User login
OSA tool uncovers risks of postoperative complications
High scores on the symptomless multivariable apnea prediction index (sMVAP) showed a strong correlation with increased risk for postsurgery complications, according to a study approved by the University of Pennsylvania, Philadelphia.
This validation helps assert the benefits of using the sMVAP as a tool to screen for obstructive sleep apnea (OSA) before elective inpatient surgeries, a test that is highly underutilized but very important, wrote M. Melanie Lyons, PhD, of the Center for Sleep and Circadian Neurobiology, University of Pennsylvania, Philadelphia, and her colleagues.
“Most patients having elective surgery are not screened for obstructive sleep apnea, even though OSA is a risk factor for postoperative complications,” wrote Dr. Lyons and her colleagues. “We observe that sMVAP correlates with higher risk for OSA, hypertension, and select postoperative complications, particularly in non-bariatric groups without routine preoperative screening for OSA.”
In a retrospective study of 40,432 patients undergoing elective surgery, high sMVAP scores were strongly correlated with postoperative complications including longer hospital stays (OR = 1.83), stays in the ICU (OR = 1.44), and respiratory complications (OR = 1.85) according to the researchers (Sleep. 2017 Jan 6. doi: 10.1093/sleep/zsw081).
Researchers separated participants into 10 categories according to the type of procedure: bariatric, orthopedic, cardiac, gastrointestinal, genitourinary, neurological, otorhinolaryngology/oral-maxillofacial/ear-nose-throat, pulmonary/thoracic, spine, and vascular.
The sMVAP calculates risk factors for OSA based on gender, age and body mass index, the researchers noted.
Those in the highest sMVAP score quintile were predominantly male (58%), with average age of 61 years, and average BMI of 40.9 kg/m2 (indicating morbid obesity). These patients reported the highest prevalence of having been previously diagnosed with OSA (26%). Comparatively, those patients in the lowest sMVAP quintile reported the lowest prevalence of an OSA diagnosis prior to undergoing their surgeries (9.3%).
Among non–bariatric surgery patients, those undergoing orthopedic procedures showed the highest correlation between complications and sMVAP scores.
The orthopedic surgery category reported a higher percentage of ICU-stay compared with bariatric surgery (14.3% vs 5.4%, P less than .0001), despite 23% of the patients who underwent an orthopedic surgery reporting previous OSA, compared with 50% of those who underwent surgery in the bariatric category.
This difference in previously reported OSA, according to Dr. Lyons and her colleagues, shows another example of the need for sMVAP in non–bariatric surgery preoperative procedure as a way to catch potentially undiagnosed OSA.
“[W]ork by Penn Bariatrics suggests that it is logical that the benefits of rigorous preoperative screening and diagnosis for OSA followed by a tailored team approach toward ensuring compliance toward treatment postoperation ... may be effective in limiting the likelihood of select postoperative complications,” the researchers wrote.
With 9.3% of all patients diagnosed with OSA, and a projected 14%-47% increase in specialty surgeries, there is an urgency in implementation of sMVAP and in conducting further studies, they noted.
This test was limited by the sample population, primarily male commercial truck drivers, the researchers noted. In addition, misclassification of OSA based on weight may have occurred in up to 20% of normal weight patients. Finally, data were collected from one hospital network, so generalizability may be limited.
Dr. M. Melanie Lyons and Dr. Junxin Li, another of the study’s authors, receive grants from the National Institutes of Health. The other authors reported no relevant disclosures.
High scores on the symptomless multivariable apnea prediction index (sMVAP) showed a strong correlation with increased risk for postsurgery complications, according to a study approved by the University of Pennsylvania, Philadelphia.
This validation helps assert the benefits of using the sMVAP as a tool to screen for obstructive sleep apnea (OSA) before elective inpatient surgeries, a test that is highly underutilized but very important, wrote M. Melanie Lyons, PhD, of the Center for Sleep and Circadian Neurobiology, University of Pennsylvania, Philadelphia, and her colleagues.
“Most patients having elective surgery are not screened for obstructive sleep apnea, even though OSA is a risk factor for postoperative complications,” wrote Dr. Lyons and her colleagues. “We observe that sMVAP correlates with higher risk for OSA, hypertension, and select postoperative complications, particularly in non-bariatric groups without routine preoperative screening for OSA.”
In a retrospective study of 40,432 patients undergoing elective surgery, high sMVAP scores were strongly correlated with postoperative complications including longer hospital stays (OR = 1.83), stays in the ICU (OR = 1.44), and respiratory complications (OR = 1.85) according to the researchers (Sleep. 2017 Jan 6. doi: 10.1093/sleep/zsw081).
Researchers separated participants into 10 categories according to the type of procedure: bariatric, orthopedic, cardiac, gastrointestinal, genitourinary, neurological, otorhinolaryngology/oral-maxillofacial/ear-nose-throat, pulmonary/thoracic, spine, and vascular.
The sMVAP calculates risk factors for OSA based on gender, age and body mass index, the researchers noted.
Those in the highest sMVAP score quintile were predominantly male (58%), with average age of 61 years, and average BMI of 40.9 kg/m2 (indicating morbid obesity). These patients reported the highest prevalence of having been previously diagnosed with OSA (26%). Comparatively, those patients in the lowest sMVAP quintile reported the lowest prevalence of an OSA diagnosis prior to undergoing their surgeries (9.3%).
Among non–bariatric surgery patients, those undergoing orthopedic procedures showed the highest correlation between complications and sMVAP scores.
The orthopedic surgery category reported a higher percentage of ICU-stay compared with bariatric surgery (14.3% vs 5.4%, P less than .0001), despite 23% of the patients who underwent an orthopedic surgery reporting previous OSA, compared with 50% of those who underwent surgery in the bariatric category.
This difference in previously reported OSA, according to Dr. Lyons and her colleagues, shows another example of the need for sMVAP in non–bariatric surgery preoperative procedure as a way to catch potentially undiagnosed OSA.
“[W]ork by Penn Bariatrics suggests that it is logical that the benefits of rigorous preoperative screening and diagnosis for OSA followed by a tailored team approach toward ensuring compliance toward treatment postoperation ... may be effective in limiting the likelihood of select postoperative complications,” the researchers wrote.
With 9.3% of all patients diagnosed with OSA, and a projected 14%-47% increase in specialty surgeries, there is an urgency in implementation of sMVAP and in conducting further studies, they noted.
This test was limited by the sample population, primarily male commercial truck drivers, the researchers noted. In addition, misclassification of OSA based on weight may have occurred in up to 20% of normal weight patients. Finally, data were collected from one hospital network, so generalizability may be limited.
