User login
Venography for stenting led to good results for patients with May-Thurner syndrome
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: May-Thurner patients treated with iliofemoral stents had significantly better (95%) clinical improvement than 21 patients not treated with stents (58%).
Data source: Retrospective analysis of a single-center registry of 65 patients with May-Thurner syndrome.
Disclosures: The authors reported that they had no disclosures.
Adaptive pneumatic compression device found comparable to compression stockings
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Vascular surgery trainees perceive weakness in venous education, case volumes
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: Of the of vascular trainees who responded to the survey, 75% reported a desire for increased venous training.
Data source: Nationwide U.S. survey of vascular trainees resulting in a 104/464 (22%) response rate.
Disclosures: The authors reported having no conflicts of interest.
Oral anticoagulation ‘reasonable’ in advanced kidney disease with A-fib
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The risk of stroke/systemic embolism in patients with advanced chronic kidney disease who were on oral anticoagulation was reduced by 49% among those not on hemodialysis and by 58% in those who were, compared with similar patients not on oral anticoagulation.
Data source: This was an observational study of nearly 50,000 patients with atrial fibrillation and stage 4 or 5 chronic kidney disease in a large U.S. administrative database.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
Dabigatran, rivaroxaban linked to slight increase in GI bleeding risk
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Novel oral anticoagulants (NOACs) receive a lot of press now. In randomized controlled trials (RCTs) comparing NOACs to warfarin for prevention of strokes and thromboembolism in atrial fibrillation (AF) and venous thromboembolism (VTE), fewer thromboembolisms are reported, but risks of gastrointestinal bleeding vary. To expand analyses for gastrointestinal bleeding, several systematic reviews and meta-analyses are reported, including this one by CS Miller et al. Their goals were to delineate risks of gastrointestinal bleeding for different NOACs compared with warfarin. What can GI clinicians now recommend about gastrointestinal bleeding for patients requiring anticoagulants? While we lack RCTs to give the highest quality of evidence about GIB as a primary outcome, conclusions now depend on the weight of evidence from recent secondary data analyses and I have some recommendations. First, although there may be differences among NOACs in risks of bleeding, all are likely to increase the risk of GI bleeding, comparable with warfarin. Some report that dabigatran and rivaroxaban have a higher risk of GI bleeding than other NOACs or warfarin, but differences are small. Second, some patients who need NOACs/warfarin have increased risks of ulcer bleeds including elderly patients and those with a history of upper GI bleeding, renal or hepatic impairment, low body weight, and concomitant antiplatelet agents. Such high-risk patients warrant treatment with a proton pump inhibitor or histamine2-receptor agonists for primary prevention while on anticoagulants. Finally, for patients with severe ulcer bleeding who require anticoagulation, warfarin or NOACs should be restarted after successful endoscopic hemostasis and proton pump inhibitors, usually within 3-5 days.
Dr. Jensen is professor of medicine at the University of California, Los Angeles; associate director of the CURE: DDRC, where he directs the Human Studies Core; a full-time staff physician in the UCLA division of digestive diseases; and a part-time staff physician in the GI section of the VA Greater Los Angeles Healthcare Center.
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Compared with conventional anticoagulants, both dabigatran and rivaroxaban conferred small but statistically significant increases in the risk of major gastrointestinal bleeding in a systematic review and meta-analysis of randomized trials reported in the November issue of Clinical Gastroenterology and Hepatology. (doi: 10.1016/j.cgh.2017.04.031)
But other novel oral anticoagulants (NOACs) showed no such effect compared with warfarin, aspirin, or placebo, reported Corey S. Miller, MD, of McGill University, Montreal, and his associates. “The potentially increased risk of GI bleeding associated with dabigatran and rivaroxaban observed in some of our subgroup analyses merits further consideration,” they wrote.
The NOACs (also known as non–vitamin K antagonist oral anticoagulants) help prevent stroke in patients with atrial fibrillation and prevent and treat venous thromboembolism. However, large AF trials have linked all except apixaban to an increased risk of major GI bleeding compared with warfarin. Dabigatran currently is the only NOAC with an approved reversal agent, “making the question of GI bleeding risk even more consequential,” the authors wrote.
They searched the MEDLINE, EMBASE, Cochrane, and ISI Web of Knowledge databases for reports of randomized trials of NOACs for approved indications published between 1980 and January 2016, which identified 43 trials of 166,289 patients. Most used warfarin as the comparator, but one study compared apixaban with aspirin and six studies compared apixaban, rivaroxaban, or dabigatran with placebo. Fifteen trials failed to specify bleeding sources and therefore could not be evaluated for the primary endpoint, the reviewers noted.
In the remaining 28 trials, 1.5% of NOAC recipients developed major GI bleeding, compared with 1.3% of recipients of conventional anticoagulants (odds ratio, 0.98; 95% confidence interval, 0.80-1.21). Five trials of dabigatran showed a 2% risk of major GI bleeding, compared with 1.4% with conventional anticoagulation, a slight but significant increase (OR, 1.27; 95% CI, 1.04-1.55). Eight trials of rivaroxaban showed a similar trend (bleeding risk, 1.7% vs. 1.3%; OR, 1.40; 95% CI, 1.15-1.70). In contrast, subgroup analyses of apixaban and edoxaban found no difference in risk of major GI bleeding versus conventional treatment.
Subgroup analyses by region found no differences except in Asia, where NOACs were associated with a significantly lower odds of major GI bleeding (0.5% and 1.2%, respectively; OR, 0.45; 95% CI, 0.22-0.91).
Most studies did not report minor or nonsevere bleeds or specify bleeding location within the GI tract, the reviewers noted. Given those caveats, NOACs and conventional anticoagulants conferred similar risks of clinically relevant nonmajor bleeding (0.6% and 0.6%, respectively), upper GI bleeding (1.5% and 1.6%), and lower GI bleeding (1.0% and 1.0%).
A post hoc analysis using a random-effects model found no significant difference in risk of major GI bleeding between either rivaroxaban or dabigatran and conventional therapy, the reviewers said. In addition, the increased risk of bleeding with dabigatran was confined to the RELY and ROCKET trials of AF, both of which exposed patients to longer treatment periods. Dabigatran is coated with tartaric acid, which might have a “direct caustic effect on the intestinal lumen,” they wrote. Also, NOACs are incompletely absorbed across the GI mucosa and therefore have some anticoagulant activity in the GI lumen, unlike warfarin or parenteral anticoagulants.
The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Compared with conventional anticoagulants, novel oral anticoagulants (NOACs) were not associated with increased risk of major gastrointestinal bleeding, with the possible exception of dabigatran and rivaroxaban.
Major finding: In the overall analysis, risk of major GI bleeding was 1.5% with NOACs and 1.3% with conventional anticoagulants (OR, 0.98; 95% CI, 0.80-1.21). In subgroup analyses, dabigatran conferred a 2% risk of major GI bleeding (OR, 1.3; 95% CI, 1.04-1.55), rivaroxaban conferred a 1.7% risk (OR, 1.40; 95% CI, 1.15-1.70).
Data source: A systematic review and meta-analysis of 43 randomized trials, comprising 166,289 patients.
Disclosures: The reviewers disclosed no funding sources. Dr. Miller and another author reported having no conflicts of interest. One author received research grants and speaker honoraria from Boehringer Ingelheim Canada, Bayer Canada, Daiichi Sankyo, Bristol Myers Squibb, and Pfizer Canada; another author disclosed serving as a consultant to Pendopharm, Boston Scientific, and Cook.
Harnessing vascular biology to rescue CVI sufferers
Despite affecting 25 million Americans, including two to six million with ulcer conditions, chronic venous insufficiency is relatively understudied compared to other vascular diseases. Yet for patients with venous leg ulcers, their condition is debilitating, painful and embarrassing.
