User login
Drug-coated balloons: The future of hemodialysis access?
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
Costs associated with the management of patients with chronic kidney disease, particularly those with end-stage renal disease requiring hemodialysis, is a huge component of the Medicare budget. The maintenance of functional vascular access in these patients remains an ongoing challenge and reduction of costs related to access failure is critical to the continued funding of the program. Traditional methods of maintaining access patency such as balloon angioplasty and bare metal stents have poor long-term outcomes, and moderate improvement is seen with the use of covered stents.
Dr. Hussain reviews the current status of drug-coated balloons (DCB) in the endovascular treatment of dysfunctional hemodialysis fistulas and grafts. Safety and efficacy data from a prospective randomized multicenter trial comparing the Bard Lutonix DCB and plain old balloon angioplasty demonstrated a significant improvement in primary patency and a 30% reduction in repeat interventions with the DCB. This led to FDA approval for the Lutonix DCB in the treatment of dysfunctional or stenotic arteriovenous fistulas. Encouraged by these results, researchers conducting ongoing international randomized trials are attempting to clarify the potential expanded indications for DCB in access stenosis. Of particular interest is the ongoing 330-patient prospective, multicenter IN-PACT trial comparing Admiral DCB to balloon angioplasty in failing arteriovenous fistulas. Both the Admiral DCB and Lutonix DCB utilize paclitaxel as the antiproliferative agent. Dr. Hussain describes the increased sensitivity of venous smooth muscle cells to paclitaxel and other antiproliferative drugs when compared with arterial smooth muscle cells. This exciting finding may explain the improved outcomes in the treatment of dialysis access lesions where pathology frequently occurs at the venous anastomosis or in the venous conduit.
Although early results with the use of DCBs are promising, ongoing clinical trials and careful analysis of data and cost effectiveness are critical to optimize outcomes in treating dialysis access dysfunction. Dr. Hussain appropriately expresses cautious optimism regarding the future of hemodialysis access with this new tool available to interventionists treating these complex patients.
Larry A. Scher, MD, is a vascular surgeon at Montefiore Einstein Center for Heart and Vascular Care, New York, and an associate medical editor for Vascular Specialist.
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Drug-coated balloons show promise of being a long-sought major advance in the endovascular treatment of stenotic arteriovenous fistulae and grafts for hemodialysis access, Syed M. Hussain, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
Something significantly better than today’s standard treatment options is needed, according to Dr. Hussain. Medicare pays out more than $50 billion annually for the treatment of patients with end-stage renal disease, and a hefty chunk of that money goes for oft-repeated procedures aimed at preserving the patency of the access sites.
“Primary patency rates leave much room for improvement,” observed Dr. Hussain, a vascular surgeon at the Christie Clinic in Champaign, Ill.
Indeed, the 50% primary patency rate at 6 months that was optimistically declared a “reasonable goal” in the 2006 Kidney Disease Outcomes Quality Initiative is actually far-fetched using the conventional tools.
“That 50% patency at 6 months would be a tall order to try to meet. Anybody in this room that does fistulography and angioplasty knows the numbers are actually a lot lower than 50%,” said Dr. Hussain.
Plain old balloon angioplasty, the standard first-line intervention for stenotic hemodialysis access sites, has a 6-month patency rate of about 30%. Bare metal stents push the rate up to about 39%. Covered stent grafts are the most effective of the conventional treatment modalities, with a 6-month patency of 51%-53%; however, they are widely considered too expensive for routine use.
Drug-coated balloons (DCBs) have been available for close to 3 years for treatment of lower extremity peripheral artery disease, where they have achieved considerable success. The Food and Drug Administration has approved three commercially available DCBs for this purpose: Bard’s Lutonix 035 AV, Medtronic’s IN.PACT Admiral, and most recently the Stellarex DCB.
In addition, the Lutonix DCB is approved for treatment of dysfunctional or stenotic arteriovenous (AV) fistulae on the strength of the positive results of the first prospective randomized multicenter trial of a DCB versus balloon angioplasty for AV access stenosis as reported at a conference in Leipzig, Germany, in 2017 and summarized by Dr. Hussain.
The pathophysiology of arterial atherosclerotic stenosis is very different from the stenosis that plagues AV access for dialysis. Arterial atherosclerotic stenosis is due to neointimal hyperplasia caused by inflammation and barotrauma secondary to angioplasty. In contrast, the neointimal hyperplasia in AV access stenosis is due to smooth muscle cell proliferation in response to nonphysiologic blood flow dynamics and shear forces between a high-pressure arterial system and the low-pressure venous system to which it has been connected, with resultant stenosis at the venous outflow anastomosis and often at the cephalic arch, Dr. Hussain explained.
Other contributors to the high rate of early stenosis in AV fistulae and grafts include traumatic balloon dilation, uremia, and repetitive traumatic needle insertion.
The breakthrough for DCBs as a potential game changer in dialysis access stenosis came with the discovery that venous smooth muscle cells are much more sensitive to paclitaxel and other antiproliferative drugs than are arterial smooth muscle cells. All three commercially available DCBs utilize paclitaxel as their active agent.
Multiple small single-center studies involving off-label use of the DCBs for dialysis access stenosis strongly suggested 6-month patency rates were higher than with balloon angioplasty. Then came the core lab-adjudicated Lutonix multicenter trial, in which 285 hemodialysis patients at 23 sites were randomized to the DCB or balloon angioplasty. Participants had to have a target lesion less than 10 cm long and had to undergo successful predilatation with high-pressure balloon angioplasty.
“The key thing to remember when we talk about dialysis grafts or fistulae is that we have to look at patency in periods of months. We can’t look at years because it’s pretty unusual to see a fistula stay open that long. So most of the time we’re trying to achieve extra months on these types of circuits,” noted Dr. Hussain.
That being said, the 8-month target lesion primary patency rate was 61.6% in the Lutonix DCB group, compared with 49.4% for percutaneous angioplasty, a statistically significant and clinically meaningful difference. Moreover, 66 interventions were required to maintain target lesion patency during that time frame in the DCB group, versus 94 in the angioplasty group; that translated to a 30% reduction in repeat interventions.
“This clearly has the potential to save a lot of money for the health care system,” he said.
The two forms of treatment were equally safe.
The expanded indication for the Lutonix DCB that resulted from this large randomized trial has triggered considerable research interest in DCBs for AV access stenosis around the world. Major ongoing randomized trials include the PAVE trial in the United Kingdom, the Spanish FISBOL trial, the APERTO trial in the Netherlands, and an Israeli randomized trial restricted to patients with cephalic arch stenosis.
Dr. Hussain is particularly excited about the ongoing 330-patient, prospective, multicenter, single-blinded clinical trial of the IN.PACT Admiral DCB versus plain balloon angioplasty. The Medtronic DCB employs a higher dosage of paclitaxel: 3.5 mcg/mm2, compared with 2.0 mcg/mm2 for the Lutonix DCB. Also, due to differences in the excipients used in the two DCBs, the paclitaxel from the IN.PACT device stays in the media of blood vessels for up to 180 days, compared with 60 days following drug delivery with the Lutonix balloon. Whether this longer period of close range antiproliferative activity will translate into a higher patency rate remains to be seen.
Dr. Hussain reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
How soon should patients with infective endocarditis be referred for valve surgery?
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
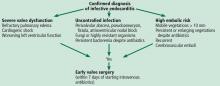
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
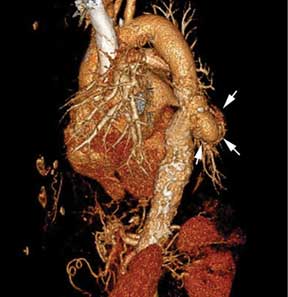
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
Infective endocarditis: Refer for expert team care as soon as possible
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
PVT after sleeve gastrectomy treatable with anticoagulants
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: Anticoagulation treatments effectively managed most portomesenteric vein thrombosis cases following laparoscopic sleeve gastrectomy.