Dr. M. Melanie Lyons and Dr. Junxin Li, another of the study’s authors, receive grants from the National Institutes of Health. The other authors reported no relevant disclosures.
High scores on the symptomless multivariable apnea prediction index (sMVAP) showed a strong correlation with increased risk for postsurgery complications, according to a study approved by the University of Pennsylvania, Philadelphia.
This validation helps assert the benefits of using the sMVAP as a tool to screen for obstructive sleep apnea (OSA) before elective inpatient surgeries, a test that is highly underutilized but very important, wrote M. Melanie Lyons, PhD, of the Center for Sleep and Circadian Neurobiology, University of Pennsylvania, Philadelphia, and her colleagues.
“Most patients having elective surgery are not screened for obstructive sleep apnea, even though OSA is a risk factor for postoperative complications,” wrote Dr. Lyons and her colleagues. “We observe that sMVAP correlates with higher risk for OSA, hypertension, and select postoperative complications, particularly in non-bariatric groups without routine preoperative screening for OSA.”
In a retrospective study of 40,432 patients undergoing elective surgery, high sMVAP scores were strongly correlated with postoperative complications including longer hospital stays (OR = 1.83), stays in the ICU (OR = 1.44), and respiratory complications (OR = 1.85) according to the researchers (Sleep. 2017 Jan 6. doi: 10.1093/sleep/zsw081).
Researchers separated participants into 10 categories according to the type of procedure: bariatric, orthopedic, cardiac, gastrointestinal, genitourinary, neurological, otorhinolaryngology/oral-maxillofacial/ear-nose-throat, pulmonary/thoracic, spine, and vascular.
The sMVAP calculates risk factors for OSA based on gender, age and body mass index, the researchers noted.
Those in the highest sMVAP score quintile were predominantly male (58%), with average age of 61 years, and average BMI of 40.9 kg/m2 (indicating morbid obesity). These patients reported the highest prevalence of having been previously diagnosed with OSA (26%). Comparatively, those patients in the lowest sMVAP quintile reported the lowest prevalence of an OSA diagnosis prior to undergoing their surgeries (9.3%).
Among non–bariatric surgery patients, those undergoing orthopedic procedures showed the highest correlation between complications and sMVAP scores.
The orthopedic surgery category reported a higher percentage of ICU-stay compared with bariatric surgery (14.3% vs 5.4%, P less than .0001), despite 23% of the patients who underwent an orthopedic surgery reporting previous OSA, compared with 50% of those who underwent surgery in the bariatric category.
This difference in previously reported OSA, according to Dr. Lyons and her colleagues, shows another example of the need for sMVAP in non–bariatric surgery preoperative procedure as a way to catch potentially undiagnosed OSA.
“[W]ork by Penn Bariatrics suggests that it is logical that the benefits of rigorous preoperative screening and diagnosis for OSA followed by a tailored team approach toward ensuring compliance toward treatment postoperation ... may be effective in limiting the likelihood of select postoperative complications,” the researchers wrote.
With 9.3% of all patients diagnosed with OSA, and a projected 14%-47% increase in specialty surgeries, there is an urgency in implementation of sMVAP and in conducting further studies, they noted.
This test was limited by the sample population, primarily male commercial truck drivers, the researchers noted. In addition, misclassification of OSA based on weight may have occurred in up to 20% of normal weight patients. Finally, data were collected from one hospital network, so generalizability may be limited.
Dr. M. Melanie Lyons and Dr. Junxin Li, another of the study’s authors, receive grants from the National Institutes of Health. The other authors reported no relevant disclosures.
FROM SLEEP
Key clinical point:
Major finding: Patients with high sMVAP scores had increased odds of complications, including extended length of stay (OR = 1.83), ICU stay (OR = 1.44), and respiratory complications (OR = 1.85).
Data source: Retrospective study of 40,432 elective surgery patient records collected from the Hospital of University of Pennsylvania, Pennsylvania Hospital, and Penn Presbyterian Medical Center between July 1, 2011, and June 30, 2014.
Disclosures: Dr. M. Melanie Lyons and Dr. Junxin Li receive grants from the National Institutes of Health. Other authors reported no relevant financial disclosures.
Insomnia: Getting to the cause, facilitating relief
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
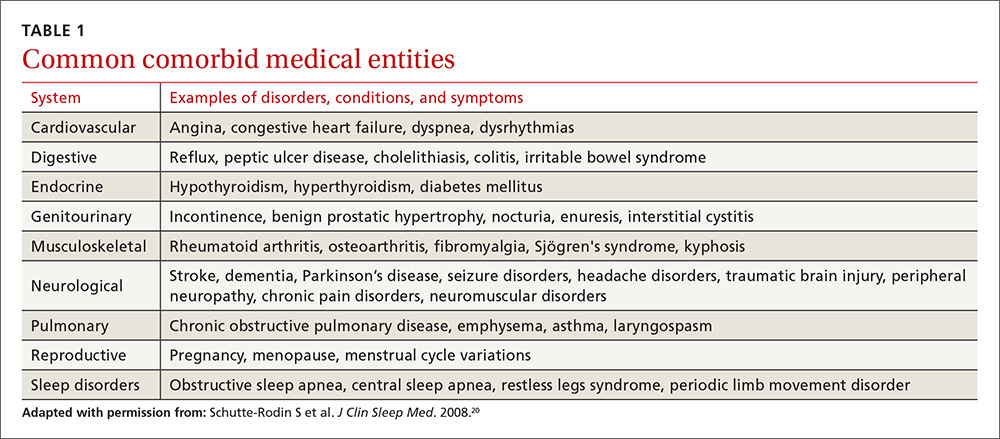
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
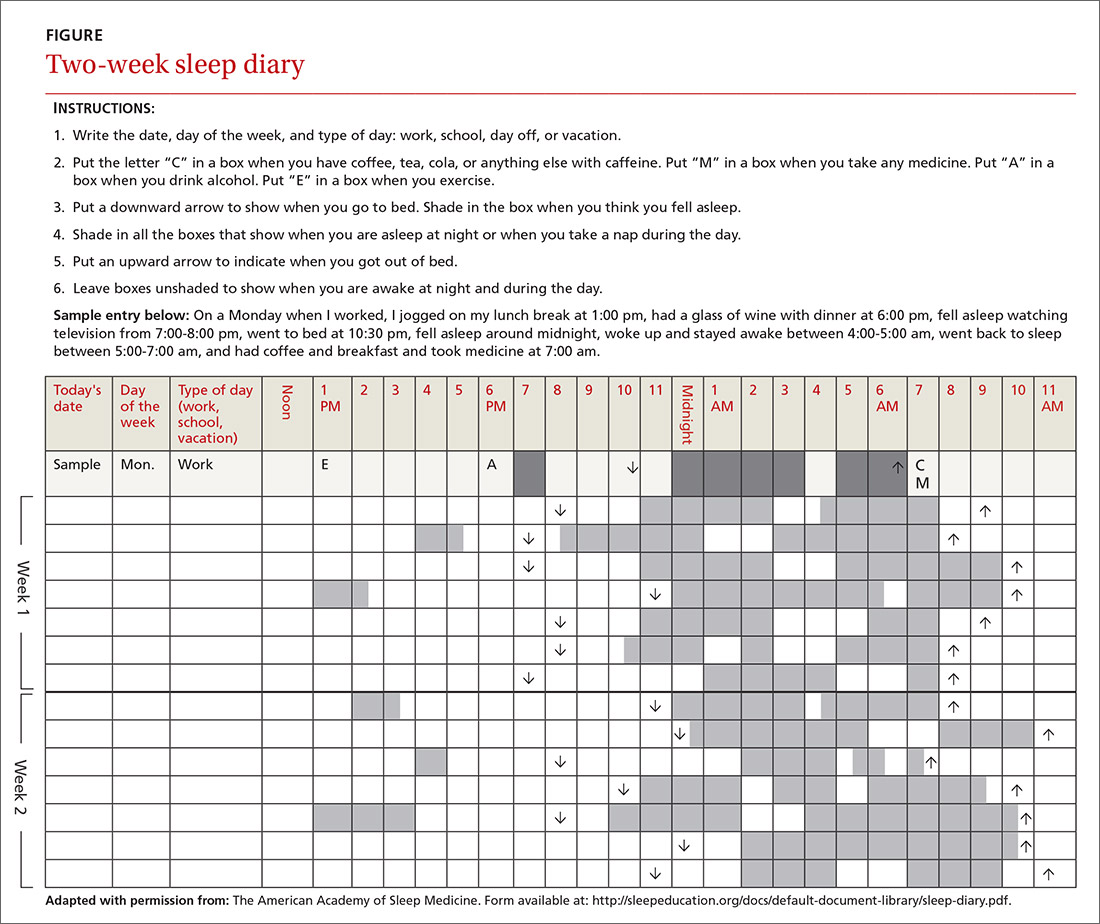
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
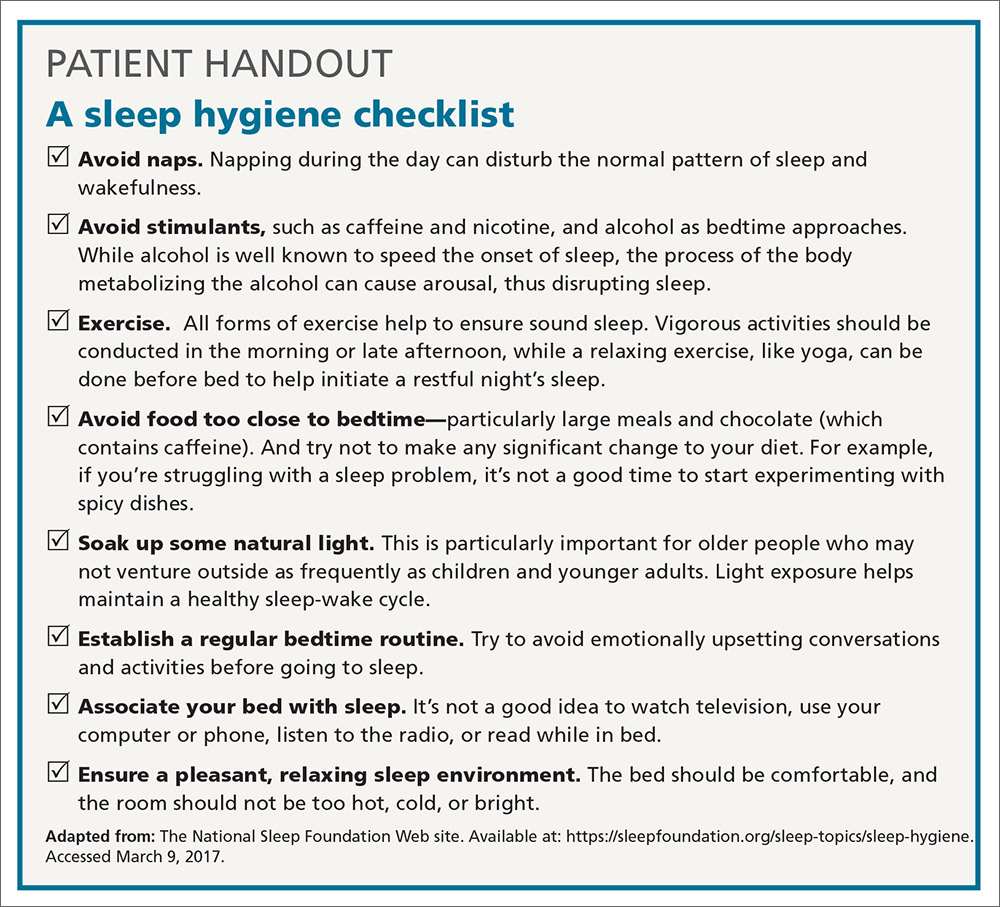
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
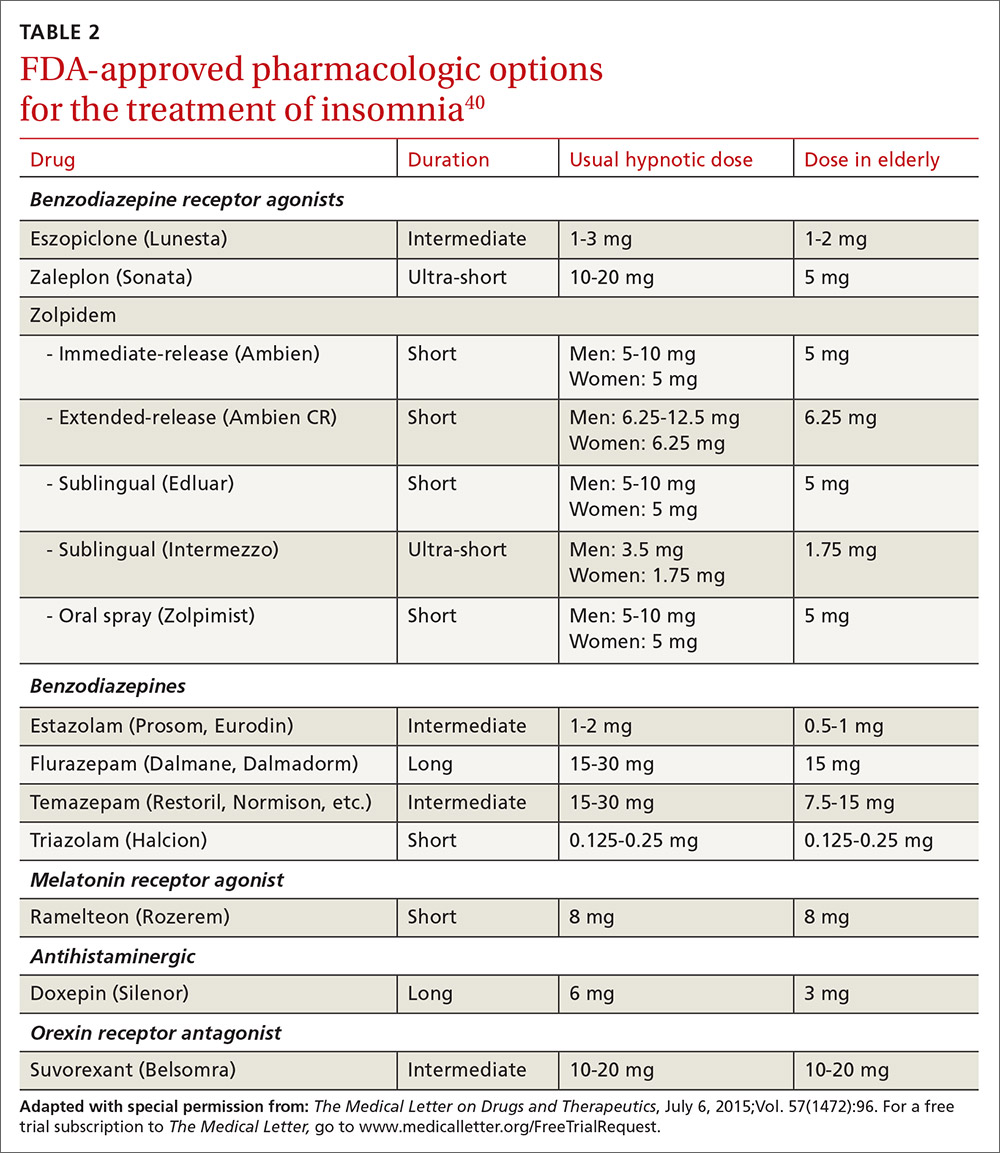
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; adam.j.sorscher@hitchcock.org.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; adam.j.sorscher@hitchcock.org.
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; adam.j.sorscher@hitchcock.org.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
PRACTICE RECOMMENDATIONS
› Recommend that patients try cognitive behavioral therapy for insomnia (CBT-I), as it is highly effective and some of its techniques can be employed in a busy family medicine clinic with little time commitment. B
› Consider pharmacotherapy for patients with chronic insomnia that persists despite CBT-I, as long as they are properly screened and followed regularly. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A veteran who is suicidal while sleeping
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
Pitolisant Reduces Cataplexy Attacks in Narcolepsy
Pitolisant, a histamine H3 receptor inverse agonist, is well tolerated and effectively reduces cataplexy, according to data published in the March issue of Lancet Neurology. If the drug’s effect is confirmed in long-term studies, pitolisant could become a first-line therapy for cataplexy in patients with narcolepsy, who currently have few treatment options, according to the authors.
Two types of drugs are used to treat cataplexy. Antidepressants are used off-label and not well supported by evidence. Sodium oxybate is effective, but may cause serious adverse events.
Zoltán Szakács, MD, a doctor at the State Health Center in Budapest, and colleagues enrolled patients with narcolepsy from 16 sleep centers in nine countries into a randomized, double-blind, placebo-controlled trial. Eligible participants were age 18, had narcolepsy with cataplexy, had at least three cataplexies per week, and had excessive daytime sleepiness. Patients were randomized to pitolisant or placebo once per day. During a three-week flexible dosing period, patients randomized to pitolisant received 5 mg for the first week, 10 mg for the second week, and for the third week, a dose of between 5 mg and 20 mg, on the basis of safety and efficacy. A four-week period of stable dosing followed. Finally, patients underwent a one-week withdrawal period.
In all, 54 patients received pitolisant, and 51 received placebo. During the stable dosing period, the weekly cataplexy rate was decreased by 75% in patients who received pitolisant and by 38% among controls. The effect was significant regardless of the patient’s stable dose of pitolisant. Use of concomitant anticataplectic treatment before study initiation did not affect the results. The drug also improved scores on the Epworth Sleepiness Scale and maintenance of wakefulness test.
The pitolisant group had a significantly higher rate of treatment-related adverse events than did the placebo group (28% vs 12%). The researchers recorded no serious adverse events, but observed one case of severe nausea in the pitolisant group.
The study’s main limitation is that the diagnosis of narcolepsy was based on nonspecific criteria, said Christian R. Baumann, Professor of Neurology at University Hospital Zurich, in an accompanying editorial. “The fact that reported mean sleep latencies in the cohort of Szakács and colleagues (4.2–4.7 min) were higher than those reported in other cohorts, including ours (2.6–2.9 min), leaves some uncertainty about patient inclusion,” he added.
Nevertheless, the study may contribute to the regulatory approval of pitolisant. “This is good news for clinicians, since we need more treatment options to better tailor individualized therapy for patients with narcolepsy,” said Dr. Baumann.
—Erik Greb
Suggested Reading
Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200-207.
Baumann CR. Wide implications of a trial on pitolisant for cataplexy. Lancet Neurol. 2017;16(3):173-174.
Pitolisant, a histamine H3 receptor inverse agonist, is well tolerated and effectively reduces cataplexy, according to data published in the March issue of Lancet Neurology. If the drug’s effect is confirmed in long-term studies, pitolisant could become a first-line therapy for cataplexy in patients with narcolepsy, who currently have few treatment options, according to the authors.
Two types of drugs are used to treat cataplexy. Antidepressants are used off-label and not well supported by evidence. Sodium oxybate is effective, but may cause serious adverse events.
Zoltán Szakács, MD, a doctor at the State Health Center in Budapest, and colleagues enrolled patients with narcolepsy from 16 sleep centers in nine countries into a randomized, double-blind, placebo-controlled trial. Eligible participants were age 18, had narcolepsy with cataplexy, had at least three cataplexies per week, and had excessive daytime sleepiness. Patients were randomized to pitolisant or placebo once per day. During a three-week flexible dosing period, patients randomized to pitolisant received 5 mg for the first week, 10 mg for the second week, and for the third week, a dose of between 5 mg and 20 mg, on the basis of safety and efficacy. A four-week period of stable dosing followed. Finally, patients underwent a one-week withdrawal period.