Dr. Ulka Sachdev is studying the condition, hoping her “bench-side research” will develop “bedside” solutions. She received a five-year, National Institutes of Health K08 and SVS Foundation grants in 2012 and an SVS Foundation Clinical Research Seed Grant in 2017. “Their ulcers are very difficult to manage,” she said. “They are large, open, painful and wet. Patients’ daily routines are negatively affected by their wounds. It’s really sad.”
Since she regularly treats CVI patients, Dr. Sachdev wondered why some patients have benign venous insufficiency that ulcerates and why some ulcers recur. “If we could determine at an earlier stage how to mitigate the risk of new ulceration or recurrence, I think it would be worth it,” she said.
In her previous, K08-supported research she hypothesized that wound healing during ischemia is promoted by inflammatory proteins released by damaged tissue. She found that certain proteins known as danger signals can be released by damaged tissue and promote regenerative effects.
“My hypothesis is that specific danger signals can be manipulated, ideally with an oral drug,” Dr. Sachdev said. “If it works, this could be a mechanism that allows a dying muscle cell to say, ‘Hey, I need help.’ This might mean that someone who cannot get a bypass or a stent might not have to face amputation.’”
Her recent grant is for studying study patterns of inflammation in chronic venous insufficiency.
The goal of her studies is to determine whether patients with benign varicose veins and those with ulcerations express inflammatory mediators that predict their response to treatment. Later, perhaps, effective treatments will be found that change these patients’ lives.
Despite affecting 25 million Americans, including two to six million with ulcer conditions, chronic venous insufficiency is relatively understudied compared to other vascular diseases. Yet for patients with venous leg ulcers, their condition is debilitating, painful and embarrassing.
Dr. Ulka Sachdev is studying the condition, hoping her “bench-side research” will develop “bedside” solutions. She received a five-year, National Institutes of Health K08 and SVS Foundation grants in 2012 and an SVS Foundation Clinical Research Seed Grant in 2017. “Their ulcers are very difficult to manage,” she said. “They are large, open, painful and wet. Patients’ daily routines are negatively affected by their wounds. It’s really sad.”
Since she regularly treats CVI patients, Dr. Sachdev wondered why some patients have benign venous insufficiency that ulcerates and why some ulcers recur. “If we could determine at an earlier stage how to mitigate the risk of new ulceration or recurrence, I think it would be worth it,” she said.
In her previous, K08-supported research she hypothesized that wound healing during ischemia is promoted by inflammatory proteins released by damaged tissue. She found that certain proteins known as danger signals can be released by damaged tissue and promote regenerative effects.
“My hypothesis is that specific danger signals can be manipulated, ideally with an oral drug,” Dr. Sachdev said. “If it works, this could be a mechanism that allows a dying muscle cell to say, ‘Hey, I need help.’ This might mean that someone who cannot get a bypass or a stent might not have to face amputation.’”
Her recent grant is for studying study patterns of inflammation in chronic venous insufficiency.
The goal of her studies is to determine whether patients with benign varicose veins and those with ulcerations express inflammatory mediators that predict their response to treatment. Later, perhaps, effective treatments will be found that change these patients’ lives.
Despite affecting 25 million Americans, including two to six million with ulcer conditions, chronic venous insufficiency is relatively understudied compared to other vascular diseases. Yet for patients with venous leg ulcers, their condition is debilitating, painful and embarrassing.
Dr. Ulka Sachdev is studying the condition, hoping her “bench-side research” will develop “bedside” solutions. She received a five-year, National Institutes of Health K08 and SVS Foundation grants in 2012 and an SVS Foundation Clinical Research Seed Grant in 2017. “Their ulcers are very difficult to manage,” she said. “They are large, open, painful and wet. Patients’ daily routines are negatively affected by their wounds. It’s really sad.”
Since she regularly treats CVI patients, Dr. Sachdev wondered why some patients have benign venous insufficiency that ulcerates and why some ulcers recur. “If we could determine at an earlier stage how to mitigate the risk of new ulceration or recurrence, I think it would be worth it,” she said.
In her previous, K08-supported research she hypothesized that wound healing during ischemia is promoted by inflammatory proteins released by damaged tissue. She found that certain proteins known as danger signals can be released by damaged tissue and promote regenerative effects.
“My hypothesis is that specific danger signals can be manipulated, ideally with an oral drug,” Dr. Sachdev said. “If it works, this could be a mechanism that allows a dying muscle cell to say, ‘Hey, I need help.’ This might mean that someone who cannot get a bypass or a stent might not have to face amputation.’”
Her recent grant is for studying study patterns of inflammation in chronic venous insufficiency.
The goal of her studies is to determine whether patients with benign varicose veins and those with ulcerations express inflammatory mediators that predict their response to treatment. Later, perhaps, effective treatments will be found that change these patients’ lives.
When the painful ‘bumps’ are calciphylaxis, what’s next?
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
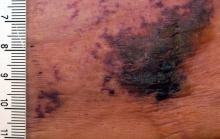
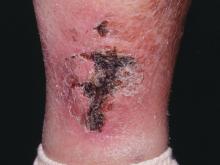
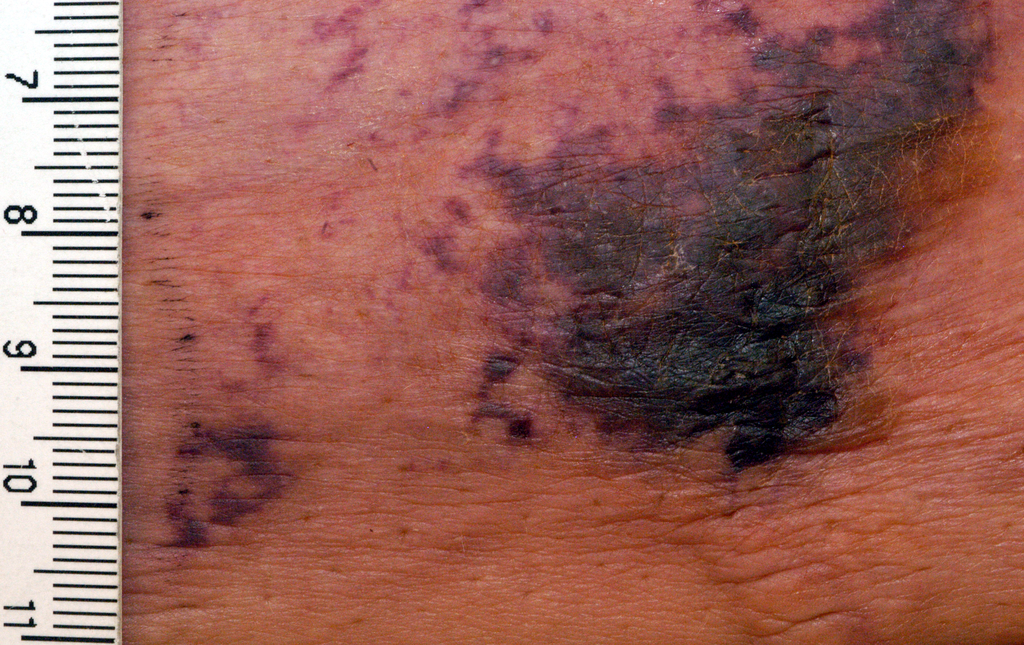
NEW YORK – When patients come to the office with painful “bumps” on the legs or elsewhere, panniculitis should be in the differential. And for some patients, said Alina Bridges, DO, the panniculitis may come with the dire diagnosis of calciphylaxis.
Calciphylaxis is an underrecognized crystal deposition disease that’s associated with panniculitis, said Dr. Bridges, speaking at the American Academy of Dermatology summer meeting. When calcium accumulates in small subcutaneous vessels, an occlusive vasculopathy is created within the dermis.
A soft-tissue radiograph of the affected area may also be helpful. Calciphylaxis shows as a fine netlike pattern of calcification, a finding that Dr. Bridges said has 90% specificity for the condition.
However, Dr. Bridges said, patients with panniculitis need a biopsy. “Careful selection of biopsy site and a deep specimen containing abundant fat obtained by incisional or excisional biopsy” is the best approach, allowing the pathologist to see the complete picture. In some cases, she said, a double-punch biopsy could also produce adequate specimens.
In addition to the calcium deposition, other pathologic findings may be lobular fat necrosis, with a pannicular vascular thrombosis. Though extravascular calcification can be seen in the panniculus, it’s not uncommon also to see intravascular calcification, said Dr. Bridges, who is the dermatopathology fellowship program director at the Mayo Clinic, Rochester, Minn.
Dr. Bridges said that the patients with calciphylaxis can present with predominant panniculitis or vasculitis, or a mixed picture; patients can also have bullae, ulcers, or livedo reticularis.
The lesions are extremely painful and become increasingly violaceous, with firm subcutaneous nodules. They are variably necrotic, and become more ulcerated over time.
Calciphylaxis is multifactorial and progressive. The prognosis is very poor for individuals with the condition, Dr. Bridges said. The median survival is 10 months, with 1-year survival rates of 46%, and just 20% of individuals with calciphylaxis surviving 2 years after diagnosis.
Gangrene is a frequent complication, and multisystem organ failure often occurs as well, she said.
Calciphylaxis most commonly occurs in individuals with chronic kidney disease and is seen in 4% of hemodialysis patients. However, it may also occur in individuals without uremia. In associations that are incompletely understood, calciphylaxis has been associated with warfarin therapy, connective tissue disorders, Crohn’s disease, liver disease, diabetes, hematologic malignancies, factor V Leiden deficiency, and protein C and S deficiency.
There’s a need for clinical suspicion of calciphylaxis when individuals with any of these conditions present with painful erythematous nodules, or with a vasculitic picture, she said.
Other, more common crystal deposition diseases can also be associated with panniculitis and can be in the differential, Dr. Bridges said. In patients with gout, sodium urate crystal deposition can occur in subcutaneous tissues.
Cutaneous oxalosis can occur as a primary disorder, when patients have metabolic errors and lack alanine-glyoxylate aminotransferase or D-glycerate dehydrogenase. Oxalosis can also be an acquired syndrome in patients with chronic renal failure who have been on long-term hemodialysis.
Although there is not a clearly effective treatment for calciphylaxis, a multitargeted, multidisciplinary approach is needed to help improve tissue health and patient quality of life. Since the primary mechanism of tissue damage is thrombotic tissue ischemia, strategies are aimed at existing clots and at preventing further clot formation.
To correct the calcium-phosphate balance, several medications have been used, including sodium thiosulfate and cinacalcet. For individuals on hemodialysis, a low-calcium dialysate may be used.
Tissue perfusion and oxygenation can be improved using tissue plasminogen activator, hyperbaric oxygen therapy, and the avoidance of warfarin if the patient requires anticoagulation.
To address wounds directly, debridement can begin with whirlpool time for patients. Surgical debridement may be required, and maggots can also help clean up wound beds.
Palliative care for patients should always include optimizing pain control and improving quality of life for patients with this serious and often life-limiting condition, Dr. Bridges said.
She reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
NEW YORK – When patients come to the office with painful “bumps” on the legs or elsewhere, panniculitis should be in the differential. And for some patients, said Alina Bridges, DO, the panniculitis may come with the dire diagnosis of calciphylaxis.
Calciphylaxis is an underrecognized crystal deposition disease that’s associated with panniculitis, said Dr. Bridges, speaking at the American Academy of Dermatology summer meeting. When calcium accumulates in small subcutaneous vessels, an occlusive vasculopathy is created within the dermis.
A soft-tissue radiograph of the affected area may also be helpful. Calciphylaxis shows as a fine netlike pattern of calcification, a finding that Dr. Bridges said has 90% specificity for the condition.
However, Dr. Bridges said, patients with panniculitis need a biopsy. “Careful selection of biopsy site and a deep specimen containing abundant fat obtained by incisional or excisional biopsy” is the best approach, allowing the pathologist to see the complete picture. In some cases, she said, a double-punch biopsy could also produce adequate specimens.
In addition to the calcium deposition, other pathologic findings may be lobular fat necrosis, with a pannicular vascular thrombosis. Though extravascular calcification can be seen in the panniculus, it’s not uncommon also to see intravascular calcification, said Dr. Bridges, who is the dermatopathology fellowship program director at the Mayo Clinic, Rochester, Minn.
Dr. Bridges said that the patients with calciphylaxis can present with predominant panniculitis or vasculitis, or a mixed picture; patients can also have bullae, ulcers, or livedo reticularis.
The lesions are extremely painful and become increasingly violaceous, with firm subcutaneous nodules. They are variably necrotic, and become more ulcerated over time.
Calciphylaxis is multifactorial and progressive. The prognosis is very poor for individuals with the condition, Dr. Bridges said. The median survival is 10 months, with 1-year survival rates of 46%, and just 20% of individuals with calciphylaxis surviving 2 years after diagnosis.
Gangrene is a frequent complication, and multisystem organ failure often occurs as well, she said.
Calciphylaxis most commonly occurs in individuals with chronic kidney disease and is seen in 4% of hemodialysis patients. However, it may also occur in individuals without uremia. In associations that are incompletely understood, calciphylaxis has been associated with warfarin therapy, connective tissue disorders, Crohn’s disease, liver disease, diabetes, hematologic malignancies, factor V Leiden deficiency, and protein C and S deficiency.
There’s a need for clinical suspicion of calciphylaxis when individuals with any of these conditions present with painful erythematous nodules, or with a vasculitic picture, she said.
Other, more common crystal deposition diseases can also be associated with panniculitis and can be in the differential, Dr. Bridges said. In patients with gout, sodium urate crystal deposition can occur in subcutaneous tissues.
Cutaneous oxalosis can occur as a primary disorder, when patients have metabolic errors and lack alanine-glyoxylate aminotransferase or D-glycerate dehydrogenase. Oxalosis can also be an acquired syndrome in patients with chronic renal failure who have been on long-term hemodialysis.
Although there is not a clearly effective treatment for calciphylaxis, a multitargeted, multidisciplinary approach is needed to help improve tissue health and patient quality of life. Since the primary mechanism of tissue damage is thrombotic tissue ischemia, strategies are aimed at existing clots and at preventing further clot formation.
To correct the calcium-phosphate balance, several medications have been used, including sodium thiosulfate and cinacalcet. For individuals on hemodialysis, a low-calcium dialysate may be used.
Tissue perfusion and oxygenation can be improved using tissue plasminogen activator, hyperbaric oxygen therapy, and the avoidance of warfarin if the patient requires anticoagulation.
To address wounds directly, debridement can begin with whirlpool time for patients. Surgical debridement may be required, and maggots can also help clean up wound beds.
Palliative care for patients should always include optimizing pain control and improving quality of life for patients with this serious and often life-limiting condition, Dr. Bridges said.
She reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
NEW YORK – When patients come to the office with painful “bumps” on the legs or elsewhere, panniculitis should be in the differential. And for some patients, said Alina Bridges, DO, the panniculitis may come with the dire diagnosis of calciphylaxis.
Calciphylaxis is an underrecognized crystal deposition disease that’s associated with panniculitis, said Dr. Bridges, speaking at the American Academy of Dermatology summer meeting. When calcium accumulates in small subcutaneous vessels, an occlusive vasculopathy is created within the dermis.
A soft-tissue radiograph of the affected area may also be helpful. Calciphylaxis shows as a fine netlike pattern of calcification, a finding that Dr. Bridges said has 90% specificity for the condition.
However, Dr. Bridges said, patients with panniculitis need a biopsy. “Careful selection of biopsy site and a deep specimen containing abundant fat obtained by incisional or excisional biopsy” is the best approach, allowing the pathologist to see the complete picture. In some cases, she said, a double-punch biopsy could also produce adequate specimens.
In addition to the calcium deposition, other pathologic findings may be lobular fat necrosis, with a pannicular vascular thrombosis. Though extravascular calcification can be seen in the panniculus, it’s not uncommon also to see intravascular calcification, said Dr. Bridges, who is the dermatopathology fellowship program director at the Mayo Clinic, Rochester, Minn.
Dr. Bridges said that the patients with calciphylaxis can present with predominant panniculitis or vasculitis, or a mixed picture; patients can also have bullae, ulcers, or livedo reticularis.
The lesions are extremely painful and become increasingly violaceous, with firm subcutaneous nodules. They are variably necrotic, and become more ulcerated over time.
Calciphylaxis is multifactorial and progressive. The prognosis is very poor for individuals with the condition, Dr. Bridges said. The median survival is 10 months, with 1-year survival rates of 46%, and just 20% of individuals with calciphylaxis surviving 2 years after diagnosis.
Gangrene is a frequent complication, and multisystem organ failure often occurs as well, she said.
Calciphylaxis most commonly occurs in individuals with chronic kidney disease and is seen in 4% of hemodialysis patients. However, it may also occur in individuals without uremia. In associations that are incompletely understood, calciphylaxis has been associated with warfarin therapy, connective tissue disorders, Crohn’s disease, liver disease, diabetes, hematologic malignancies, factor V Leiden deficiency, and protein C and S deficiency.
There’s a need for clinical suspicion of calciphylaxis when individuals with any of these conditions present with painful erythematous nodules, or with a vasculitic picture, she said.
Other, more common crystal deposition diseases can also be associated with panniculitis and can be in the differential, Dr. Bridges said. In patients with gout, sodium urate crystal deposition can occur in subcutaneous tissues.
Cutaneous oxalosis can occur as a primary disorder, when patients have metabolic errors and lack alanine-glyoxylate aminotransferase or D-glycerate dehydrogenase. Oxalosis can also be an acquired syndrome in patients with chronic renal failure who have been on long-term hemodialysis.
Although there is not a clearly effective treatment for calciphylaxis, a multitargeted, multidisciplinary approach is needed to help improve tissue health and patient quality of life. Since the primary mechanism of tissue damage is thrombotic tissue ischemia, strategies are aimed at existing clots and at preventing further clot formation.
To correct the calcium-phosphate balance, several medications have been used, including sodium thiosulfate and cinacalcet. For individuals on hemodialysis, a low-calcium dialysate may be used.
Tissue perfusion and oxygenation can be improved using tissue plasminogen activator, hyperbaric oxygen therapy, and the avoidance of warfarin if the patient requires anticoagulation.
To address wounds directly, debridement can begin with whirlpool time for patients. Surgical debridement may be required, and maggots can also help clean up wound beds.
Palliative care for patients should always include optimizing pain control and improving quality of life for patients with this serious and often life-limiting condition, Dr. Bridges said.
She reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
VA cohort study: Individualize SSI prophylaxis based on patient factors
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
FROM PLOS MEDICINE
Key clinical point:
Major finding: The SSI incidence was 0.95% vs. 1.48% with combination vs. single agent–therapy in cardiac surgery patients. Acute kidney injuries occurred in 23.75% of all surgery patients receiving combination prophylaxis, compared with 20.79% and 13.93% with vancomycin or a beta-lactam, respectively.
Data source: A retrospective cohort study of more than 70,000 surgical procedures.
Disclosures: This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated grant from Merck Pharmaceuticals in 2013.
Bilateral cellulitis on legs? Think venous stasis dermatitis
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Major bleeding deaths may outweigh VTE risk in older cancer patients
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
AT EHA 2017
Key clinical point: In cancer patients older than 65, the risk of major bleeding from anticoagulants may outweigh the risk of venous thromboembolism.
Major finding: 7-day mortality rates were 11% for major bleeding vs 0.5% for recurrent VTE.
Data source: Retrospective cohort study of 6,967 cancer patients age 65 and older from Ontario, Canada.
Disclosures: The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.