights issue in 1997.
During the 2023-24 legislative sessions, 19 states (including Ms. Milano’s home state of Pennsylvania) considered aid-in-dying legislation, according to the advocacy group Compassion & Choices. Delaware was the sole state to pass it, but the governor has yet to act on it.
Ms. Sandeen said that many states initially pass restrictive laws — requiring 21-day wait times and psychiatric evaluations, for instance — only to eventually repeal provisions that prove unduly onerous. That makes her optimistic that more states will eventually follow Vermont and Oregon, she said.
Ms. Milano would have preferred to travel to neighboring New Jersey, where aid in dying has been legal since 2019, but its residency requirement made that a nonstarter. And though Oregon has more providers than the largely rural state of Vermont, Ms. Milano opted for the 9-hour car ride to Burlington because it was less physically and financially draining than a cross-country trip.
The logistics were key because Ms. Milano knew she’d have to return. When she traveled to Vermont in May 2023 with her husband and her brother, she wasn’t near death. She figured that the next time she was in Vermont, it would be to request the medication. Then she’d have to wait 15 days to receive it.
The waiting period is standard to ensure that a person has what Dr. Barnard calls “thoughtful time to contemplate the decision,” although she said most have done that long before. Some states have shortened the period or, like Oregon, have a waiver option.
That waiting period can be hard on patients, on top of being away from their health care team, home, and family. Blanke said he has seen as many as 25 relatives attend the death of an Oregon resident, but out-of-staters usually bring only one person. And while finding a place to die can be a problem for Oregonians who are in care homes or hospitals that prohibit aid in dying, it’s especially challenging for nonresidents.
When Oregon lifted its residency requirement, Dr. Blanke advertised on Craigslist and used the results to compile a list of short-term accommodations, including Airbnbs, willing to allow patients to die there. Nonprofits in states with aid-in-dying laws also maintain such lists, Ms. Sandeen said.
Ms. Milano hasn’t gotten to the point where she needs to find a place to take the meds and end her life. In fact, because she had a relatively healthy year after her first trip to Vermont, she let her 6-month approval period lapse.
In June, though, she headed back to open another 6-month window. This time, she went with a girlfriend who has a camper van. They drove 6 hours to cross the state border, stopping at a playground and gift shop before sitting in a parking lot where Ms. Milano had a Zoom appointment with her doctors rather than driving 3 more hours to Burlington to meet in person.
“I don’t know if they do GPS tracking or IP address kind of stuff, but I would have been afraid not to be honest,” she said.
That’s not all that scares her. She worries she’ll be too sick to return to Vermont when she is ready to die. And, even if she can get there, she wonders whether she’ll have the courage to take the medication. About one-third of people approved for assisted death don’t follow through, Dr. Blanke said. For them, it’s often enough to know they have the meds — the control — to end their lives when they want.
Ms. Milano said she is grateful she has that power now while she’s still healthy enough to travel and enjoy life. “I just wish more people had the option,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
In the 18 months after Francine Milano was diagnosed with a recurrence of the ovarian cancer she thought she’d beaten 20 years ago, she traveled twice from her home in Pennsylvania to Vermont. She went not to ski, hike, or leaf-peep, but to arrange to die.
Dying with medical assistance wasn’t an option when Ms. Milano learned in early 2023 that her disease was incurable. At that point, she would have had to travel to Switzerland — or live in the District of Columbia or one of the 10 states where medical aid in dying was legal.
But Vermont lifted its residency requirement in May 2023, followed by Oregon two months later. (Montana effectively allows aid in dying through a 2009 court decision, but that ruling doesn’t spell out rules around residency. And though New York and California recently considered legislation that would allow out-of-staters to secure aid in dying, neither provision passed.)
Despite the limited options and the challenges — such as finding doctors in a new state, figuring out where to die, and traveling when too sick to walk to the next room, let alone climb into a car — dozens have made the trek to the two states that have opened their doors to terminally ill nonresidents seeking aid in dying.
At least 26 people have traveled to Vermont to die, representing nearly 25% of the reported assisted deaths in the state from May 2023 through this June, according to the Vermont Department of Health. In Oregon, 23 out-of-state residents died using medical assistance in 2023, just over 6% of the state total, according to the Oregon Health Authority.
Oncologist Charles Blanke, MD, whose clinic in Portland is devoted to end-of-life care, said he thinks that Oregon’s total is likely an undercount and he expects the numbers to grow. Over the past year, he said, he’s seen two to four out-of-state patients a week — about one-quarter of his practice — and fielded calls from across the U.S., including New York, the Carolinas, Florida, and “tons from Texas.” But just because patients are willing to travel doesn’t mean it’s easy or that they get their desired outcome.
“The law is pretty strict about what has to be done,” Dr. Blanke said.
As in other states that allow what some call physician-assisted death or assisted suicide, Oregon and Vermont require patients to be assessed by two doctors. Patients must have less than six months to live, be mentally and cognitively sound, and be physically able to ingest the drugs to end their lives. Charts and records must be reviewed in the state; neglecting to do so constitutes practicing medicine out of state, which violates medical licensing requirements. For the same reason, the patients must be in the state for the initial exam, when they request the drugs, and when they ingest them.
State legislatures impose those restrictions as safeguards — to balance the rights of patients seeking aid in dying with a legislative imperative not to pass laws that are harmful to anyone, said Peg Sandeen, CEO of the group Death With Dignity. Like many aid-in-dying advocates, however, she said such rules create undue burdens for people who are already suffering.
Diana Barnard, MD, a Vermont palliative care physician, said some patients cannot even come for their appointments. “They end up being sick or not feeling like traveling, so there’s rescheduling involved,” she said. “It’s asking people to use a significant part of their energy to come here when they really deserve to have the option closer to home.”
Those opposed to aid in dying include religious groups that say taking a life is immoral, and medical practitioners who argue their job is to make people more comfortable at the end of life, not to end the life itself.
Anthropologist Anita Hannig, who interviewed dozens of terminally ill patients while researching her 2022 book, “The Day I Die: The Untold Story of Assisted Dying in America,” said she doesn’t expect federal legislation to settle the issue anytime soon. As the Supreme Court did with abortion in 2022, it ruled assisted dying to be a states’ rights issue in 1997.
During the 2023-24 legislative sessions, 19 states (including Ms. Milano’s home state of Pennsylvania) considered aid-in-dying legislation, according to the advocacy group Compassion & Choices. Delaware was the sole state to pass it, but the governor has yet to act on it.
Ms. Sandeen said that many states initially pass restrictive laws — requiring 21-day wait times and psychiatric evaluations, for instance — only to eventually repeal provisions that prove unduly onerous. That makes her optimistic that more states will eventually follow Vermont and Oregon, she said.
Ms. Milano would have preferred to travel to neighboring New Jersey, where aid in dying has been legal since 2019, but its residency requirement made that a nonstarter. And though Oregon has more providers than the largely rural state of Vermont, Ms. Milano opted for the 9-hour car ride to Burlington because it was less physically and financially draining than a cross-country trip.
The logistics were key because Ms. Milano knew she’d have to return. When she traveled to Vermont in May 2023 with her husband and her brother, she wasn’t near death. She figured that the next time she was in Vermont, it would be to request the medication. Then she’d have to wait 15 days to receive it.
The waiting period is standard to ensure that a person has what Dr. Barnard calls “thoughtful time to contemplate the decision,” although she said most have done that long before. Some states have shortened the period or, like Oregon, have a waiver option.
That waiting period can be hard on patients, on top of being away from their health care team, home, and family. Blanke said he has seen as many as 25 relatives attend the death of an Oregon resident, but out-of-staters usually bring only one person. And while finding a place to die can be a problem for Oregonians who are in care homes or hospitals that prohibit aid in dying, it’s especially challenging for nonresidents.
When Oregon lifted its residency requirement, Dr. Blanke advertised on Craigslist and used the results to compile a list of short-term accommodations, including Airbnbs, willing to allow patients to die there. Nonprofits in states with aid-in-dying laws also maintain such lists, Ms. Sandeen said.
Ms. Milano hasn’t gotten to the point where she needs to find a place to take the meds and end her life. In fact, because she had a relatively healthy year after her first trip to Vermont, she let her 6-month approval period lapse.
In June, though, she headed back to open another 6-month window. This time, she went with a girlfriend who has a camper van. They drove 6 hours to cross the state border, stopping at a playground and gift shop before sitting in a parking lot where Ms. Milano had a Zoom appointment with her doctors rather than driving 3 more hours to Burlington to meet in person.
“I don’t know if they do GPS tracking or IP address kind of stuff, but I would have been afraid not to be honest,” she said.
That’s not all that scares her. She worries she’ll be too sick to return to Vermont when she is ready to die. And, even if she can get there, she wonders whether she’ll have the courage to take the medication. About one-third of people approved for assisted death don’t follow through, Dr. Blanke said. For them, it’s often enough to know they have the meds — the control — to end their lives when they want.
Ms. Milano said she is grateful she has that power now while she’s still healthy enough to travel and enjoy life. “I just wish more people had the option,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Breast Cancer Index Predicts Benefit of Ovarian Function Suppression in Premenopausal Women
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Radiation Therapy Underused After Nipple-Sparing Mastectomy
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
BRCA Mutations in Men: Important but Often Overlooked
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
One in Ten Chronic Pain Patients May Develop Opioid Use Disorder
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FIT Screening Cuts Colorectal Cancer Mortality by One Third
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.