In all, 54 patients received pitolisant, and 51 received placebo. During the stable dosing period, the weekly cataplexy rate was decreased by 75% in patients who received pitolisant and by 38% among controls. The effect was significant regardless of the patient’s stable dose of pitolisant. Use of concomitant anticataplectic treatment before study initiation did not affect the results. The drug also improved scores on the Epworth Sleepiness Scale and maintenance of wakefulness test.
The pitolisant group had a significantly higher rate of treatment-related adverse events than did the placebo group (28% vs 12%). The researchers recorded no serious adverse events, but observed one case of severe nausea in the pitolisant group.
The study’s main limitation is that the diagnosis of narcolepsy was based on nonspecific criteria, said Christian R. Baumann, Professor of Neurology at University Hospital Zurich, in an accompanying editorial. “The fact that reported mean sleep latencies in the cohort of Szakács and colleagues (4.2–4.7 min) were higher than those reported in other cohorts, including ours (2.6–2.9 min), leaves some uncertainty about patient inclusion,” he added.
Nevertheless, the study may contribute to the regulatory approval of pitolisant. “This is good news for clinicians, since we need more treatment options to better tailor individualized therapy for patients with narcolepsy,” said Dr. Baumann.
—Erik Greb
Suggested Reading
Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200-207.
Baumann CR. Wide implications of a trial on pitolisant for cataplexy. Lancet Neurol. 2017;16(3):173-174.
Pitolisant, a histamine H3 receptor inverse agonist, is well tolerated and effectively reduces cataplexy, according to data published in the March issue of Lancet Neurology. If the drug’s effect is confirmed in long-term studies, pitolisant could become a first-line therapy for cataplexy in patients with narcolepsy, who currently have few treatment options, according to the authors.
Two types of drugs are used to treat cataplexy. Antidepressants are used off-label and not well supported by evidence. Sodium oxybate is effective, but may cause serious adverse events.
Zoltán Szakács, MD, a doctor at the State Health Center in Budapest, and colleagues enrolled patients with narcolepsy from 16 sleep centers in nine countries into a randomized, double-blind, placebo-controlled trial. Eligible participants were age 18, had narcolepsy with cataplexy, had at least three cataplexies per week, and had excessive daytime sleepiness. Patients were randomized to pitolisant or placebo once per day. During a three-week flexible dosing period, patients randomized to pitolisant received 5 mg for the first week, 10 mg for the second week, and for the third week, a dose of between 5 mg and 20 mg, on the basis of safety and efficacy. A four-week period of stable dosing followed. Finally, patients underwent a one-week withdrawal period.
In all, 54 patients received pitolisant, and 51 received placebo. During the stable dosing period, the weekly cataplexy rate was decreased by 75% in patients who received pitolisant and by 38% among controls. The effect was significant regardless of the patient’s stable dose of pitolisant. Use of concomitant anticataplectic treatment before study initiation did not affect the results. The drug also improved scores on the Epworth Sleepiness Scale and maintenance of wakefulness test.
The pitolisant group had a significantly higher rate of treatment-related adverse events than did the placebo group (28% vs 12%). The researchers recorded no serious adverse events, but observed one case of severe nausea in the pitolisant group.
The study’s main limitation is that the diagnosis of narcolepsy was based on nonspecific criteria, said Christian R. Baumann, Professor of Neurology at University Hospital Zurich, in an accompanying editorial. “The fact that reported mean sleep latencies in the cohort of Szakács and colleagues (4.2–4.7 min) were higher than those reported in other cohorts, including ours (2.6–2.9 min), leaves some uncertainty about patient inclusion,” he added.
Nevertheless, the study may contribute to the regulatory approval of pitolisant. “This is good news for clinicians, since we need more treatment options to better tailor individualized therapy for patients with narcolepsy,” said Dr. Baumann.
—Erik Greb
Suggested Reading
Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200-207.
Baumann CR. Wide implications of a trial on pitolisant for cataplexy. Lancet Neurol. 2017;16(3):173-174.
Guideline Provides Recommendations for Pharmacologic Treatment of Chronic Insomnia
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Light therapy eases Parkinson’s-related sleep disturbances
Light therapy significantly reduced excessive daytime sleepiness, improved sleep quality, decreased overnight awakenings, shortened sleep latency, enhanced daytime alertness and activity level, and improved motor symptoms in patients with Parkinson’s disease, according to a report published online Feb. 20 in JAMA Neurology.
The noninvasive, nonpharmacologic treatment was well tolerated, and patient adherence was excellent in a small, multicenter, randomized controlled trial. Light therapy is widely available as a treatment for several sleep and psychiatric disorders and is “relatively easy to prescribe and incorporate into a clinical practice,” said Aleksandar Videnovic, MD, of the department of neurology at Massachusetts General Hospital and the division of sleep medicine at Harvard Medical School, both in Boston, and his associates.
To assess the safety and efficacy of light therapy as a novel treatment for PD, they studied 31 adults (age range, 32-77 years) who had a mean disease duration of 6 years. These study participants were randomly assigned to use 1 hour of exposure to 10,000 lux of bright light (16 patients in the intervention group) or 1 hour of exposure to less than 300 lux of dim red light (15 control subjects) every morning and every afternoon for 2 weeks.
The study participants – 13 men and 18 women – also wore actigraphy monitors all day and all night, completed daily sleep diaries, and noted daytime sleepiness in a log every 2 hours, 3 days per week.
Bright light significantly improved excessive daytime sleepiness as measured by the Epworth Sleepiness Scale and self-reported alertness during wake time, as well as several sleep metrics such as overall sleep quality, overnight awakenings, and ease of falling asleep. All the patients in the intervention group reported being more refreshed in the mornings during the study period, as compared with baseline.
Light therapy also improved overall PD severity as measured by the Unified Parkinson’s Disease Rating Scale, particularly in scores related to activities of daily living and motor symptoms. Moreover, this effect persisted during the 2-week washout period after treatment was discontinued, Dr. Videnovic and his associates said (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5192).
The treatment was well tolerated. In the intervention group, one patient reported headache and another sleepiness, and in the control group one patient reported itchy eyes. The effects resolved spontaneously, and neither lead to treatment withdrawal.
“Based on these results, the next logical step is to optimize various parameters of light therapy (e.g., intensity, duration, and wavelength) not only for impaired sleep and alertness but also for other motor and nonmotor manifestation of PD,” the investigators wrote.
A major limitation of this study was that exposure to ambient light throughout the day was not measured. Some people in the control group received as much or even more light exposure than those assigned to bright-light therapy. “Future studies may be more strict in controlling such exposures,” Dr. Videnovic and his associates said.
This study was supported by the National Parkinson Foundation and the National Institutes of Health. Dr. Videnovic reported having no relevant financial disclosures. One of his associates reported ties to Merck, Phillips, Eisai, and Teva.
The study by Dr. Videnovic and his associates is important because it introduces a new concept into the much-studied phenomenon of sleep disturbances in Parkinson’s disease.
The authors demonstrated that chronobiological interventions can be used therapeutically in PD. Accounting for circadian physiology also sets a new standard for future studies of sleep, nighttime wakefulness, and daytime function not only in PD but, it is hoped, in other diseases as well.
Birgit Högl, MD, is with the department of neurology at the Medical University of Innsbruck (Austria). She reported receiving honoraria as a speaker, advisory board member, or consultant from UCB, Otsuka, Lundbeck, Lilly, Axovant, AbbVie, Mundipharma, Benevolent Bio, and Janssen Cilag, and travel support from Habel Medizintechnik and Vivisol. Dr. Högl made these remarks in an editorial (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5519) accompanying the report by Dr. Videnovic and his colleagues.
The study by Dr. Videnovic and his associates is important because it introduces a new concept into the much-studied phenomenon of sleep disturbances in Parkinson’s disease.
The authors demonstrated that chronobiological interventions can be used therapeutically in PD. Accounting for circadian physiology also sets a new standard for future studies of sleep, nighttime wakefulness, and daytime function not only in PD but, it is hoped, in other diseases as well.
Birgit Högl, MD, is with the department of neurology at the Medical University of Innsbruck (Austria). She reported receiving honoraria as a speaker, advisory board member, or consultant from UCB, Otsuka, Lundbeck, Lilly, Axovant, AbbVie, Mundipharma, Benevolent Bio, and Janssen Cilag, and travel support from Habel Medizintechnik and Vivisol. Dr. Högl made these remarks in an editorial (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5519) accompanying the report by Dr. Videnovic and his colleagues.
The study by Dr. Videnovic and his associates is important because it introduces a new concept into the much-studied phenomenon of sleep disturbances in Parkinson’s disease.
The authors demonstrated that chronobiological interventions can be used therapeutically in PD. Accounting for circadian physiology also sets a new standard for future studies of sleep, nighttime wakefulness, and daytime function not only in PD but, it is hoped, in other diseases as well.
Birgit Högl, MD, is with the department of neurology at the Medical University of Innsbruck (Austria). She reported receiving honoraria as a speaker, advisory board member, or consultant from UCB, Otsuka, Lundbeck, Lilly, Axovant, AbbVie, Mundipharma, Benevolent Bio, and Janssen Cilag, and travel support from Habel Medizintechnik and Vivisol. Dr. Högl made these remarks in an editorial (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5519) accompanying the report by Dr. Videnovic and his colleagues.
Light therapy significantly reduced excessive daytime sleepiness, improved sleep quality, decreased overnight awakenings, shortened sleep latency, enhanced daytime alertness and activity level, and improved motor symptoms in patients with Parkinson’s disease, according to a report published online Feb. 20 in JAMA Neurology.
The noninvasive, nonpharmacologic treatment was well tolerated, and patient adherence was excellent in a small, multicenter, randomized controlled trial. Light therapy is widely available as a treatment for several sleep and psychiatric disorders and is “relatively easy to prescribe and incorporate into a clinical practice,” said Aleksandar Videnovic, MD, of the department of neurology at Massachusetts General Hospital and the division of sleep medicine at Harvard Medical School, both in Boston, and his associates.
To assess the safety and efficacy of light therapy as a novel treatment for PD, they studied 31 adults (age range, 32-77 years) who had a mean disease duration of 6 years. These study participants were randomly assigned to use 1 hour of exposure to 10,000 lux of bright light (16 patients in the intervention group) or 1 hour of exposure to less than 300 lux of dim red light (15 control subjects) every morning and every afternoon for 2 weeks.
The study participants – 13 men and 18 women – also wore actigraphy monitors all day and all night, completed daily sleep diaries, and noted daytime sleepiness in a log every 2 hours, 3 days per week.
Bright light significantly improved excessive daytime sleepiness as measured by the Epworth Sleepiness Scale and self-reported alertness during wake time, as well as several sleep metrics such as overall sleep quality, overnight awakenings, and ease of falling asleep. All the patients in the intervention group reported being more refreshed in the mornings during the study period, as compared with baseline.
Light therapy also improved overall PD severity as measured by the Unified Parkinson’s Disease Rating Scale, particularly in scores related to activities of daily living and motor symptoms. Moreover, this effect persisted during the 2-week washout period after treatment was discontinued, Dr. Videnovic and his associates said (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5192).
The treatment was well tolerated. In the intervention group, one patient reported headache and another sleepiness, and in the control group one patient reported itchy eyes. The effects resolved spontaneously, and neither lead to treatment withdrawal.
“Based on these results, the next logical step is to optimize various parameters of light therapy (e.g., intensity, duration, and wavelength) not only for impaired sleep and alertness but also for other motor and nonmotor manifestation of PD,” the investigators wrote.
A major limitation of this study was that exposure to ambient light throughout the day was not measured. Some people in the control group received as much or even more light exposure than those assigned to bright-light therapy. “Future studies may be more strict in controlling such exposures,” Dr. Videnovic and his associates said.
This study was supported by the National Parkinson Foundation and the National Institutes of Health. Dr. Videnovic reported having no relevant financial disclosures. One of his associates reported ties to Merck, Phillips, Eisai, and Teva.
Light therapy significantly reduced excessive daytime sleepiness, improved sleep quality, decreased overnight awakenings, shortened sleep latency, enhanced daytime alertness and activity level, and improved motor symptoms in patients with Parkinson’s disease, according to a report published online Feb. 20 in JAMA Neurology.
The noninvasive, nonpharmacologic treatment was well tolerated, and patient adherence was excellent in a small, multicenter, randomized controlled trial. Light therapy is widely available as a treatment for several sleep and psychiatric disorders and is “relatively easy to prescribe and incorporate into a clinical practice,” said Aleksandar Videnovic, MD, of the department of neurology at Massachusetts General Hospital and the division of sleep medicine at Harvard Medical School, both in Boston, and his associates.
