User login
Framingham: Arterial stiffening not inevitable with aging
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
The most reassuring finding of Niiranen et al. is that healthy vascular aging can be achieved. It can even be found in the elderly, albeit much less commonly than among adults in their 50s and 60s.
The clinical consequences of these findings are not yet clear, and we don’t yet know what to advise patients who have healthy vascular aging. But this study paves the way for research into protective factors, not just risk factors, for CVD.
Future research in this area will not only improve our understanding of the pathophysiology of vascular disease but also will offer new predictive, diagnostic, and therapeutic tools.
Gemma Currie, MD, and Christian Delles, MD, are at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, Scotland. Their work is supported by the British Heart Foundation and the European Commission, and they reported having no relevant financial disclosures. Dr. Currie and Dr. Delles made these remarks in an editorial accompanying Dr. Niiranen’s report (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09122).
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 and older participating in certain cohorts of the Framingham Heart Study, according to a report published online May 30 in Hypertension.
Researchers studied healthy vascular aging – the absence of age-related increases in arterial stiffness and blood pressure – using data collected from 3,196 older adults (mean age, 62 years) participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study. Aortic pulse wave velocity was used as a surrogate marker for arterial stiffness, said Teemu J. Niiranen, MD, PhD, and his associates in the Framingham Heart Study.
Overall, 566 men and women (17.7%) showed healthy vascular aging. The prevalence of healthy vascular aging was 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older. The cardiovascular risk factors most strongly associated with healthy vascular aging were a low body mass index, an absence of diabetes, and the use of lipid-lowering therapy, Dr. Niiranen and his associates said (Hypertens. 2017 May 30. doi: 10.1161/hypertensionaha.117.09026).
During a mean follow-up of 10 years, 391 study participants developed cardiovascular disease. People with healthy vascular aging were at substantially lower risk than were other participants for CVD (hazard ratio, 0.34), even after the data were adjusted to account for other traditional CVD risk factors. This highlights the importance of arterial stiffness in the pathogenesis of CVD, they added.
Although there are no specific therapies to prevent or treat arterial stiffening at this time, it seems reasonable that standard lifestyle changes and treatments aimed at reducing CVD – particularly maintaining a healthy weight, staving off diabetes, and using lipid-lowering medications – may prevent or delay arterial stiffening, Dr. Niiranen and his associates said.
The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering, Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
FROM HYPERTENSION
Key clinical point: Arterial stiffening is not an inevitable part of aging and did not occur in 18% of 3,196 men and women aged 50 years and older participating in certain cohorts of the Framingham Heart Study.
Major finding: The prevalence of healthy vascular aging was 18% overall, 30.3% in people aged 50-59 years, 7.4% in those aged 60-69 years, and 1% in those aged 70 years and older.
Data source: A secondary analysis of data accumulated for 3,196 adults participating in the 1998-2001, the 2005-2008, the Offspring, and the Third-Generation cohorts of the Framingham Heart Study.
Disclosures: The National Heart, Lung, and Blood Institutes’s Framingham Heart Study, the National Institutes of Health, and Boston University supported this work. Dr. Niiranen reported having no relevant financial disclosures; one of his associates reported owning Cardiovascular Engineering Inc., which develops and manufactures devices to measure vascular stiffness, as well as having ties to Merck, Novartis, Philips, and Servier.
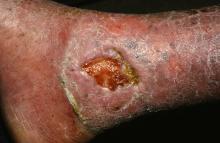
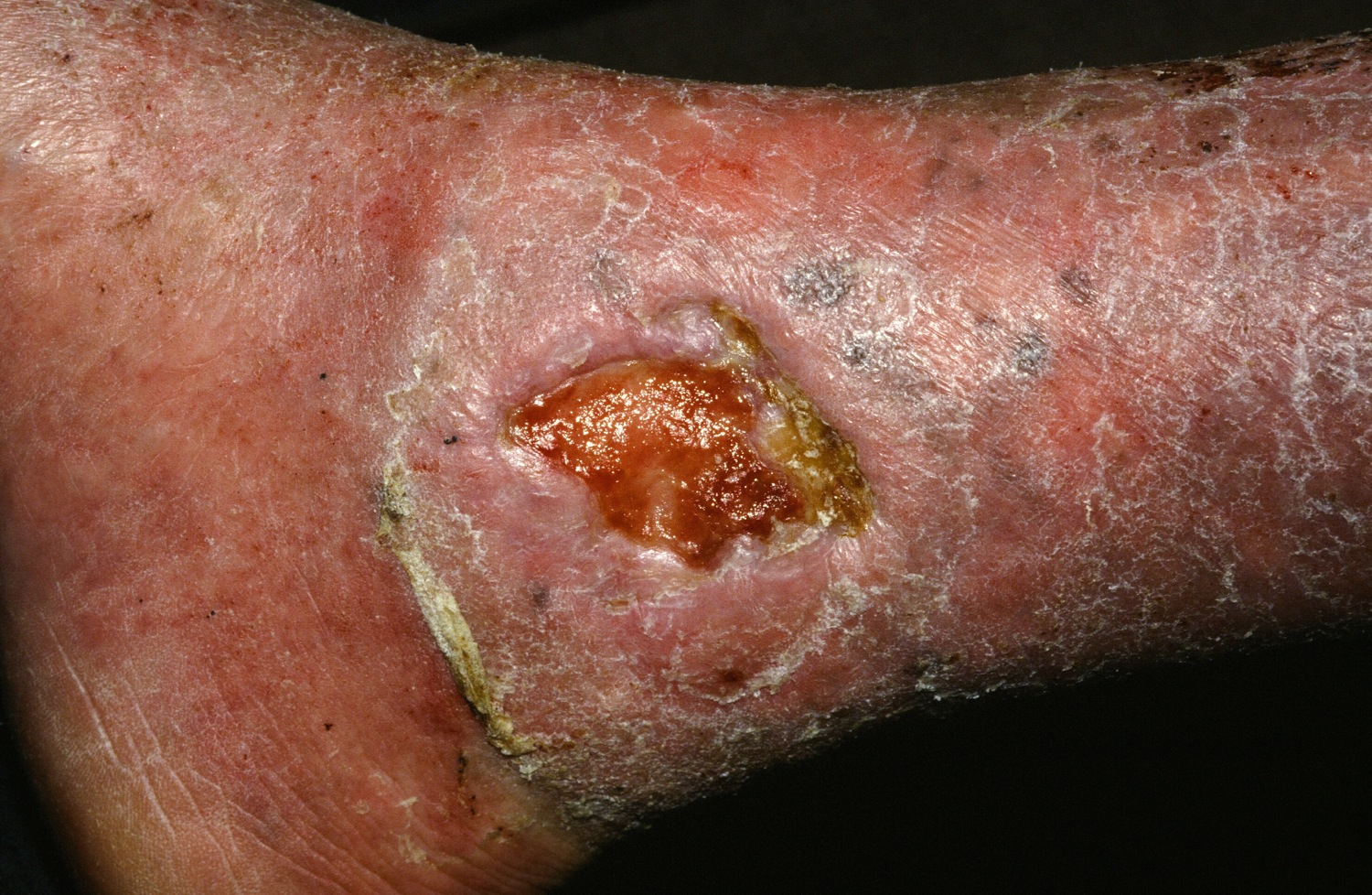
Stem cell therapy significantly improves ulcer healing
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
At SID 2017
Key clinical point: Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy.
Major finding: Neither control group achieved meaningful wound closure by week 24, while stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week (P less than .0005 for difference in healing rates among groups).
Data source: A randomized, controlled, double-blind pilot trial of 11 patients.
Disclosures: The National Institutes of Health supported the study. Dr. Grada had no conflicts of interest.
New DES hailed for smallest coronary vessels
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
AT EUROPCR
Key clinical point:
Major finding: At 12 months’ follow-up, the key outcomes were a 3% rate of target vessel MI, a 2% rate of clinically driven target lesion revascularization, no stent thrombosis, and no cardiac deaths.
Data source: A prospective, multicenter, open-label trial in 101 patients who underwent percutaneous coronary intervention for coronary lesions with a reference vessel diameter of less than 2.25 mm.
Disclosures: The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant to and paid speaker on behalf of that company as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
FDA approves first specific treatment for giant cell arteritis
The Food and Drug Administration has approved subcutaneous tocilizumab (Actemra) for the treatment of giant cell arteritis, according to a May 22 announcement from the agency.
Giant cell arteritis is a type of vasculitis that inflames blood vessels in the head, causing arteries to narrow or become irregular. The temporal arteries are the most commonly affected blood vessels, but giant cell arteritis can also affect other large blood vessels such as the aorta. Tocilizumab is the first drug specifically intended to treat giant cell arteritis. The standard treatment has typically been high doses of corticosteroids, tapered over time.
The FDA’s approval of tocilizumab was based on results from a double-blind, placebo-controlled study of 251 patients with giant cell arteritis. After 1 year, patients who received tocilizumab and tapered prednisone achieved remission at a higher rate than did patients who received placebo and tapered prednisone. Safety was consistent with tocilizumab’s known safety profile.
Subcutaneous tocilizumab is also approved for moderate to severely active rheumatoid arthritis. The intravenous formulation is approved for the treatment of moderate to severely active rheumatoid arthritis, systemic juvenile idiopathic arthritis, and polyarticular juvenile idiopathic arthritis.
The FDA granted both Breakthrough Therapy and Priority Review designations to this supplemental new drug application of tocilizumab.
The Food and Drug Administration has approved subcutaneous tocilizumab (Actemra) for the treatment of giant cell arteritis, according to a May 22 announcement from the agency.
Giant cell arteritis is a type of vasculitis that inflames blood vessels in the head, causing arteries to narrow or become irregular. The temporal arteries are the most commonly affected blood vessels, but giant cell arteritis can also affect other large blood vessels such as the aorta. Tocilizumab is the first drug specifically intended to treat giant cell arteritis. The standard treatment has typically been high doses of corticosteroids, tapered over time.
The FDA’s approval of tocilizumab was based on results from a double-blind, placebo-controlled study of 251 patients with giant cell arteritis. After 1 year, patients who received tocilizumab and tapered prednisone achieved remission at a higher rate than did patients who received placebo and tapered prednisone. Safety was consistent with tocilizumab’s known safety profile.
Subcutaneous tocilizumab is also approved for moderate to severely active rheumatoid arthritis. The intravenous formulation is approved for the treatment of moderate to severely active rheumatoid arthritis, systemic juvenile idiopathic arthritis, and polyarticular juvenile idiopathic arthritis.
The FDA granted both Breakthrough Therapy and Priority Review designations to this supplemental new drug application of tocilizumab.
The Food and Drug Administration has approved subcutaneous tocilizumab (Actemra) for the treatment of giant cell arteritis, according to a May 22 announcement from the agency.
Giant cell arteritis is a type of vasculitis that inflames blood vessels in the head, causing arteries to narrow or become irregular. The temporal arteries are the most commonly affected blood vessels, but giant cell arteritis can also affect other large blood vessels such as the aorta. Tocilizumab is the first drug specifically intended to treat giant cell arteritis. The standard treatment has typically been high doses of corticosteroids, tapered over time.
The FDA’s approval of tocilizumab was based on results from a double-blind, placebo-controlled study of 251 patients with giant cell arteritis. After 1 year, patients who received tocilizumab and tapered prednisone achieved remission at a higher rate than did patients who received placebo and tapered prednisone. Safety was consistent with tocilizumab’s known safety profile.
Subcutaneous tocilizumab is also approved for moderate to severely active rheumatoid arthritis. The intravenous formulation is approved for the treatment of moderate to severely active rheumatoid arthritis, systemic juvenile idiopathic arthritis, and polyarticular juvenile idiopathic arthritis.
The FDA granted both Breakthrough Therapy and Priority Review designations to this supplemental new drug application of tocilizumab.
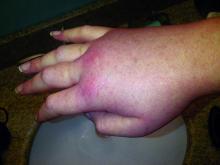
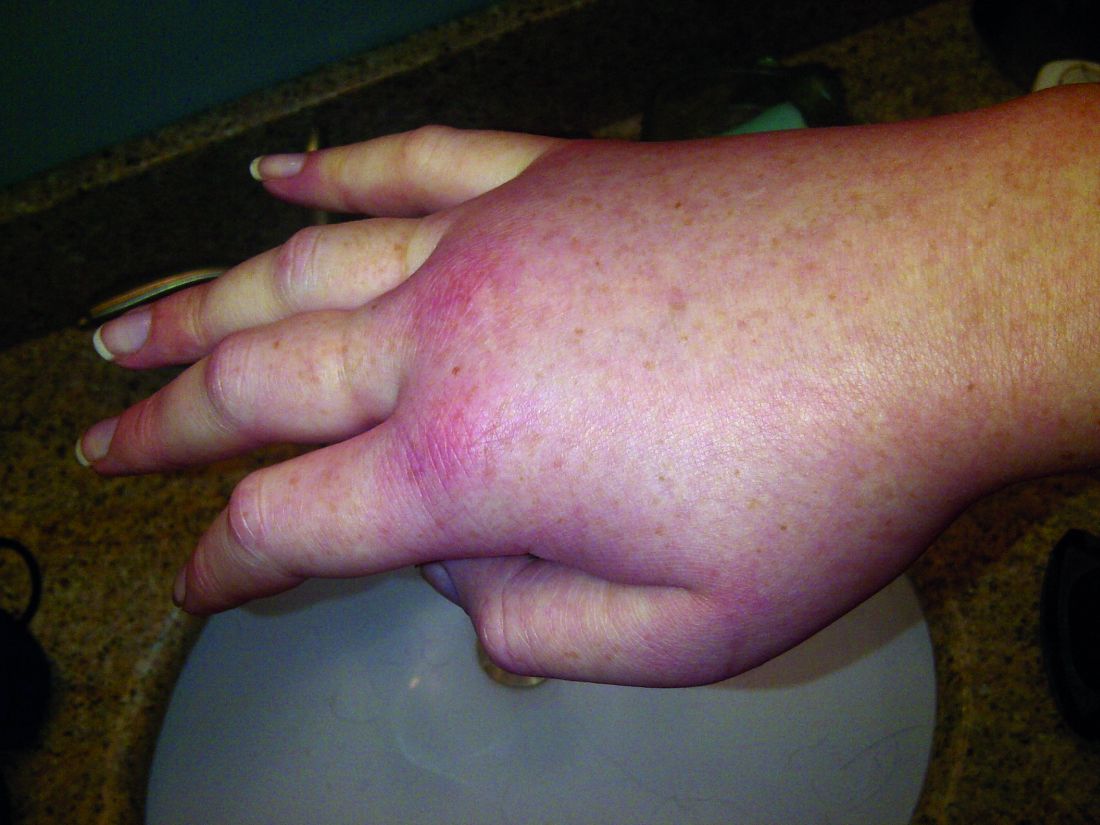
Cutaneous manifestations can signify severe systemic disease in ANCA-associated vasculitis
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
AT SID 2017
Key clinical point: Skin lesions can be a red flag for severe systemic disease in patients with ANCA-associated vasculitis.
Major finding: Among patients with granulomatosis with polyangiitis or eosinophilic granulomatosis with polyangiitis, the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (HR, 2.0, P less than .03). The hazard ratio was not elevated in patients with microscopic polyangiitis.
Data source: A cross-sectional study of 1,184 patients with ANCA-associated vasculitis from 130 centers worldwide.
Disclosures: Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
New ACR-EULAR diagnostic criteria proposed for ANCA-associated vasculitides
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
AT RHEUMATOLOGY 2017
Key clinical point: Provisional classification criteria have been developed to identify patients with granulomatosis with polyangiitis.
Major finding: The nine-item classification criteria model has an area under the curve of 0.98, with high sensitivity (90.7%) and specificity (93.5%).
Data source: More than 1,400 vasculitis cases submitted to the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study.
Disclosures: Dr. Robson reported having no conflicts of interest.
Blood donor age, sex do not affect recipient survival
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
Key clinical point: The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation.
Major finding: The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99.
Data source: A retrospective cohort study involving 968,264 transfusion recipients in Sweden and Denmark during a 10-year period.
Disclosures: The Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, Karolinska Institutet’s Strategic Research Program, and the Danish Council for Independent Research supported the study. Dr. Edgren and his associates reported having no relevant financial disclosures.
Tissue, peripheral eosinophilia correlate with UC activity, severity
Evidence linking tissue and peripheral eosinophilia with ulcerative colitis (UC) activity and severity was found in a retrospective chart review of pediatric UC cases.
Further, the review found both types of eosinophilia linked with the need for step-up therapy or corticosteroid therapy in the first year following UC diagnosis.
Sara Morgenstern, MD, of Tel Aviv University, and her coauthors reviewed all pediatric UC cases diagnosed between ages 0 and 17 years at the Schneider Children’s Hospital of Israel, Petah Tikva, between 1990 and 2015. Of 96 children diagnosed with UC by colonoscopy and followed for a median of 13 years, 31 had severe eosinophilia at the time of diagnosis, compared with 40 who had mild eosinophilia, and 25 who had a normal tissue eosinophil count. After remission, 77 had a normal eosinophilia and 19 had mild eosinophilia.
“At diagnosis, at follow-up with histologic activity and at follow-up with histologic remission, peripheral eosinophilia was demonstrated in 27%, 30% and 8%, respectively,” Dr. Morgenstern and her coauthors wrote. In the control group, 5% had peripheral eosinophilia, a significant difference (Dig Liver Dis. 2017 Feb;49[2]:170-4).
Disease activity and severity, as measured using the Pediatric UC Activity Index score, correlated significantly with tissue and blood eosinophil counts at diagnosis (P = .02 and P = .01, respectively). Disease activity and severity also correlated significantly with corticosteroid therapy, immunomodulatory therapy, and biologic therapy during the first year following diagnosis (P = .018, .04, and .05 for tissue eosinophilia; P = .013, .01, and .04 for peripheral eosinophilia, respectively).
“These findings may suggest that both tissue and peripheral eosinophilia may serve as a diagnostic marker for disease activity, severity, and short-term outcomes also in the pediatric population,” they wrote.
Dr. Morgenstern and her coauthors had no relevant financial disclosures.
Evidence linking tissue and peripheral eosinophilia with ulcerative colitis (UC) activity and severity was found in a retrospective chart review of pediatric UC cases.
Further, the review found both types of eosinophilia linked with the need for step-up therapy or corticosteroid therapy in the first year following UC diagnosis.
Sara Morgenstern, MD, of Tel Aviv University, and her coauthors reviewed all pediatric UC cases diagnosed between ages 0 and 17 years at the Schneider Children’s Hospital of Israel, Petah Tikva, between 1990 and 2015. Of 96 children diagnosed with UC by colonoscopy and followed for a median of 13 years, 31 had severe eosinophilia at the time of diagnosis, compared with 40 who had mild eosinophilia, and 25 who had a normal tissue eosinophil count. After remission, 77 had a normal eosinophilia and 19 had mild eosinophilia.
“At diagnosis, at follow-up with histologic activity and at follow-up with histologic remission, peripheral eosinophilia was demonstrated in 27%, 30% and 8%, respectively,” Dr. Morgenstern and her coauthors wrote. In the control group, 5% had peripheral eosinophilia, a significant difference (Dig Liver Dis. 2017 Feb;49[2]:170-4).
Disease activity and severity, as measured using the Pediatric UC Activity Index score, correlated significantly with tissue and blood eosinophil counts at diagnosis (P = .02 and P = .01, respectively). Disease activity and severity also correlated significantly with corticosteroid therapy, immunomodulatory therapy, and biologic therapy during the first year following diagnosis (P = .018, .04, and .05 for tissue eosinophilia; P = .013, .01, and .04 for peripheral eosinophilia, respectively).
“These findings may suggest that both tissue and peripheral eosinophilia may serve as a diagnostic marker for disease activity, severity, and short-term outcomes also in the pediatric population,” they wrote.
Dr. Morgenstern and her coauthors had no relevant financial disclosures.
Evidence linking tissue and peripheral eosinophilia with ulcerative colitis (UC) activity and severity was found in a retrospective chart review of pediatric UC cases.
Further, the review found both types of eosinophilia linked with the need for step-up therapy or corticosteroid therapy in the first year following UC diagnosis.
Sara Morgenstern, MD, of Tel Aviv University, and her coauthors reviewed all pediatric UC cases diagnosed between ages 0 and 17 years at the Schneider Children’s Hospital of Israel, Petah Tikva, between 1990 and 2015. Of 96 children diagnosed with UC by colonoscopy and followed for a median of 13 years, 31 had severe eosinophilia at the time of diagnosis, compared with 40 who had mild eosinophilia, and 25 who had a normal tissue eosinophil count. After remission, 77 had a normal eosinophilia and 19 had mild eosinophilia.
“At diagnosis, at follow-up with histologic activity and at follow-up with histologic remission, peripheral eosinophilia was demonstrated in 27%, 30% and 8%, respectively,” Dr. Morgenstern and her coauthors wrote. In the control group, 5% had peripheral eosinophilia, a significant difference (Dig Liver Dis. 2017 Feb;49[2]:170-4).
Disease activity and severity, as measured using the Pediatric UC Activity Index score, correlated significantly with tissue and blood eosinophil counts at diagnosis (P = .02 and P = .01, respectively). Disease activity and severity also correlated significantly with corticosteroid therapy, immunomodulatory therapy, and biologic therapy during the first year following diagnosis (P = .018, .04, and .05 for tissue eosinophilia; P = .013, .01, and .04 for peripheral eosinophilia, respectively).
“These findings may suggest that both tissue and peripheral eosinophilia may serve as a diagnostic marker for disease activity, severity, and short-term outcomes also in the pediatric population,” they wrote.
Dr. Morgenstern and her coauthors had no relevant financial disclosures.
FROM DIGESTIVE AND LIVER DISEASE
Key clinical point:
Major finding: At diagnosis, at follow-up with histologic activity, and at follow-up with histologic remission, peripheral eosinophilia was demonstrated in 27%, 30%, and 8%, respectively.
Data source: Ninety-six children diagnosed with UC by colonoscopy.
Disclosures: Dr. Morgenstern and her coauthors had no relevant financial disclosures.
Vascular surgeons underutilize palliative care planning
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
FROM JAMA SURGERY
Key clinical point:
Major finding: Of the 111 patients studied, 81 died on palliative care, but only 15 presented an advanced directive.
Data source: A retrospective cohort study of the records of patients aged 18-99 years who died in the vascular surgery service at Oregon Health and Science University Hospital from 2005-2014.
Disclosures: The authors reported no financial disclosures.
Lanadelumab reduced hereditary angioedema attacks by 88%-100%
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
dermnews@frontlinemedcom.com
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
dermnews@frontlinemedcom.com
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
dermnews@frontlinemedcom.com
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100%.
Major finding: All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no angioedema attacks during the study period.
Data source: A multicenter, randomized, double-blind, placebo-controlled phase Ib trial involving 37 adults who had hereditary angioedema with C1 inhibitor deficiency.
Disclosures: The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.