User login
Add-on antipsychotic beats switching meds in older adults with resistant depression
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAGP 2023
Antipsychotic cuts Alzheimer’s-related agitation
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
AT AAGP 2023
Cognitive remediation training reduces aggression in schizophrenia
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.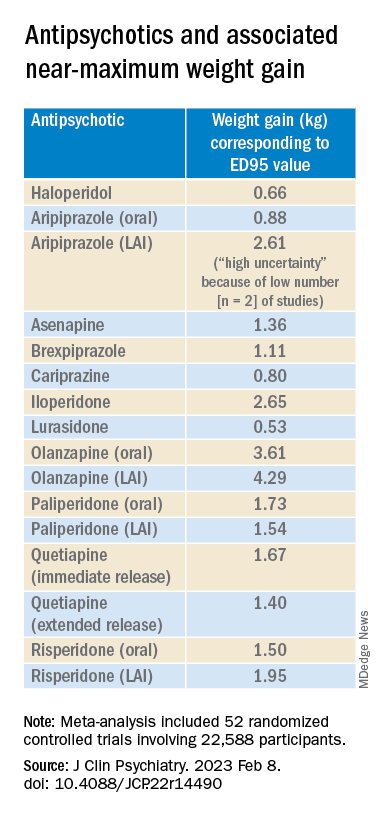
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Encephalitis linked to psychosis, suicidal thoughts
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROPSYCHIATRY AND CLINICAL NEUROSCIENCES
Thyroid hormones predict psychotic depression in MDD patients
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
Visual hallucinations: Differentiating psychiatric and neurologic causes
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.
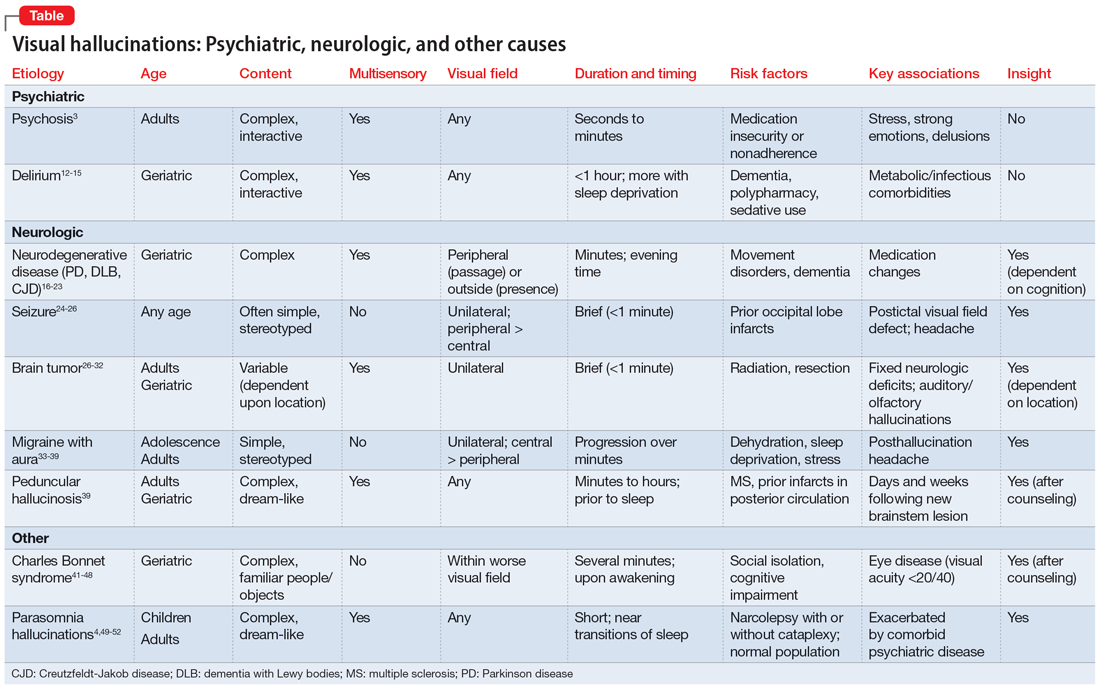
Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
Prodromal symptoms of schizophrenia: What to look for
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Immunodeficiencies tied to psychiatric disorders in offspring
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.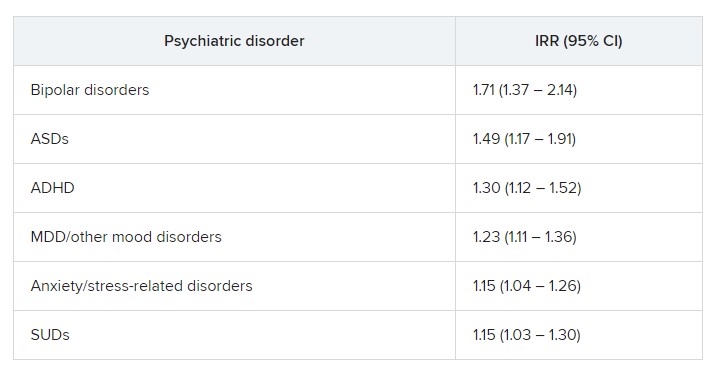
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
No benefit of long-acting antipsychotics in schizophrenia?
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY