User login
Official Newspaper of the American College of Surgeons
Antipsychotic drugs failed to shorten ICU delirium
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Antipsychotics do not reduce the duration or incidence of delirium in intensive care.
Major finding: Patients treated with antipsychotics showed similar median days without delirium or coma, compared with those treated with placebo.
Study details: A randomized, double-blind, placebo-controlled trial in 566 intensive care patients.
Disclosures: The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors were supported by the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
Source: Girard TD et al. N Engl J Med. 2018 Oct 22. doi: 10.1056/NEJMoa1808217.
Vascular emergencies on the rise, but more patients surviving
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Rates of endovascular repair for nontraumatic vascular emergencies rose sharply.
Major finding: Endovascular repair rates for nontraumatic vascular emergencies climbed from 24% to 36% of cases from 2005 to 2014 (P for trend, less than .0001).
Study details: A 10-year sample of hospitalizations for nontraumatic vascular emergencies from the U.S. National Inpatient Sample.
Disclosures: Dr. Vogel reported no outside sources of funding and no conflicts of interest.
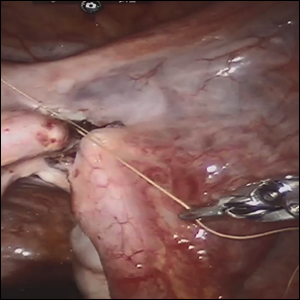
Robot-assisted laparoscopic tubal anastomosis following sterilization

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
Experts: Kavanaugh may have lasting impact on health care
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
Daptomycin/fosfomycin: A new standard for MRSA bacteremia?
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
SAN FRANCISCO – Daptomycin plus fosfomycin is more effective than daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia, according to a multicenter, randomized trial from Spain.
“I think this is really an important study; I think it will change clinical practice for this infection” once it’s published, said lead investigator Miquel Pujol, MD, PhD, clinical head of infectious diseases at Bellvitge University Hospital in Barcelona.
The current standard for MRSA bacteremia is daptomycin (Cubicin) or vancomycin (Vancocin) monotherapy on both sides of the Atlantic, but mortality rates are way too high, more than 30% in some reviews. Dr. Pujol and his colleagues wanted to find something better.
Their lab work showed that daptomycin and fosfomycin (Monurol) were synergistic and rapidly bactericidal against MRSA, and anecdotal experience in Spain suggested the drugs improved bacteremia outcomes, so they decided to put the combination to the test.
They randomized 74 MRSA bacteremia patients to the combination, daptomycin 10mg/kg IV daily plus fosfomycin 2g IV q 6h. They randomized 81 other subjects to standard of care with daptomycin monotherapy, also at 10mg/kg IV daily. Treatment was 10-14 days for uncomplicated and 28-42 days for complicated bacteremia.
The open-label trial was conducted at 18 medical centers in Spain, where fosfomycin was discovered in dirt samples in the late 1960s and remains a matter of pride.
At day 7, 69 of the 74 combination patients (93.2%) were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, versus 62 of 81 patients (76.5%) on monotherapy (absolute difference 16.7%; 95% confidence interval, 5.4%-27.7%). Three people in the combination arm (4.1%) had died by day 7, versus six on monotherapy (7.4%).
Six weeks after the end of treatment at the test-of-cure visit, 40 of 74 combination patients (54.1%) were alive with resolution of all clinical signs and symptoms, negative blood cultures, and no previous or subsequent relapses; just 34 of 81 patients (42%) in the monotherapy arm hit that mark. The 12.1% difference was not statistically significant, nor was the difference in 12-week survival.
However, patients in the combination arm were 70% less likely to have complicated bacteremia at the test-of-cure visit (9.5% vs. 28.4%; relative risk 0.3; 95% CI, 0.2-0.7). There were no cases of persistent or recurrent infection in the combination arm, but nine persistent (11.1%) and five recurrent (6.2%) cases with daptomycin monotherapy. The differences were statistically significant.
The subjects all had at least one positive MRSA blood culture within 72 hours of randomization. Exclusion criteria included MRSA pneumonia, prosthetic valve endocarditis, end-stage liver disease, and moderate to severe heart failure.
There were no significant baseline differences between the groups. About half the subjects were men, and the mean age was about 73 years. The mean Charlson Comorbidity Index score was a bit under 4, and the mean Pitt bacteremia score a bit over 1. The leading source of infection was vascular catheter; acquisition was thought to be nosocomial in more than 40% of patients.
There were no discontinuations from drug side effects in the daptomycin arm, but there were five in the combination arm, including two for heart failure, two for respiratory insufficiency, and one for GI bleeding. Even so, the benefit outweighed the risk, Dr. Pujol said.
Intravenous fosfomycin is available in Europe, but the drug is approved in the United States only as an oral formulation. That could change soon; Nabriva Therapeutics plans to file its IV formulation (Contepo) for Food and Drug Administration approval in late 2018.
Though it is not standard of practice yet, the combination is increasingly being used in Spain for MRSA bacteremia, according to Dr. Pujol. “Patients probably need the combination [at least] initially, especially if they have complicated bacteremia” or fail monotherapy, he said at ID week, an annual scientific meeting on infectious diseases.
The work was funded by the Spanish government. Dr. Pujol said he had no relevant disclosures.
SOURCE: Pujol M et al. 2018 ID Week abstract LB3
REPORTING FROM ID WEEK 2018
Key clinical point:
Major finding: At day 93% of the combination patients were alive with clinical improvement, clearance of bacteremia, and no subsequent relapse, vs. 77% on monotherapy.
Study details: Randomized, open label trial in 155 patients with MRSA bacteremia.
Disclosures: The work was funded by the Spanish government. The lead investigator said he had no relevant disclosures.
Source: Pujol M et al. 2018 ID Week, Abstract LB3
Booming economy helps flatten Medicaid enrollment and limit costs, states report
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Claudication, CLI differ significantly in hospital readmission, costs, mortality
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
FROM ANNALS OF VASCULAR SURGERY
Key clinical point: CLI was significantly more expensive and showed higher mortality rates compared with claudication.
Major finding: The 30-day readmission/any readmission rate was 9.0%/21.5% and 19.3%/40.4%, for claudication and CLI, respectively.
Study details: An analysis of more than 90,000 patients in the Nationwide Readmission Database in 2013 and 2014.
Disclosures: The authors did not report disclosures but indicated that the study did not receive any outside funding.
Source: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
Study: Problems persist with APMs
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
TV ads must trumpet drug prices, Trump administration says. Pharma tries a Plan B.
effectively setting the stage for months or possibly years of battle with the powerful industry.
The proposal, released late in the day, would require pharmaceutical companies to include in its television advertising the price of any drug that cost more than $35 a month. The price should be listed at the end of the advertisement in “a legible manner,” the rule states. It goes on to explain that the price should be presented against a contrasting background in a way that is easy to read.
Health and Human Services Secretary Alex M. Azar II, nodding to an industry proposal announced earlier in the day, said voluntary moves are not enough.
“We will not wait for an industry with so many conflicting and perverse incentives to reform itself,” Azar told the audience gathered at the National Academy of Sciences in Washington.
If approved, the proposed rule has no government enforcement mechanism that would force the companies to comply. Rather, it depends on shaming, noting that federal regulators would post a list of companies violating the rule. It would depend on the private sector to police itself with litigation.
“It is noteworthy that the government is unwilling to take enforcement action,” said Rachel Sachs, an associate professor of law at Washington University, St. Louis, and expert in drug-pricing regulation. The rule might never be finalized, she added.
“It will take many months if not years for this regulation to be implemented and free from the cloud of litigation that will follow it. And the administration knows that,” Ms. Sachs said.
Earlier on Oct. 15, the pharmaceutical industry trade group went on the offensive in anticipation of Mr. Azar’s speech by announcing its own plans.
“Putting list prices in isolation in the advertisements themselves would be misleading or confusing,” argued Stephen J. Ubl, CEO of the Pharmaceutical Researchers and Manufacturers of America, or PhRMA, the major trade group for branded drugs.
Instead, Mr. Ubl, whose trade group represents the largest pharmaceutical manufacturers on the globe, promised that pharma companies would direct consumers to websites that include a drug’s list price and estimates of what people can expect to pay, which can vary widely depending on coverage.
Drug manufacturers would voluntarily opt in to this disclosure starting next spring, he said. Mr. Ubl remained strongly critical of the White House proposal.
The Trump administration’s proposal comes weeks before midterm elections in which health care is a top voter concern. Polling from the Kaiser Family Foundation suggests most voters support forcing price transparency in drug advertisements. (Kaiser Health News is an editorially independent program of the foundation.)
The White House’s plan, which was teased in President Trump’s blueprint this summer, has won praise from insurance groups and the American Medical Association.
Sen. Chuck Grassley (R-Iowa) and Sen. Dick Durbin (D-Ill.) also proposed the plan in the Senate last month, but it failed to garner enough support.
Experts pointed out a host of complications, suggesting that neither PhRMA’s approach nor the White House’s would fully explain to consumers what they’ll actually pay for drugs.
On Oct. 15, Sen. Grassley applauded Mr. Azar’s announcement, saying it was a “common-sense way to lower prices.”
But Dale Cooke, a consultant who works with drug companies trying to meet Food and Drug Administration requirements for advertising, warned there is no reason to believe posting prices would help drive down prices.
“No one has ever explained to me why this would work,” Mr. Cooke said. “What’s the mechanism by which this results in lower drug prices?”
Even more, it could be confusing for patients, Mr. Cooke said. The proposed rule seemed to acknowledge this danger, he said, noting, “On the other hand, consumers, intimidated and confused by high list prices, may be deterred from contacting their physicians about drugs or medical conditions.”
A drug’s list price – the metric HHS wants to emphasize – often bears little relationship to what a patient pays at the drugstore. Insurance plans and pharmacy benefit managers often negotiate cheaper prices than the list price. Some patients qualify for other discounts. And often patients pay only what their copay or deductible requires at any given time.
Other consumers could be stuck paying the full cost, depending on how their insurance plan is designed, or if they don’t have coverage.
“The system is very opaque, very complicated, and importantly, there isn’t a huge relationship between list prices for drugs and what patients will expect to pay out-of-pocket,” said Adrienne Faerber, PhD, a lecturer at the Dartmouth Institute for Health Policy and Clinical Practice who researches drug marketing.
But the industry’s strategy, she said, also appeared lacking.
Under PhRMA’s plan, drugmakers would not standardize how they display their information. Where consumers go could vary on Pfizer’s website versus Merck’s to learn about the list price and the range of out-of-pocket costs. That, Ms. Faerber argued, would make it difficult for people to unearth relevant information.
PhRMA also announced it is partnering with patient advocacy groups to create a “patient affordability platform,” which could help patients search for costs and insurance coverage options.
Mr. Ubl cast PhRMA’s proposal as a way to address more effectively the government and public concern about drug price transparency.
Pharmaceutical manufacturers rely heavily on national advertising and together represent the third-highest spender in national television advertising, according to Michael Leszega, a manager of market intelligence at consulting firm Magna.
At certain times of day, pharmaceutical ads make up more than 40 percent of TV advertisements. And those commercials stand out because they are generally longer, with a long list of side effects and warnings the pharmaceutical industry must tag on at the end.
Those disclaimers highlight another challenge for the administration: legal action.
The rule notes its legal justification was based on the responsibility of the Centers for Medicare & Medicaid Services to ensure the health coverage programs that it administers – Medicare and Medicaid – must be operated in a manner that “minimizes reasonable expenditures.”
Sachs noted that the argument may be weak because most drugs are marketed to a wider audience than Medicare and Medicaid beneficiaries.
A body of Supreme Court decisions dictate how disclaimers and disclosures can be required, said constitutional law expert Robert Corn-Revere. He filed a “friend of the court” brief in a 2011 U.S. Supreme Court case related to commercial speech and the pharmaceutical industry.
Generally, the administration’s requirement must meet the standards of being purely factual, noncontroversial, and not burdensome, Mr. Corn-Revere said.
On the question of whether requiring drug prices be listed in advertising violates the First Amendment’s free-speech guarantee, Corn-Revere said it “all comes down to the specifics.”
Mr. Ubl, when asked earlier about legal action, didn’t rule out the possibility. “We believe there are substantial statutory and constitutional principles that arise” from requiring list-price disclosure, Mr. Ubl said, adding: “We do have concerns about that approach.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
effectively setting the stage for months or possibly years of battle with the powerful industry.
The proposal, released late in the day, would require pharmaceutical companies to include in its television advertising the price of any drug that cost more than $35 a month. The price should be listed at the end of the advertisement in “a legible manner,” the rule states. It goes on to explain that the price should be presented against a contrasting background in a way that is easy to read.
Health and Human Services Secretary Alex M. Azar II, nodding to an industry proposal announced earlier in the day, said voluntary moves are not enough.
“We will not wait for an industry with so many conflicting and perverse incentives to reform itself,” Azar told the audience gathered at the National Academy of Sciences in Washington.
If approved, the proposed rule has no government enforcement mechanism that would force the companies to comply. Rather, it depends on shaming, noting that federal regulators would post a list of companies violating the rule. It would depend on the private sector to police itself with litigation.
“It is noteworthy that the government is unwilling to take enforcement action,” said Rachel Sachs, an associate professor of law at Washington University, St. Louis, and expert in drug-pricing regulation. The rule might never be finalized, she added.
“It will take many months if not years for this regulation to be implemented and free from the cloud of litigation that will follow it. And the administration knows that,” Ms. Sachs said.
Earlier on Oct. 15, the pharmaceutical industry trade group went on the offensive in anticipation of Mr. Azar’s speech by announcing its own plans.
“Putting list prices in isolation in the advertisements themselves would be misleading or confusing,” argued Stephen J. Ubl, CEO of the Pharmaceutical Researchers and Manufacturers of America, or PhRMA, the major trade group for branded drugs.
Instead, Mr. Ubl, whose trade group represents the largest pharmaceutical manufacturers on the globe, promised that pharma companies would direct consumers to websites that include a drug’s list price and estimates of what people can expect to pay, which can vary widely depending on coverage.
Drug manufacturers would voluntarily opt in to this disclosure starting next spring, he said. Mr. Ubl remained strongly critical of the White House proposal.
The Trump administration’s proposal comes weeks before midterm elections in which health care is a top voter concern. Polling from the Kaiser Family Foundation suggests most voters support forcing price transparency in drug advertisements. (Kaiser Health News is an editorially independent program of the foundation.)
The White House’s plan, which was teased in President Trump’s blueprint this summer, has won praise from insurance groups and the American Medical Association.
Sen. Chuck Grassley (R-Iowa) and Sen. Dick Durbin (D-Ill.) also proposed the plan in the Senate last month, but it failed to garner enough support.
Experts pointed out a host of complications, suggesting that neither PhRMA’s approach nor the White House’s would fully explain to consumers what they’ll actually pay for drugs.
On Oct. 15, Sen. Grassley applauded Mr. Azar’s announcement, saying it was a “common-sense way to lower prices.”
But Dale Cooke, a consultant who works with drug companies trying to meet Food and Drug Administration requirements for advertising, warned there is no reason to believe posting prices would help drive down prices.
“No one has ever explained to me why this would work,” Mr. Cooke said. “What’s the mechanism by which this results in lower drug prices?”
Even more, it could be confusing for patients, Mr. Cooke said. The proposed rule seemed to acknowledge this danger, he said, noting, “On the other hand, consumers, intimidated and confused by high list prices, may be deterred from contacting their physicians about drugs or medical conditions.”
A drug’s list price – the metric HHS wants to emphasize – often bears little relationship to what a patient pays at the drugstore. Insurance plans and pharmacy benefit managers often negotiate cheaper prices than the list price. Some patients qualify for other discounts. And often patients pay only what their copay or deductible requires at any given time.
Other consumers could be stuck paying the full cost, depending on how their insurance plan is designed, or if they don’t have coverage.
“The system is very opaque, very complicated, and importantly, there isn’t a huge relationship between list prices for drugs and what patients will expect to pay out-of-pocket,” said Adrienne Faerber, PhD, a lecturer at the Dartmouth Institute for Health Policy and Clinical Practice who researches drug marketing.
But the industry’s strategy, she said, also appeared lacking.
Under PhRMA’s plan, drugmakers would not standardize how they display their information. Where consumers go could vary on Pfizer’s website versus Merck’s to learn about the list price and the range of out-of-pocket costs. That, Ms. Faerber argued, would make it difficult for people to unearth relevant information.
PhRMA also announced it is partnering with patient advocacy groups to create a “patient affordability platform,” which could help patients search for costs and insurance coverage options.
Mr. Ubl cast PhRMA’s proposal as a way to address more effectively the government and public concern about drug price transparency.
Pharmaceutical manufacturers rely heavily on national advertising and together represent the third-highest spender in national television advertising, according to Michael Leszega, a manager of market intelligence at consulting firm Magna.
At certain times of day, pharmaceutical ads make up more than 40 percent of TV advertisements. And those commercials stand out because they are generally longer, with a long list of side effects and warnings the pharmaceutical industry must tag on at the end.
Those disclaimers highlight another challenge for the administration: legal action.
The rule notes its legal justification was based on the responsibility of the Centers for Medicare & Medicaid Services to ensure the health coverage programs that it administers – Medicare and Medicaid – must be operated in a manner that “minimizes reasonable expenditures.”
Sachs noted that the argument may be weak because most drugs are marketed to a wider audience than Medicare and Medicaid beneficiaries.
A body of Supreme Court decisions dictate how disclaimers and disclosures can be required, said constitutional law expert Robert Corn-Revere. He filed a “friend of the court” brief in a 2011 U.S. Supreme Court case related to commercial speech and the pharmaceutical industry.
Generally, the administration’s requirement must meet the standards of being purely factual, noncontroversial, and not burdensome, Mr. Corn-Revere said.
On the question of whether requiring drug prices be listed in advertising violates the First Amendment’s free-speech guarantee, Corn-Revere said it “all comes down to the specifics.”
Mr. Ubl, when asked earlier about legal action, didn’t rule out the possibility. “We believe there are substantial statutory and constitutional principles that arise” from requiring list-price disclosure, Mr. Ubl said, adding: “We do have concerns about that approach.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
effectively setting the stage for months or possibly years of battle with the powerful industry.
The proposal, released late in the day, would require pharmaceutical companies to include in its television advertising the price of any drug that cost more than $35 a month. The price should be listed at the end of the advertisement in “a legible manner,” the rule states. It goes on to explain that the price should be presented against a contrasting background in a way that is easy to read.
Health and Human Services Secretary Alex M. Azar II, nodding to an industry proposal announced earlier in the day, said voluntary moves are not enough.
“We will not wait for an industry with so many conflicting and perverse incentives to reform itself,” Azar told the audience gathered at the National Academy of Sciences in Washington.
If approved, the proposed rule has no government enforcement mechanism that would force the companies to comply. Rather, it depends on shaming, noting that federal regulators would post a list of companies violating the rule. It would depend on the private sector to police itself with litigation.
“It is noteworthy that the government is unwilling to take enforcement action,” said Rachel Sachs, an associate professor of law at Washington University, St. Louis, and expert in drug-pricing regulation. The rule might never be finalized, she added.
“It will take many months if not years for this regulation to be implemented and free from the cloud of litigation that will follow it. And the administration knows that,” Ms. Sachs said.
Earlier on Oct. 15, the pharmaceutical industry trade group went on the offensive in anticipation of Mr. Azar’s speech by announcing its own plans.
“Putting list prices in isolation in the advertisements themselves would be misleading or confusing,” argued Stephen J. Ubl, CEO of the Pharmaceutical Researchers and Manufacturers of America, or PhRMA, the major trade group for branded drugs.
Instead, Mr. Ubl, whose trade group represents the largest pharmaceutical manufacturers on the globe, promised that pharma companies would direct consumers to websites that include a drug’s list price and estimates of what people can expect to pay, which can vary widely depending on coverage.
Drug manufacturers would voluntarily opt in to this disclosure starting next spring, he said. Mr. Ubl remained strongly critical of the White House proposal.
The Trump administration’s proposal comes weeks before midterm elections in which health care is a top voter concern. Polling from the Kaiser Family Foundation suggests most voters support forcing price transparency in drug advertisements. (Kaiser Health News is an editorially independent program of the foundation.)
The White House’s plan, which was teased in President Trump’s blueprint this summer, has won praise from insurance groups and the American Medical Association.
Sen. Chuck Grassley (R-Iowa) and Sen. Dick Durbin (D-Ill.) also proposed the plan in the Senate last month, but it failed to garner enough support.
Experts pointed out a host of complications, suggesting that neither PhRMA’s approach nor the White House’s would fully explain to consumers what they’ll actually pay for drugs.
On Oct. 15, Sen. Grassley applauded Mr. Azar’s announcement, saying it was a “common-sense way to lower prices.”
But Dale Cooke, a consultant who works with drug companies trying to meet Food and Drug Administration requirements for advertising, warned there is no reason to believe posting prices would help drive down prices.
“No one has ever explained to me why this would work,” Mr. Cooke said. “What’s the mechanism by which this results in lower drug prices?”
Even more, it could be confusing for patients, Mr. Cooke said. The proposed rule seemed to acknowledge this danger, he said, noting, “On the other hand, consumers, intimidated and confused by high list prices, may be deterred from contacting their physicians about drugs or medical conditions.”
A drug’s list price – the metric HHS wants to emphasize – often bears little relationship to what a patient pays at the drugstore. Insurance plans and pharmacy benefit managers often negotiate cheaper prices than the list price. Some patients qualify for other discounts. And often patients pay only what their copay or deductible requires at any given time.
Other consumers could be stuck paying the full cost, depending on how their insurance plan is designed, or if they don’t have coverage.
“The system is very opaque, very complicated, and importantly, there isn’t a huge relationship between list prices for drugs and what patients will expect to pay out-of-pocket,” said Adrienne Faerber, PhD, a lecturer at the Dartmouth Institute for Health Policy and Clinical Practice who researches drug marketing.
But the industry’s strategy, she said, also appeared lacking.
Under PhRMA’s plan, drugmakers would not standardize how they display their information. Where consumers go could vary on Pfizer’s website versus Merck’s to learn about the list price and the range of out-of-pocket costs. That, Ms. Faerber argued, would make it difficult for people to unearth relevant information.
PhRMA also announced it is partnering with patient advocacy groups to create a “patient affordability platform,” which could help patients search for costs and insurance coverage options.
Mr. Ubl cast PhRMA’s proposal as a way to address more effectively the government and public concern about drug price transparency.
Pharmaceutical manufacturers rely heavily on national advertising and together represent the third-highest spender in national television advertising, according to Michael Leszega, a manager of market intelligence at consulting firm Magna.
At certain times of day, pharmaceutical ads make up more than 40 percent of TV advertisements. And those commercials stand out because they are generally longer, with a long list of side effects and warnings the pharmaceutical industry must tag on at the end.
Those disclaimers highlight another challenge for the administration: legal action.
The rule notes its legal justification was based on the responsibility of the Centers for Medicare & Medicaid Services to ensure the health coverage programs that it administers – Medicare and Medicaid – must be operated in a manner that “minimizes reasonable expenditures.”
Sachs noted that the argument may be weak because most drugs are marketed to a wider audience than Medicare and Medicaid beneficiaries.
A body of Supreme Court decisions dictate how disclaimers and disclosures can be required, said constitutional law expert Robert Corn-Revere. He filed a “friend of the court” brief in a 2011 U.S. Supreme Court case related to commercial speech and the pharmaceutical industry.
Generally, the administration’s requirement must meet the standards of being purely factual, noncontroversial, and not burdensome, Mr. Corn-Revere said.
On the question of whether requiring drug prices be listed in advertising violates the First Amendment’s free-speech guarantee, Corn-Revere said it “all comes down to the specifics.”
Mr. Ubl, when asked earlier about legal action, didn’t rule out the possibility. “We believe there are substantial statutory and constitutional principles that arise” from requiring list-price disclosure, Mr. Ubl said, adding: “We do have concerns about that approach.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Need blood STAT? Call for a drone
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
AT AABB 2018