Major finding: PVT is rare (0.3%) but occurs more frequently with laparoscopic sleeve gastrectomy, compared with other bariatric surgery procedures.
Study details: A multicenter, retrospective study conducted in Australia from 2007 to 2016 with 5,951 adult obese patients who received elective laparoscopic sleeve gastrectomy.
Disclosures: The authors reported no conflicts of interest.
Source: Tan SBM et al. Surg Obes Relat Dis. Mar 2018;14:271-6.
Hidden lesion easily missed on chest radiography
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
Jessica Stempe
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
A 46-year-old man with poorly controlled hypertension presented with the sudden onset of chest pain and shortness of breath. His blood pressure was 158/96 mm Hg and his left ventricular ejection fraction was less than 20%. He was admitted to the hospital for newly diagnosed heart failure.
SACCULAR AORTIC ANEURYSMS
This case shows the value of carefully examining the chest radiograph, especially behind the heart.
Saccular aneurysms of the descending thoracic aorta are rare and can be easily missed if asymptomatic. They are less common than fusiform aneurysms and may be more prone to rupture. Shang et al1 identified atherosclerosis as the most frequent cause of saccular aneurysms of the thoracic aorta. However, they can be caused by other inflammatory conditions and infections.1
Without surgical repair, thoracic aortic aneurysms larger than 6 cm have higher rates of expansion and rupture (a 20% 6-year cumulative risk) than smaller ones.2 Mid-descending aortic aneurysms expand faster than those of the ascending aorta.3
Indications for surgery include rupture, severe chest pain, compressive symptoms, large size (eg, ≥ 5.5 cm for asymptomatic descending thoracic aneurysms), and rapid growth rate (≥ 10 mm per year), all of which are associated with a higher mortality rate.4
Endovascular grafting should be strongly considered in patients who have significant comorbidities, but this approach may have poorer long-term outcomes compared with open surgery. Blood pressure should be lowered as far as the patient can tolerate without adverse effects, usually with a beta-blocker along with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.4
Statin therapy to lower the low-density lipoprotein cholesterol level to less than 70 mg/dL is also needed to reduce the risk of complications and cardiovascular disease.
All patients with saccular aortic aneurysm should be followed closely and be evaluated for surgery.
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Shang EK, Nathan DP, Boonn WW, et al. A modern experience with saccular aortic aneurysms. J Vasc Surg 2013; 57(1):84–88. doi:10.1016/j.jvs.2012.07.002
- Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994; 107(5):1323–1333.
- Bonser RS, Pagano D, Lewis ME, et al. Clinical and patho-anatomical factors affecting expansion of thoracic aortic aneurysms. Heart 2000; 84(3):277–283.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
Jessica Stempe
Jessica Stempe
Perioperative interruption of dual antiplatelet therapy
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
In reply: Perioperative interruption of dual antiplatelet therapy
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
Femoral artery endarterectomy still ‘gold standard’
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
CHICAGO – Additional higher quality supporting evidence is needed before , Jeffrey J. Siracuse, MD, FACS, asserted at a symposium on vascular surgery sponsored by Northwestern University.
“Open surgery in the CFA [common femoral artery] is probably still the gold standard in most cases,” said Dr. Siracuse, a vascular surgeon at Boston University.
He was quick to note that others would disagree. Stenting and other endovascular interventions in the CFA are booming in popularity, particularly among cardiologists, interventional radiologists, and the patients to whom the clinicians present the option in a favorable light. But this enthusiasm is based almost entirely on small, single-center, retrospective studies conducted in patients with heterogeneous profiles. The one prospective randomized multicenter trial of stenting versus surgery for CFA stenosis published to date – the French TECCO study – has a number of key limitations, flaws, and unanswered questions, which endovascular proponents have overlooked in their enthusiasm to promote an “endo-first” approach in the CFA, according to Dr. Siracuse.
“Everyone’s pretty much jumping on the bandwagon now. I think endovascular therapy of the CFA is here to stay. You’re going to see more people doing it, and potentially doing it incorrectly,” he predicted.
“The biggest thing I worry about with stenting is covering or jailing out the deep femoral artery. On multiple occasions – including a case just 2 weeks ago – I’ve taken out stents placed in the CFA by others that developed in-stent hyperplasia to the extent that the entire stent goes down, the DFA is covered, and now all of a sudden you’ve lost all flow to the leg. That’s my biggest concern with stenting,” he said.
Dr. Siracuse has other reservations as well. The CFA has traditionally been considered a “no-stent zone” because of the unique biomechanical stresses the artery is subjected to as a result of torsion, flexion, and extension at the hip joint. These forces render the area particularly vulnerable to neointimal hyperplasia, acute thrombosis, and stent fracture.
In addition, he noted, CFA endarterectomy for atherosclerotic lesions is a mature, well-established operation with an excellent track record for safety and durability. Dr. Siracuse’s review of procedural safety in 1,513 patients in the American College of Surgeons National Surgical Quality Improvement Project database during 2007-2010 showed a 30-day mortality of 1.5% and a 7.9% rate of major or minor complications (Vasc Endovascular Surg. 2014 Jan;48[1]:27-33).
In contrast, his review of 1,014 patients who underwent nonemergent endovascular CFA interventions for CFA stenosis without acute limb ischemia in the Vascular Quality Initiative registry demonstrated a 1-year patency rate of 85.3%, significantly lower than historically observed patency rates for endarterectomy. The 30-day mortality rate of 1.6% associated with endovascular interventions was essentially the same as in his earlier analysis of endarterectomy in the ACS NSQIP database, and the average 1.5-day hospital length of stay was shorter than with open surgery. Of considerable concern, however, stent implantation, which was performed in 35% of the endovascular interventions, was an independent predictor of amputation or death, with an associated 195% increased risk (J Vasc Surg. 2017 Apr;[4]:1039-46).
The travails of TECCO
The 17-center French TECCO study randomized 117 patients with de novo CFA atherosclerotic lesions to treatment via self-expanding stents or open surgery. A total of 98 participants were Rutherford stage 3, making TECCO primarily a study of claudicants. The primary outcome – the 30-day combined rate of morbidity and mortality – occurred in 26% of the surgical patients, a significantly higher rate than the 12.5% in the stent population. After a median follow-up of 24 months, the rates of primary patency, target lesion and extremity revascularization, and sustained clinical improvement were similar in the two groups (JACC Cardiovasc Interv. 2017 Jul 10;10[13]:1344-54).
The TECCO findings were hailed by endovascular therapy partisans as a big win. However, closer examination tells a different story, according to Dr. Siracuse.
There was no 30-day mortality in this rather small study. All 16 morbidity events occurring in the open surgery group within 30 days were relatively minor: 10 cases of delayed wound healing, 4 cases of postoperative paresthesia requiring medication, and 2 cases of lymphorrhea lasting longer than 3 days. In contrast, the seven morbidity events in the stent group included a complication requiring urgent open surgical repair at the time of stenting, one stent fracture, and a major amputation.
“The investigators didn’t elaborate on that major amputation, but I thought it was a little alarming because you should not have a major amputation with CFA interventions for claudicants,” the vascular surgeon commented. “Really, do people care about a lymphatic leak or do they care about amputation? I think more needs to be fleshed out about what really happened in that case.”
He was also puzzled by the hospital lengths of stay: a mean of 3.2 days in the stent group and 6.3 days in the open surgery group. “I think those lengths of stay are astounding. Very high and unusual,” he observed.
Dr. Siracuse predicted that much-needed high-quality data comparing treatments of the CFA will be provided by the BEST-CLI trial (Best Endovascular versus Surgical Treatment for Critical Limb Ischemia), which has been updated to include both open and endovascular interventions.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Primary livedo reticularis of the abdomen
Livedo reticularis can be the manifestation of a wide range of conditions: hematologic and hypercoagulable states, embolic events, connective tissue disease, infection, vasculitis, malignancy, neurologic and endocrine conditions, and medication effects.1 Our patient had no recent history of vascular procedures or peripheral eosinophilia to suggest cholesterol embolization, and he had not recently started taking any new medications. His current medications included aspirin 81 mg, atorvastatin 40 mg, amlodipine 10 mg, and insulin glargine 20 units. Tests for cryoglobulin and antiphospholipid antibodies were negative. There was no evidence of malignancy, and evaluations for infectious and autoimmune diseases were negative.
Biopsy study of a skin lesion showed features consistent with livedo reticularis, with no evidence of vasculitis. The lesions resolved without definitive therapy by hospital day 3. This, in addition to other features of the lesions (eg, uniformity, unbroken reticular segments) and the extensive negative workup for systemic disease, suggested primary livedo reticularis.
CAUSES, TYPES, SUBTYPES
Livedo reticularis results from changes in the cutaneous microvasculature, composed of central arterioles that drain into an interconnecting, netlike venous plexus.1,2 Conditions such as arteriolar deoxygenation and venous plexus venodilation that result in a prominent venous plexus can give rise to clinical livedo reticularis.3
Primary livedo reticularis is thought to occur from spontaneous arteriolar vasospasm. It is a diagnosis of exclusion. An evaluation for underlying disease is important, as livedo reticularis can be associated with the range of conditions listed above.
In our patient, methamphetamine was considered a possible cause, but the findings of livedo reticularis were delayed and persisted longer than expected if they were drug-related.
Livedo racemosa
Distinguishing livedo reticularis from livedo racemosa is important. Livedo racemosa is always secondary and is often associated with antiphospholipid syndrome. It is present in 25% of cases of primary antiphospholipid syndrome and in up to 70% of cases of antiphospholipid syndrome associated with systemic lupus erythematosus.5 The reticular pattern of livedo racemosa is permanent and often has irregular and incomplete segments of reticular lattice, with a distribution that is more generalized, involving the trunk or buttocks (or both) in addition to the extremities.3,4 Consequently, a thorough history and physical examination are needed to guide additional workup.
- Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol 2005; 52:1009–1019.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa. A literature review. J Neurol 2005; 252:1155–1166.
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol 2006; 33:2379–2382.
- Dean SM. Livedo reticularis and related disorders. Curr Treat Options Cardiovasc Med 2011; 13:179–191.
- Chadachan V, Dean SM, Eberhardt RT. Cutaneous changes in peripheral arterial vascular disease. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Education; 2012.
Livedo reticularis can be the manifestation of a wide range of conditions: hematologic and hypercoagulable states, embolic events, connective tissue disease, infection, vasculitis, malignancy, neurologic and endocrine conditions, and medication effects.1 Our patient had no recent history of vascular procedures or peripheral eosinophilia to suggest cholesterol embolization, and he had not recently started taking any new medications. His current medications included aspirin 81 mg, atorvastatin 40 mg, amlodipine 10 mg, and insulin glargine 20 units. Tests for cryoglobulin and antiphospholipid antibodies were negative. There was no evidence of malignancy, and evaluations for infectious and autoimmune diseases were negative.
Biopsy study of a skin lesion showed features consistent with livedo reticularis, with no evidence of vasculitis. The lesions resolved without definitive therapy by hospital day 3. This, in addition to other features of the lesions (eg, uniformity, unbroken reticular segments) and the extensive negative workup for systemic disease, suggested primary livedo reticularis.
CAUSES, TYPES, SUBTYPES
Livedo reticularis results from changes in the cutaneous microvasculature, composed of central arterioles that drain into an interconnecting, netlike venous plexus.1,2 Conditions such as arteriolar deoxygenation and venous plexus venodilation that result in a prominent venous plexus can give rise to clinical livedo reticularis.3
Primary livedo reticularis is thought to occur from spontaneous arteriolar vasospasm. It is a diagnosis of exclusion. An evaluation for underlying disease is important, as livedo reticularis can be associated with the range of conditions listed above.
In our patient, methamphetamine was considered a possible cause, but the findings of livedo reticularis were delayed and persisted longer than expected if they were drug-related.
Livedo racemosa
Distinguishing livedo reticularis from livedo racemosa is important. Livedo racemosa is always secondary and is often associated with antiphospholipid syndrome. It is present in 25% of cases of primary antiphospholipid syndrome and in up to 70% of cases of antiphospholipid syndrome associated with systemic lupus erythematosus.5 The reticular pattern of livedo racemosa is permanent and often has irregular and incomplete segments of reticular lattice, with a distribution that is more generalized, involving the trunk or buttocks (or both) in addition to the extremities.3,4 Consequently, a thorough history and physical examination are needed to guide additional workup.
Livedo reticularis can be the manifestation of a wide range of conditions: hematologic and hypercoagulable states, embolic events, connective tissue disease, infection, vasculitis, malignancy, neurologic and endocrine conditions, and medication effects.1 Our patient had no recent history of vascular procedures or peripheral eosinophilia to suggest cholesterol embolization, and he had not recently started taking any new medications. His current medications included aspirin 81 mg, atorvastatin 40 mg, amlodipine 10 mg, and insulin glargine 20 units. Tests for cryoglobulin and antiphospholipid antibodies were negative. There was no evidence of malignancy, and evaluations for infectious and autoimmune diseases were negative.
Biopsy study of a skin lesion showed features consistent with livedo reticularis, with no evidence of vasculitis. The lesions resolved without definitive therapy by hospital day 3. This, in addition to other features of the lesions (eg, uniformity, unbroken reticular segments) and the extensive negative workup for systemic disease, suggested primary livedo reticularis.
CAUSES, TYPES, SUBTYPES
Livedo reticularis results from changes in the cutaneous microvasculature, composed of central arterioles that drain into an interconnecting, netlike venous plexus.1,2 Conditions such as arteriolar deoxygenation and venous plexus venodilation that result in a prominent venous plexus can give rise to clinical livedo reticularis.3
Primary livedo reticularis is thought to occur from spontaneous arteriolar vasospasm. It is a diagnosis of exclusion. An evaluation for underlying disease is important, as livedo reticularis can be associated with the range of conditions listed above.
In our patient, methamphetamine was considered a possible cause, but the findings of livedo reticularis were delayed and persisted longer than expected if they were drug-related.
Livedo racemosa
Distinguishing livedo reticularis from livedo racemosa is important. Livedo racemosa is always secondary and is often associated with antiphospholipid syndrome. It is present in 25% of cases of primary antiphospholipid syndrome and in up to 70% of cases of antiphospholipid syndrome associated with systemic lupus erythematosus.5 The reticular pattern of livedo racemosa is permanent and often has irregular and incomplete segments of reticular lattice, with a distribution that is more generalized, involving the trunk or buttocks (or both) in addition to the extremities.3,4 Consequently, a thorough history and physical examination are needed to guide additional workup.
- Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol 2005; 52:1009–1019.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa. A literature review. J Neurol 2005; 252:1155–1166.
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol 2006; 33:2379–2382.
- Dean SM. Livedo reticularis and related disorders. Curr Treat Options Cardiovasc Med 2011; 13:179–191.
- Chadachan V, Dean SM, Eberhardt RT. Cutaneous changes in peripheral arterial vascular disease. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Education; 2012.
- Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol 2005; 52:1009–1019.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa. A literature review. J Neurol 2005; 252:1155–1166.
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol 2006; 33:2379–2382.
- Dean SM. Livedo reticularis and related disorders. Curr Treat Options Cardiovasc Med 2011; 13:179–191.
- Chadachan V, Dean SM, Eberhardt RT. Cutaneous changes in peripheral arterial vascular disease. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Education; 2012.
Correction: Update on VTE
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”