To assess the safety and efficacy of light therapy as a novel treatment for PD, they studied 31 adults (age range, 32-77 years) who had a mean disease duration of 6 years. These study participants were randomly assigned to use 1 hour of exposure to 10,000 lux of bright light (16 patients in the intervention group) or 1 hour of exposure to less than 300 lux of dim red light (15 control subjects) every morning and every afternoon for 2 weeks.
The study participants – 13 men and 18 women – also wore actigraphy monitors all day and all night, completed daily sleep diaries, and noted daytime sleepiness in a log every 2 hours, 3 days per week.
Bright light significantly improved excessive daytime sleepiness as measured by the Epworth Sleepiness Scale and self-reported alertness during wake time, as well as several sleep metrics such as overall sleep quality, overnight awakenings, and ease of falling asleep. All the patients in the intervention group reported being more refreshed in the mornings during the study period, as compared with baseline.
Light therapy also improved overall PD severity as measured by the Unified Parkinson’s Disease Rating Scale, particularly in scores related to activities of daily living and motor symptoms. Moreover, this effect persisted during the 2-week washout period after treatment was discontinued, Dr. Videnovic and his associates said (JAMA Neurol. 2017 Feb 20. doi: 10.1001/jamaneurol.2016.5192).
The treatment was well tolerated. In the intervention group, one patient reported headache and another sleepiness, and in the control group one patient reported itchy eyes. The effects resolved spontaneously, and neither lead to treatment withdrawal.
“Based on these results, the next logical step is to optimize various parameters of light therapy (e.g., intensity, duration, and wavelength) not only for impaired sleep and alertness but also for other motor and nonmotor manifestation of PD,” the investigators wrote.
A major limitation of this study was that exposure to ambient light throughout the day was not measured. Some people in the control group received as much or even more light exposure than those assigned to bright-light therapy. “Future studies may be more strict in controlling such exposures,” Dr. Videnovic and his associates said.
This study was supported by the National Parkinson Foundation and the National Institutes of Health. Dr. Videnovic reported having no relevant financial disclosures. One of his associates reported ties to Merck, Phillips, Eisai, and Teva.
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: Compared with a control condition, bright light significantly improved excessive daytime sleepiness as measured by the Epworth Sleepiness Scale and self-reported alertness during wake time.
Data source: A randomized controlled trial involving 31 adults with Parkinson’s disease–related sleep disturbances.
Disclosures: This study was supported by the National Parkinson Foundation and the National Institutes of Health. Dr. Videnovic reported having no relevant financial disclosures. One of his associates reported ties to Merck, Phillips, Eisai, and Teva.
Sleep-Disordered Breathing in the Active-Duty Military Population and Its Civilian Care Cost
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
Sleep-disordered breathing (SDB) is a continuum of symptoms that range from primary snoring with upper airway resistance to frank obstruction seen in obstructive sleep apnea (OSA). This disease spectrum has been reported to affect 10% to 17% of men and 3% to 9% of women in the general population.1 The specific incidence of OSA has been estimated to be about 2% to 4% of the general adult population.2,3 Sleep-disordered breathing often leads to poor sleep quality, which has been associated with many medical comorbidities, including vascular disease, hypertension, major cardiac events, cardiomyopathies, impaired concentration, reduced psychomotor vigilance and cognition, and daytime somnolence.1,2,4-6 Furthermore, there is evidence that the prevalence of SDB continues to grow among the general population.1 However, the prevalence of SDB in various populations (eg, pediatric vs adult, varying body mass index, country of origin) varies widely due to the multifactorial nature of the risk factors and the difficulty in diagnosing SDB.
Some of the more intuitive medical sequelae of SDB are daytime somnolence and subsequent impaired concentration for those with disrupted sleep patterns. Medical literature has paid specific attention to cohorts of personnel who may be at heightened risk from impaired concentration or inability to focus. These populations include but are not limited to sleep-deprived resident physicians, firefighters, truck drivers, and heavy-machine operators.7,8
Military service members represent a distinct cohort that often is relied on to maintain vigilance even in austere environments. Concentration is paramount in order to perform combat operations or tasks that involve operating heavy machinery, such as nuclear submarines, aircraft, or tanks. Given the myriad of unique operational demands on service members, SDB can have detrimental consequences on an individual’s health and his or her military readiness and training. Ultimately, SDB may degrade a unit’s effectiveness and perhaps the country’s military capability.
Active-duty military service members seem to be more susceptible to clinically relevant sleep conditions. In the military, causes of disruptions in normal sleep patterns are multifactorial. Medical literature focuses on circadian disruptions due to shift work and frequent travel, frequent alternating use of caffeine and sedatives, exposure to combat/trauma, and chronic sleep deprivation.9-11 Studies have been published that focus on service members who have returned from combat deployment.10,12,13 However, these studies do not explore the overall burden of disease, and there are no specific data to suggest the prevalence, annual incidence, or associated costs.
To quantify this disease burden in the military, this study focused on the subset of sleep disorders that impact respiration during sleep and determined the prevalence and annual incidence for the entire active-duty population. Additionally, the authors fill a void in the literature by determining the financial burden of SDB on civilian care expenditures.
Methods
This study was a retrospective review of administrative military health care data spanning fiscal years (FYs) 2009 to 2013 (October 1, 2008 to September 30, 2013). The study protocol was approved by the Naval Medical Center Portsmouth Institutional Review Board, and approval was given to waive informed consent. The Health Analysis Department at the Navy and Marine Corps Public Health Center (NMCPHC) obtained and analyzed data from the Military Health System (MHS) Management Analysis and Reporting Tool (M2). The M2 system is an ad hoc query tool used for viewing population, clinical, and financial MHS data, including care received within military treatment facilities (MTFs) and care purchased through TRICARE at civilian facilities. Both inpatient and outpatient health care records were included.
The population included all active-duty service members and guard/reserve members on active duty within all military services, including air force, army, coast guard, and navy branches, between FY 2009 and FY 2013. The authors identified service members with SDB as those with at least 1 ICD-9 diagnosis code related to SDB: obstructive sleep apnea (327.23); sleep-related hypoventilation/hypoxemia (327.26); and other organic sleep disorder (327.80).
Due to the transient nature of the military population, a monthly average over the 5 years of the study determined the overall number of service members eligible for care (1,717,227 service members).
Data Analysis
Prevalence of diagnosed SDB per FY was calculated as the number of service members who received at least 1 SDB diagnostic code between October 1, 2008 and September 30, 2013, over the average total active-duty population. Incidence per year was calculated as the number of new cases per FY, using 2009 as the baseline. Data were stratified by demographic and enrollment information for diagnosed service members and analyzed using SAS 9.4 (Cary, NC) software.
Direct costs associated with SDB treatment fall into 2 categories for service members: (1) care delivered by civilian providers, calculated based on the amount TRICARE paid for the service, using insurance claim data; and (2) care received at MTFs by military providers. Costs for care at MTFs cannot be calculated, as the total cost amount for a single record is not directly attributed to SDB diagnosis.
Results
A total of 197,183 service members were diagnosed with SDB from FY 2009 to FY 2013. Both the annual incidence and prevalence of SDB for the active-duty military population showed upward trends for each of the years evaluated (Figure 1).
Notably, 72% of service members seen for SDB ranged in age from 25 to 44 years (Table).
Discussion
This study shows that the prevalence and incidence of SDB in the active-duty population are less than those reported for the civilian populace as a whole but are still greater than expected for an otherwise healthy and young population. Furthermore, the burden of disease and the cost to diagnose and treat have steadily increased for each of the past 5 fiscal years that were assessed.
The data show an upward trend in the incidence and prevalence of SDB in the military from FY 2009 to FY 2013 for reasons that are not clear but likely with many confounding contributions. As the spectrum of SDB has become better defined and the detrimental sequelae are better understood, it is likely that both service members and health care providers are more aware of the symptoms and more importantly, the potential for interventions that improve quality of life. It is also important to note that the U.S. military is a very transient organization with a nearly constant turnover between new enlistees/officers and those leaving the service or retiring after 20 years of service. Thus, despite an annual incidence of nearly 3% throughout the years evaluated, the annual increase in prevalence is not necessarily commensurate.
The FY 2013 prevalence (4.2%) and civilian care costs ($98,259,519) present traditional indications of the disease burden. Both metrics represent a sizable and increasing disease burden for the military. It is also important to note that these costs reflect only the short-term expenses for initial diagnosis and therapy. These costs in no way reflect the care for the long-term medical sequelae that have been recently linked to uncontrolled SDB/OSA, such as heart and vascular diseases, hypertension, and increased stroke risk. Additional costs will continue to grow.
Perhaps the most validated predictive factor for diagnosis of SDB or OSA is body habitus as measured by body mass index (BMI). In particular, nearly 60% to 90% of patients with OSA are obese.2 Weight gain seems to increase the OSA severity, whereas losing weight decreases it.14-16 Although the U.S. military employs height and weight standards that preclude those with persistently overweight or obese BMIs from continued service, these standards often are not rigid, and there are overweight or even obese active-duty members. Interestingly, despite a population that essentially controls for the most predictive risk factor, the prevalence of SDB is still approximately 1 in 20 (4.9%) in FY 2013.
Given the significant burden of disease represented by the incidence, prevalence, and cost data determined in this study and the growing recognition of long-term complications from poorly controlled SDB, it has become evident that more efficacious interventions are needed. Modern treatments for SDB can be classified as surgical or nonsurgical but with no single modality fitting the need for all patients secondary to poor adherence and/or limited efficacy.17-20 However, to mitigate the impact on military readiness and taxpayer-funded health care costs, it may be appropriate to begin exploring therapeutic options beyond the current standard of care. For example, an invasive and costly onetime surgical intervention using an implantable device to stimulate the hypoglossal nerve to open a person’s airway during inspiration is being investigated in a younger, nonobese cohort of patients.21 Further research is warranted into this specific model of therapeutic intervention and others for service members.
Limitations
Limitations in this study include possible reporting errors due to improper or insufficient medical coding as well as data entry errors at the clinic that may exist within medical billing databases. Therefore, the results of this analysis may be over- or underrepresented. The increase in incidence and prevalence may not necessarily reflect an increasing number of people who have the disease. The increase could be a result of better SDB detection practices or incentives to be diagnosed with SDB (VA disability claims upon retirement). The assumption is made that procedures corresponding with SDB diagnoses are directly related to SDB, and any costs incurred from those procedures are due to SDB.
It is important to note variability between services and institutions within the DoD in the diagnosis and treatment of SDB. Specifically, some institutions use ambulatory polysomnograms, or studies done at home, and autotitration of continuous positive airway pressure, whereas others require more costly hospital-based studies and laboratory titration. Another confounder in the cost data is the number of diagnoses and treatment deferred to the network as a result of the relatively small number of sleep-trained physicians within the military.
Conclusion
As the field of sleep medicine continues to develop its literature, it is becoming clearer that the detrimental sequelae of SDB are varied and pose significant short- and long-term risks. Active-duty service members represent a subset of the population with consequences that are potentially graver than those of civilians, especially when they are expected to operate complicated machinery or to make rapid and critical decisions in battle.
The prevalence and incidence of SDB increased each year during a 5-year review and currently affects 1 in 20 service members. Furthermore, the cost of civilian care for this disease process was nearly $100 million in FY 2012 to FY 2013, suggesting a growing financial burden for taxpayers. Further research is warranted to fully appreciate the impact of SDB on both service members and the U.S. military.
Acknowledgments
The authors thank the U.S. Navy and specifically the support within the Department of Otolaryngology at the Naval Medical Center Portsmouth for the time and effort allotted for completion of this study. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the Navy and Marine Corps Public Health Center (NMCPHC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and NMCPHC.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
3. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
4. Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316.
5. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028.
6. Gilat H, Vinker S, Buda I, Soudry E, Shani M, Bachar G. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45.
7. Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med. 2008;9(2):129-136.
8. Barger LK, Rajaratnam SM, Wang W, et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med. 2015;11(3):233-240.
9. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
10. Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
11. Capaldi VF 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879-888.
12. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
13. Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230-235.
14. Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048-1054.
15. Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97(8):896-897.
16. Loube DI, Loube AA, Mitler MM. Weight loss for obstructive sleep apnea: the optimal therapy for obese patients. J Am Diet Assoc. 1994;94(11):1291-1295.
17. Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med. 2015;3(5):397-403.
18. Mysliwiec V, Capaldi VF, 2nd, Gill J, et al. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475-482.
19. Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of patients with obstructive sleep apnea. Respir Care. 2013;58(9):1467-1473.
20. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178.
21. Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep. 2015;38(5):735-744.
Sleep apnea may induce distinct form of atrial fibrillation
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
Key clinical point: Atrial fibrillation associated with sleep apnea appears to have features that should be addressed specifically for sustained rhythm control.
Major finding: AF patients with sleep apnea have more non–pulmonary vein triggers after ablation than do those without sleep apnea (41.8% vs. 11.6%).
Data source: A prospective multicenter observational study.
Disclosures: Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
USPSTF punts on sleep apnea screening
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Curb AF recurrences through risk factor modification
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS