User login
New kids on the block for migraine treatment and prophylaxis
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
Where do you stand on the Middle East conflict?
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
AI in medicine has a major Cassandra problem
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
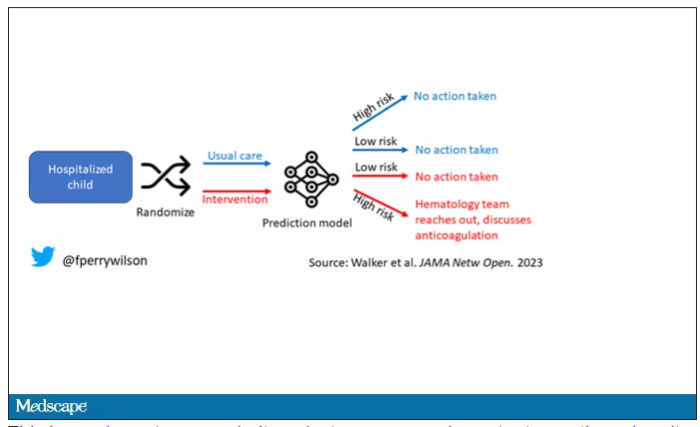
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
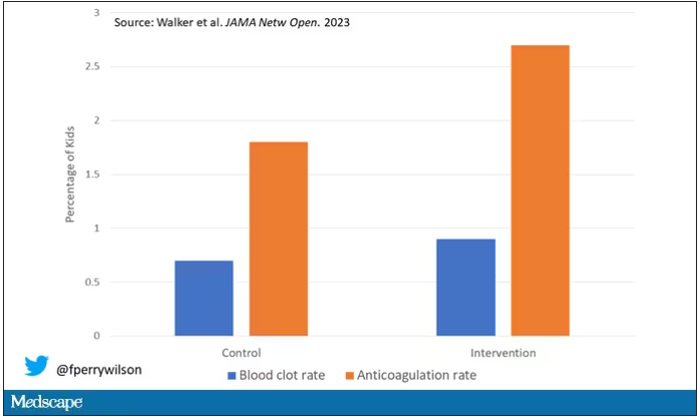
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
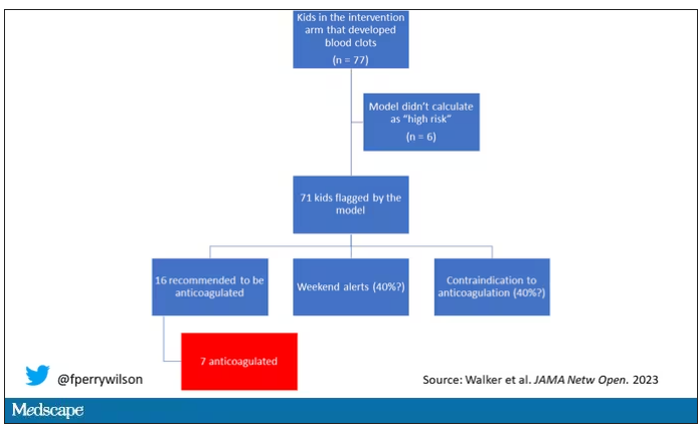
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Debate: Is lasting remission of type 2 diabetes feasible in the real-world setting?
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
Making time to care for patients with diabetes
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Suits or joggers? A doctor’s dress code
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.
In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.
In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.
In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
Trading one’s eggs for a service discount raises tough issues, says ethicist
This transcript has been edited for clarity.
I had a case come to me of a 32-year-old resident who works in a hospital near where I am and was very interested in freezing her eggs. She wasn’t married and was getting worried that maybe she wouldn’t have a partner soon. She was also getting worried that the potential ability of her eggs to be fertilized would begin to decline, which is a phenomenon that does occur with age. She thought, I’m 32; maybe I should freeze my eggs now, as it’s better than to try freezing them when I’m 35 or 37. The potency may be far less.
There are many programs out there now. There have been academic programs for a long time that have been doing egg freezing, and there are many children who have been born successfully. However, it’s also true that people freeze their eggs when they’re 40 years old, and the likelihood of their “working,” if you will, is far less. I wouldn’t say it’s impossible, but age matters. This medical resident knew that and she decided to look into egg freezing.
Well, it turned out that egg freezing is not something that her student insurance plan – or most insurance plans in general – covers. The opportunity to do this is probably going to cost her about $10,000. There are many new egg-freezing infertility programs that have stared up that aren’t part of hospitals. There are clinics that are run for profit. They sometimes encourage women to freeze their eggs.
The student resident quickly found out that there were companies near her who would do egg freezing but would cut a deal if she agreed to take drugs to super-ovulate, make a large number of eggs, and they would be procured if she agreed to give half of them to other women who needed eggs for their infertility treatment. She could keep half and she could get very discounted treatment of egg freezing.
That may be a deal that she’s going to accept. She doesn’t have a path forward. She’s worried about freezing her eggs right now. But there are many ethical considerations that really have to be thought through here.
First and foremost, she’s giving eggs to others. They’re going to use them to try to make children. They can’t make their own eggs, for some reason. She’s going to have some biologically related kids out there. It used to be that you could say to someone who donated sperm or eggs that this will be anonymous.
But in today’s day and age with 23andMe, Ancestry, and better genetic testing, there’s a pretty good likelihood that somebody is going to find out that the person they thought was their biological mom isn’t, and they have someone out there who was the person who, in this case, donated an egg.
Is she willing to risk having that connection, that contact, to have someone enter her life in the future? It’s a situation where she’s donating the eggs, but I’ll tell you that the clinic is going to make far more money using the donated eggs, probably getting $10,000 or $15,000 a cycle with people who are trying to have a child. They’ll make much more money than she’s going to get by donating.
She may get a $5,000 discount, if you will, but the clinic has a business interest. The more they get women involved in bartering their eggs, the more they’re going to profit. In a sense, she’s being coerced, perhaps – I’m going to put it glibly – to sell cheaply. She probably is getting undervalue, even though she needs a path to do this egg freezing.
The other big issue is that we don’t know that egg freezing is going to work for her until someone tries to use those eggs. She may have her own infertility problem not due to age but to other things. Approximately 8%-9% of couples do have infertility problems, sometimes related to gametes. She may never get a partner. Maybe she doesn’t want to use these eggs on her own as a single mom. All of these issues have to be talked through.
What really troubles me here is not so much that someone would choose to barter their eggs, but that they don’t get counseling. They don’t get independent advice about thinking this all through. It’s turning into a business. A business has a commodity – her eggs – that they want. She’s getting more and more desperate, willing to cut a deal to get where she needs to be, but perhaps is not really thinking through all of the ethical dimensions that bartering or trading one’s eggs in order to gain access to freezing entails.
We have to set up a system where there’s independent advice and independent counseling; otherwise, I think we’re closer to exploitation.
A version of this article first appeared on Medscape.com.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use.
This transcript has been edited for clarity.
I had a case come to me of a 32-year-old resident who works in a hospital near where I am and was very interested in freezing her eggs. She wasn’t married and was getting worried that maybe she wouldn’t have a partner soon. She was also getting worried that the potential ability of her eggs to be fertilized would begin to decline, which is a phenomenon that does occur with age. She thought, I’m 32; maybe I should freeze my eggs now, as it’s better than to try freezing them when I’m 35 or 37. The potency may be far less.
There are many programs out there now. There have been academic programs for a long time that have been doing egg freezing, and there are many children who have been born successfully. However, it’s also true that people freeze their eggs when they’re 40 years old, and the likelihood of their “working,” if you will, is far less. I wouldn’t say it’s impossible, but age matters. This medical resident knew that and she decided to look into egg freezing.
Well, it turned out that egg freezing is not something that her student insurance plan – or most insurance plans in general – covers. The opportunity to do this is probably going to cost her about $10,000. There are many new egg-freezing infertility programs that have stared up that aren’t part of hospitals. There are clinics that are run for profit. They sometimes encourage women to freeze their eggs.
The student resident quickly found out that there were companies near her who would do egg freezing but would cut a deal if she agreed to take drugs to super-ovulate, make a large number of eggs, and they would be procured if she agreed to give half of them to other women who needed eggs for their infertility treatment. She could keep half and she could get very discounted treatment of egg freezing.
That may be a deal that she’s going to accept. She doesn’t have a path forward. She’s worried about freezing her eggs right now. But there are many ethical considerations that really have to be thought through here.
First and foremost, she’s giving eggs to others. They’re going to use them to try to make children. They can’t make their own eggs, for some reason. She’s going to have some biologically related kids out there. It used to be that you could say to someone who donated sperm or eggs that this will be anonymous.
But in today’s day and age with 23andMe, Ancestry, and better genetic testing, there’s a pretty good likelihood that somebody is going to find out that the person they thought was their biological mom isn’t, and they have someone out there who was the person who, in this case, donated an egg.
Is she willing to risk having that connection, that contact, to have someone enter her life in the future? It’s a situation where she’s donating the eggs, but I’ll tell you that the clinic is going to make far more money using the donated eggs, probably getting $10,000 or $15,000 a cycle with people who are trying to have a child. They’ll make much more money than she’s going to get by donating.
She may get a $5,000 discount, if you will, but the clinic has a business interest. The more they get women involved in bartering their eggs, the more they’re going to profit. In a sense, she’s being coerced, perhaps – I’m going to put it glibly – to sell cheaply. She probably is getting undervalue, even though she needs a path to do this egg freezing.
The other big issue is that we don’t know that egg freezing is going to work for her until someone tries to use those eggs. She may have her own infertility problem not due to age but to other things. Approximately 8%-9% of couples do have infertility problems, sometimes related to gametes. She may never get a partner. Maybe she doesn’t want to use these eggs on her own as a single mom. All of these issues have to be talked through.
What really troubles me here is not so much that someone would choose to barter their eggs, but that they don’t get counseling. They don’t get independent advice about thinking this all through. It’s turning into a business. A business has a commodity – her eggs – that they want. She’s getting more and more desperate, willing to cut a deal to get where she needs to be, but perhaps is not really thinking through all of the ethical dimensions that bartering or trading one’s eggs in order to gain access to freezing entails.
We have to set up a system where there’s independent advice and independent counseling; otherwise, I think we’re closer to exploitation.
A version of this article first appeared on Medscape.com.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use.
This transcript has been edited for clarity.
I had a case come to me of a 32-year-old resident who works in a hospital near where I am and was very interested in freezing her eggs. She wasn’t married and was getting worried that maybe she wouldn’t have a partner soon. She was also getting worried that the potential ability of her eggs to be fertilized would begin to decline, which is a phenomenon that does occur with age. She thought, I’m 32; maybe I should freeze my eggs now, as it’s better than to try freezing them when I’m 35 or 37. The potency may be far less.
There are many programs out there now. There have been academic programs for a long time that have been doing egg freezing, and there are many children who have been born successfully. However, it’s also true that people freeze their eggs when they’re 40 years old, and the likelihood of their “working,” if you will, is far less. I wouldn’t say it’s impossible, but age matters. This medical resident knew that and she decided to look into egg freezing.
Well, it turned out that egg freezing is not something that her student insurance plan – or most insurance plans in general – covers. The opportunity to do this is probably going to cost her about $10,000. There are many new egg-freezing infertility programs that have stared up that aren’t part of hospitals. There are clinics that are run for profit. They sometimes encourage women to freeze their eggs.
The student resident quickly found out that there were companies near her who would do egg freezing but would cut a deal if she agreed to take drugs to super-ovulate, make a large number of eggs, and they would be procured if she agreed to give half of them to other women who needed eggs for their infertility treatment. She could keep half and she could get very discounted treatment of egg freezing.
That may be a deal that she’s going to accept. She doesn’t have a path forward. She’s worried about freezing her eggs right now. But there are many ethical considerations that really have to be thought through here.
First and foremost, she’s giving eggs to others. They’re going to use them to try to make children. They can’t make their own eggs, for some reason. She’s going to have some biologically related kids out there. It used to be that you could say to someone who donated sperm or eggs that this will be anonymous.
But in today’s day and age with 23andMe, Ancestry, and better genetic testing, there’s a pretty good likelihood that somebody is going to find out that the person they thought was their biological mom isn’t, and they have someone out there who was the person who, in this case, donated an egg.
Is she willing to risk having that connection, that contact, to have someone enter her life in the future? It’s a situation where she’s donating the eggs, but I’ll tell you that the clinic is going to make far more money using the donated eggs, probably getting $10,000 or $15,000 a cycle with people who are trying to have a child. They’ll make much more money than she’s going to get by donating.
She may get a $5,000 discount, if you will, but the clinic has a business interest. The more they get women involved in bartering their eggs, the more they’re going to profit. In a sense, she’s being coerced, perhaps – I’m going to put it glibly – to sell cheaply. She probably is getting undervalue, even though she needs a path to do this egg freezing.
The other big issue is that we don’t know that egg freezing is going to work for her until someone tries to use those eggs. She may have her own infertility problem not due to age but to other things. Approximately 8%-9% of couples do have infertility problems, sometimes related to gametes. She may never get a partner. Maybe she doesn’t want to use these eggs on her own as a single mom. All of these issues have to be talked through.
What really troubles me here is not so much that someone would choose to barter their eggs, but that they don’t get counseling. They don’t get independent advice about thinking this all through. It’s turning into a business. A business has a commodity – her eggs – that they want. She’s getting more and more desperate, willing to cut a deal to get where she needs to be, but perhaps is not really thinking through all of the ethical dimensions that bartering or trading one’s eggs in order to gain access to freezing entails.
We have to set up a system where there’s independent advice and independent counseling; otherwise, I think we’re closer to exploitation.
A version of this article first appeared on Medscape.com.
Dr. Caplan is director, division of medical ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use.
How PCPs are penalized for positive outcomes from lifestyle change
The Centers for Medicare & Medicaid Services 2022 National Quality Strategy is described as an “ambitious long-term initiative that aims to promote the highest quality outcomes and safest care for all individuals.” It is a commendable goal for an overburdened U.S. health care system that spends more than other high-income counties yet experiences poorer outcomes. But whole-person, person-centered care cannot be achieved under current misaligned quality measures that fail to measure what we purport to value: the quintuple aim of improved health outcomes, cost savings, patient satisfaction, clinician well-being, and health equity.
Lifestyle first
Clinical practice guidelines for many chronic diseases recommend lifestyle intervention as the first and optimal treatment. A growing body of evidence supports lifestyle behavior interventions to treat and, when used intensively, even reverse common chronic conditions such as cardiovascular disease, obesity, and type 2 diabetes, while also providing effective prevention for those conditions. However, no current quality measures consider lifestyle interventions. In fact, some quality measures unintentionally penalize physicians for successfully treating or reversing disease through lifestyle behavior interventions while rewarding clinicians for meeting process measures – usually adherence to medication – regardless of whether health outcomes improved.
Rewarding medication adherence for the treatment of diseases in which lifestyle is a primary therapy (such as hypertension), combined with other health care constraints (lack of lifestyle education, time to spend with patients, and infrastructure support) incentivizes physicians to skip the conversation about lifestyle changes and go straight to medication prescription. Meanwhile, the clinician who takes the extra time to guide a patient toward lifestyle interventions that could treat their current disease and prevent future diseases – without side effects – is penalized.
Misaligned quality measures like these can stifle clinical judgment and risk reducing the practice of medicine to mindless box-checking. In many cases, patients are not even informed that lifestyle behavior change may be a treatment option (much less the first recommended option) for their conditions. This delivery of care is not person-centered and, in fact, may raise questions about the adequacy of informed treatment consent.
Reimbursement barriers
Lifestyle medicine is a growing medical specialty that uses therapeutic lifestyle interventions as a primary modality to treat chronic conditions. Since certification began in 2017, almost 2500 US physicians and 1000 nonphysician health professionals have earned certification. Health systems, including the U.S. military, are increasingly integrating lifestyle medicine. There have been advancements since one survey found that more than half of lifestyle medicine clinicians reported receiving no reimbursement for lifestyle behavior interventions. However, barriers, especially in fee-for-service systems, still inhibit many patients from receiving insurance coverage for comprehensive, interdisciplinary, and whole-person treatments called intensive therapeutic lifestyle change (ITLC) programs.
Existing comprehensive lifestyle programs that patients are eligible for (ie, the Diabetes Prevention Program and intensive behavioral therapy) are often so poorly reimbursed that clinicians and health systems decline to offer them. An example of a well-reimbursed ITLC program is intensive cardiac rehabilitation (ICR), which remains underutilized and limited to a narrow segment of patients, despite ICR›s proven benefits for managing comorbid risk factors such as hemoglobin A1c and weight. Even when lifestyle intervention programs are available and patients are eligible to participate (often through shared medical appointments), patient copays for the frequent visits required to achieve and sustain behavior change – or the lack of reimbursement for interdisciplinary team members – discourage engagement.
Penalizing successful outcomes
Despite the fact that lifestyle behaviors are top contributors to health and, conversely, contribute to up to 80% of chronic diseases, few quality measures focus on screening for lifestyle factors or treating diseases with lifestyle interventions. An example of an existing quality measure is screening or treatment for harmful substance use.
Specific quality measures that penalize lifestyle medicine approaches include pharmacotherapy for type 2 diabetes, dyslipidemia, osteoporosis, and gout as well as approaches to rheumatoid arthritis.
Statins offer a useful example of the conundrum faced by clinicians who want to offer lifestyle interventions. A lifestyle medicine primary care physician had a patient covered by Medicare Advantage who was diagnosed with hyperlipidemia. The patient had total cholesterol of 226 and a triglycerides level of 132. Instead of prescribing the routine statin, the physician prescribed lifestyle behavior modifications. Within 3 weeks, the patient›s total cholesterol improved to 171 and triglycerides to 75. This was a great success for the delighted patient. However, the CMS 5-Star Rating System assigned the primary care physician a grade of C rather than A, which put the physician›s 5-star rating at risk. Why? Because the system bases its score largely on medication compliance. The physician was penalized despite achieving the optimal health outcome, and at a lower cost than with medication. This misalignment does not incentivize patient-centered care because it disregards patient preference, shared decision-making, and evidence-based practice.
Risk adjustment
Rather than automatically managing disease with ever-increasing quantities of costly medications and procedures, lifestyle medicine clinicians first pursue a goal of health restoration when appropriate. But Medicare risk adjustment incentivizes physicians to manage rather than reverse disease. How much Medicare pays health plans is determined in part by how sick the patients are; the sicker the patient, the more Medicare pays, because those patients› costs are expected to be higher. This ensures that health plans are not penalized for enrolling sicker patients. But a physician utilizing diet alone to achieve remission in a patient with type 2 diabetes is penalized financially because, when the risk is adjusted, diabetes is no longer listed among the patient›s conditions. So, Medicare pays the physician less money. That misalignment incentivizes clinicians to manage the symptoms of type 2 diabetes rather than achieve remission, despite remission being the ideal clinical outcome.
Realigning quality measures
Quality measures were developed to quantify health care processes and outcomes, and to ensure the delivery of safe care to all patients. However, over time the number of quality measures has swelled to 2500, evolving into a confusing, time-consuming, and even soul-crushing responsibility for the physician.
Instead of relying heavily on process measures, we must incentivize outcome measures that honor patient autonomy and allow clinicians to offer lifestyle intervention as the first line of treatment. Risk-score calculations should be adjusted so that we stop incentivizing disease management and penalizing disease reversal.
CMS’s proposed development of “a universal foundation” of quality measures is an opportunity to begin the realignment of quality measures and values. This foundation is intended to establish more consistent and meaningful measures, reduce clinician burnout by streamlining the reporting process, and advance health equity. For this change to be successful, it is vital that lifestyle behavior interventions – optimal nutrition, physical activity, restorative sleep, social connections, stress management, and avoidance of harmful substances – become the foundation of universal quality measures. This will ensure that every clinician is incentivized to discuss lifestyle behaviors with patients and pursue the first clinical step recommended by clinical practice guidelines for most chronic diseases. Only then can we truly deliver high-value, whole-person, person-centered care and achieve the quintuple aim.
Dr. Patel is president-elect, American College of Lifestyle Medicine; Lifestyle Medicine Medical Director, Wellvana Health, Midland, Tex. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Medicare & Medicaid Services 2022 National Quality Strategy is described as an “ambitious long-term initiative that aims to promote the highest quality outcomes and safest care for all individuals.” It is a commendable goal for an overburdened U.S. health care system that spends more than other high-income counties yet experiences poorer outcomes. But whole-person, person-centered care cannot be achieved under current misaligned quality measures that fail to measure what we purport to value: the quintuple aim of improved health outcomes, cost savings, patient satisfaction, clinician well-being, and health equity.
Lifestyle first
Clinical practice guidelines for many chronic diseases recommend lifestyle intervention as the first and optimal treatment. A growing body of evidence supports lifestyle behavior interventions to treat and, when used intensively, even reverse common chronic conditions such as cardiovascular disease, obesity, and type 2 diabetes, while also providing effective prevention for those conditions. However, no current quality measures consider lifestyle interventions. In fact, some quality measures unintentionally penalize physicians for successfully treating or reversing disease through lifestyle behavior interventions while rewarding clinicians for meeting process measures – usually adherence to medication – regardless of whether health outcomes improved.
Rewarding medication adherence for the treatment of diseases in which lifestyle is a primary therapy (such as hypertension), combined with other health care constraints (lack of lifestyle education, time to spend with patients, and infrastructure support) incentivizes physicians to skip the conversation about lifestyle changes and go straight to medication prescription. Meanwhile, the clinician who takes the extra time to guide a patient toward lifestyle interventions that could treat their current disease and prevent future diseases – without side effects – is penalized.
Misaligned quality measures like these can stifle clinical judgment and risk reducing the practice of medicine to mindless box-checking. In many cases, patients are not even informed that lifestyle behavior change may be a treatment option (much less the first recommended option) for their conditions. This delivery of care is not person-centered and, in fact, may raise questions about the adequacy of informed treatment consent.
Reimbursement barriers
Lifestyle medicine is a growing medical specialty that uses therapeutic lifestyle interventions as a primary modality to treat chronic conditions. Since certification began in 2017, almost 2500 US physicians and 1000 nonphysician health professionals have earned certification. Health systems, including the U.S. military, are increasingly integrating lifestyle medicine. There have been advancements since one survey found that more than half of lifestyle medicine clinicians reported receiving no reimbursement for lifestyle behavior interventions. However, barriers, especially in fee-for-service systems, still inhibit many patients from receiving insurance coverage for comprehensive, interdisciplinary, and whole-person treatments called intensive therapeutic lifestyle change (ITLC) programs.
Existing comprehensive lifestyle programs that patients are eligible for (ie, the Diabetes Prevention Program and intensive behavioral therapy) are often so poorly reimbursed that clinicians and health systems decline to offer them. An example of a well-reimbursed ITLC program is intensive cardiac rehabilitation (ICR), which remains underutilized and limited to a narrow segment of patients, despite ICR›s proven benefits for managing comorbid risk factors such as hemoglobin A1c and weight. Even when lifestyle intervention programs are available and patients are eligible to participate (often through shared medical appointments), patient copays for the frequent visits required to achieve and sustain behavior change – or the lack of reimbursement for interdisciplinary team members – discourage engagement.
Penalizing successful outcomes
Despite the fact that lifestyle behaviors are top contributors to health and, conversely, contribute to up to 80% of chronic diseases, few quality measures focus on screening for lifestyle factors or treating diseases with lifestyle interventions. An example of an existing quality measure is screening or treatment for harmful substance use.
Specific quality measures that penalize lifestyle medicine approaches include pharmacotherapy for type 2 diabetes, dyslipidemia, osteoporosis, and gout as well as approaches to rheumatoid arthritis.
Statins offer a useful example of the conundrum faced by clinicians who want to offer lifestyle interventions. A lifestyle medicine primary care physician had a patient covered by Medicare Advantage who was diagnosed with hyperlipidemia. The patient had total cholesterol of 226 and a triglycerides level of 132. Instead of prescribing the routine statin, the physician prescribed lifestyle behavior modifications. Within 3 weeks, the patient›s total cholesterol improved to 171 and triglycerides to 75. This was a great success for the delighted patient. However, the CMS 5-Star Rating System assigned the primary care physician a grade of C rather than A, which put the physician›s 5-star rating at risk. Why? Because the system bases its score largely on medication compliance. The physician was penalized despite achieving the optimal health outcome, and at a lower cost than with medication. This misalignment does not incentivize patient-centered care because it disregards patient preference, shared decision-making, and evidence-based practice.
Risk adjustment
Rather than automatically managing disease with ever-increasing quantities of costly medications and procedures, lifestyle medicine clinicians first pursue a goal of health restoration when appropriate. But Medicare risk adjustment incentivizes physicians to manage rather than reverse disease. How much Medicare pays health plans is determined in part by how sick the patients are; the sicker the patient, the more Medicare pays, because those patients› costs are expected to be higher. This ensures that health plans are not penalized for enrolling sicker patients. But a physician utilizing diet alone to achieve remission in a patient with type 2 diabetes is penalized financially because, when the risk is adjusted, diabetes is no longer listed among the patient›s conditions. So, Medicare pays the physician less money. That misalignment incentivizes clinicians to manage the symptoms of type 2 diabetes rather than achieve remission, despite remission being the ideal clinical outcome.
Realigning quality measures
Quality measures were developed to quantify health care processes and outcomes, and to ensure the delivery of safe care to all patients. However, over time the number of quality measures has swelled to 2500, evolving into a confusing, time-consuming, and even soul-crushing responsibility for the physician.
Instead of relying heavily on process measures, we must incentivize outcome measures that honor patient autonomy and allow clinicians to offer lifestyle intervention as the first line of treatment. Risk-score calculations should be adjusted so that we stop incentivizing disease management and penalizing disease reversal.
CMS’s proposed development of “a universal foundation” of quality measures is an opportunity to begin the realignment of quality measures and values. This foundation is intended to establish more consistent and meaningful measures, reduce clinician burnout by streamlining the reporting process, and advance health equity. For this change to be successful, it is vital that lifestyle behavior interventions – optimal nutrition, physical activity, restorative sleep, social connections, stress management, and avoidance of harmful substances – become the foundation of universal quality measures. This will ensure that every clinician is incentivized to discuss lifestyle behaviors with patients and pursue the first clinical step recommended by clinical practice guidelines for most chronic diseases. Only then can we truly deliver high-value, whole-person, person-centered care and achieve the quintuple aim.
Dr. Patel is president-elect, American College of Lifestyle Medicine; Lifestyle Medicine Medical Director, Wellvana Health, Midland, Tex. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Medicare & Medicaid Services 2022 National Quality Strategy is described as an “ambitious long-term initiative that aims to promote the highest quality outcomes and safest care for all individuals.” It is a commendable goal for an overburdened U.S. health care system that spends more than other high-income counties yet experiences poorer outcomes. But whole-person, person-centered care cannot be achieved under current misaligned quality measures that fail to measure what we purport to value: the quintuple aim of improved health outcomes, cost savings, patient satisfaction, clinician well-being, and health equity.
Lifestyle first
Clinical practice guidelines for many chronic diseases recommend lifestyle intervention as the first and optimal treatment. A growing body of evidence supports lifestyle behavior interventions to treat and, when used intensively, even reverse common chronic conditions such as cardiovascular disease, obesity, and type 2 diabetes, while also providing effective prevention for those conditions. However, no current quality measures consider lifestyle interventions. In fact, some quality measures unintentionally penalize physicians for successfully treating or reversing disease through lifestyle behavior interventions while rewarding clinicians for meeting process measures – usually adherence to medication – regardless of whether health outcomes improved.
Rewarding medication adherence for the treatment of diseases in which lifestyle is a primary therapy (such as hypertension), combined with other health care constraints (lack of lifestyle education, time to spend with patients, and infrastructure support) incentivizes physicians to skip the conversation about lifestyle changes and go straight to medication prescription. Meanwhile, the clinician who takes the extra time to guide a patient toward lifestyle interventions that could treat their current disease and prevent future diseases – without side effects – is penalized.
Misaligned quality measures like these can stifle clinical judgment and risk reducing the practice of medicine to mindless box-checking. In many cases, patients are not even informed that lifestyle behavior change may be a treatment option (much less the first recommended option) for their conditions. This delivery of care is not person-centered and, in fact, may raise questions about the adequacy of informed treatment consent.
Reimbursement barriers
Lifestyle medicine is a growing medical specialty that uses therapeutic lifestyle interventions as a primary modality to treat chronic conditions. Since certification began in 2017, almost 2500 US physicians and 1000 nonphysician health professionals have earned certification. Health systems, including the U.S. military, are increasingly integrating lifestyle medicine. There have been advancements since one survey found that more than half of lifestyle medicine clinicians reported receiving no reimbursement for lifestyle behavior interventions. However, barriers, especially in fee-for-service systems, still inhibit many patients from receiving insurance coverage for comprehensive, interdisciplinary, and whole-person treatments called intensive therapeutic lifestyle change (ITLC) programs.
Existing comprehensive lifestyle programs that patients are eligible for (ie, the Diabetes Prevention Program and intensive behavioral therapy) are often so poorly reimbursed that clinicians and health systems decline to offer them. An example of a well-reimbursed ITLC program is intensive cardiac rehabilitation (ICR), which remains underutilized and limited to a narrow segment of patients, despite ICR›s proven benefits for managing comorbid risk factors such as hemoglobin A1c and weight. Even when lifestyle intervention programs are available and patients are eligible to participate (often through shared medical appointments), patient copays for the frequent visits required to achieve and sustain behavior change – or the lack of reimbursement for interdisciplinary team members – discourage engagement.
Penalizing successful outcomes
Despite the fact that lifestyle behaviors are top contributors to health and, conversely, contribute to up to 80% of chronic diseases, few quality measures focus on screening for lifestyle factors or treating diseases with lifestyle interventions. An example of an existing quality measure is screening or treatment for harmful substance use.
Specific quality measures that penalize lifestyle medicine approaches include pharmacotherapy for type 2 diabetes, dyslipidemia, osteoporosis, and gout as well as approaches to rheumatoid arthritis.
Statins offer a useful example of the conundrum faced by clinicians who want to offer lifestyle interventions. A lifestyle medicine primary care physician had a patient covered by Medicare Advantage who was diagnosed with hyperlipidemia. The patient had total cholesterol of 226 and a triglycerides level of 132. Instead of prescribing the routine statin, the physician prescribed lifestyle behavior modifications. Within 3 weeks, the patient›s total cholesterol improved to 171 and triglycerides to 75. This was a great success for the delighted patient. However, the CMS 5-Star Rating System assigned the primary care physician a grade of C rather than A, which put the physician›s 5-star rating at risk. Why? Because the system bases its score largely on medication compliance. The physician was penalized despite achieving the optimal health outcome, and at a lower cost than with medication. This misalignment does not incentivize patient-centered care because it disregards patient preference, shared decision-making, and evidence-based practice.
Risk adjustment
Rather than automatically managing disease with ever-increasing quantities of costly medications and procedures, lifestyle medicine clinicians first pursue a goal of health restoration when appropriate. But Medicare risk adjustment incentivizes physicians to manage rather than reverse disease. How much Medicare pays health plans is determined in part by how sick the patients are; the sicker the patient, the more Medicare pays, because those patients› costs are expected to be higher. This ensures that health plans are not penalized for enrolling sicker patients. But a physician utilizing diet alone to achieve remission in a patient with type 2 diabetes is penalized financially because, when the risk is adjusted, diabetes is no longer listed among the patient›s conditions. So, Medicare pays the physician less money. That misalignment incentivizes clinicians to manage the symptoms of type 2 diabetes rather than achieve remission, despite remission being the ideal clinical outcome.
Realigning quality measures
Quality measures were developed to quantify health care processes and outcomes, and to ensure the delivery of safe care to all patients. However, over time the number of quality measures has swelled to 2500, evolving into a confusing, time-consuming, and even soul-crushing responsibility for the physician.
Instead of relying heavily on process measures, we must incentivize outcome measures that honor patient autonomy and allow clinicians to offer lifestyle intervention as the first line of treatment. Risk-score calculations should be adjusted so that we stop incentivizing disease management and penalizing disease reversal.
CMS’s proposed development of “a universal foundation” of quality measures is an opportunity to begin the realignment of quality measures and values. This foundation is intended to establish more consistent and meaningful measures, reduce clinician burnout by streamlining the reporting process, and advance health equity. For this change to be successful, it is vital that lifestyle behavior interventions – optimal nutrition, physical activity, restorative sleep, social connections, stress management, and avoidance of harmful substances – become the foundation of universal quality measures. This will ensure that every clinician is incentivized to discuss lifestyle behaviors with patients and pursue the first clinical step recommended by clinical practice guidelines for most chronic diseases. Only then can we truly deliver high-value, whole-person, person-centered care and achieve the quintuple aim.
Dr. Patel is president-elect, American College of Lifestyle Medicine; Lifestyle Medicine Medical Director, Wellvana Health, Midland, Tex. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Male patients with breast cancer: Special considerations and gender-specific concerns
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Fatima Cardoso, MD: Today we will be discussing breast cancer in male patients. To join me in this discussion, I have Sharon Giordano and Oliver Bogler. I will ask, to start, that we briefly introduce ourselves.
I’m Fatima Cardoso. I’m a medical oncologist based in Lisbon, Portugal. I have had a special interest in this topic for a couple of years. Sharon?
Sharon H. Giordano, MD, MPH, FASCO: I’m Sharon Giordano. I practice at the University of Texas MD Anderson Cancer Center. I’m also a medical oncologist and treat most of the male breast cancer patients that are seen here.
Oliver Bogler, PhD: I’m Oliver Bogler. I’m a cancer biologist by background and an 11-year survivor of breast cancer. Dr. Giordano was my oncologist during the active phase of my treatment. It’s great to be here with you.
Special considerations surrounding male patients
- Dr. Cardoso: Sharon, when you are treating breast cancer in a male patient, what specific considerations do you have?
- Dr. Giordano: As we all know, breast cancer in men is a rare disease. It makes up about 1 in 1,000 cases of breast cancer.
Often, what we need to do and what we end up doing is extrapolating as much as possible from clinical trials that were conducted in female patients with breast cancer. I think that’s one of the major challenges we face in treating the disease. There have been international efforts to try to put together standardized treatment approaches.
For example, ASCO has created guidelines for the management of male breast cancer. NCCN also has a special page on considerations for treatment of men with breast cancer. I would encourage people to look at those resources if questions do come up on the topic.
Dr. Cardoso: Perhaps we can also mention that the latest clinical trials fortunately have been allowing for male patients to be included, which is very important so that we can start having some data on the new drugs. I think that’s also relevant.
Dr. Giordano: That’s a great point because, historically, most of the trials explicitly excluded men. I don’t know if it was intentional or they just wrote the trials saying “women with breast cancer,” because that’s what most people thought of. I think it’s a great effort by the FDA and by investigators to make sure now that men are included in the trials. That will help build our evidence base.
Dr. Cardoso: Oliver, 11 years ago, you faced the diagnosis and you went through this. Can you speak a little bit about this challenge of going through what is considered a rare disease, but also a disease that is very much associated with the female gender traditionally?
Dr. Bogler: Gladly. For me, it was particularly odd because my wife, at the time that I was diagnosed, was a 5-year survivor of breast cancer. It took me some time to even think that the lump I felt might be the same disease. That seemed very unlikely, statistically, and also odd.
I have to say that I was protected from much of the fish-out-of-water experience that many men have because I both worked and was treated at MD Anderson, where Dr Giordano has a large practice, so my colleagues and my friends were not surprised that a man could get this disease.
Many of the patients I met had that experience, difficulty convincing their primary care physician or even their first-line oncologist that this could be the case. I just want to connect to what you both said, which is that 10 years ago, inclusion of men in clinical trials was not standard. It is a fantastic development to see that because unless we include men, we won’t learn about that type of breast cancer.
Dr. Cardoso: Even if only a few are entering each trial, at least it allows us to see if the drug behaves the same way or if there is any strange behavior of the drug in a male patient. It’s already one step forward. You were going to mention something, Sharon?
Dr. Giordano: I was going to say that, anecdotally, I’ve heard the experience that Oliver referred to, of many men feeling not so much uncomfortable with the diagnosis – although that does happen – but not having an obvious fit within the health care system.
For example, going to get their mammogram as part of their diagnostic workup and whoever might be taking them back saying, “Oh, no, this is Mrs. Jones, not Mr.,” and trying to argue with them that it’s not really meant for them. I had a patient – and this guy had a great sense of humor – who had a biopsy done and the instructions were to place this pink, floral ice pack inside your bra.
Even the materials that we have are gender specific. I think those things all together can certainly contribute to a man feeling like a fish out of water.
Dr. Cardoso: Actually, I fought in my institution because they wanted to call the Breast and Gynecology Unit the Women’s Unit. I said that there is no way you can call it the Women’s Unit because we have male patients. There are small things that we can do in our institutions to try to decrease the stigma and to make it less awkward for a man to be in a waiting room that says Women’s Clinic or something similar to that.
The importance of a support system
Dr. Cardoso: I wanted Oliver, perhaps, to mention experiences that you may have heard from other men. Some men do not feel that comfortable speaking about the disease. Also, some of them do not feel comfortable after treatment to go to the beach, to show the scar, and to show what happens after you have radiation.
Some men actually take it quite heavily, psychologically speaking. Have you encountered some of these men?
Dr. Bogler: Definitely. I think it leads to men not accessing the support opportunities – their family, their friends, or the support groups – and staying away from those because of this feeling of not wanting to share about it. That can be damaging. Cancer treatment is usually a tough road for most people, and the long-term consequences of hormone therapy – most men have hormone-driven disease – can be significant. I agree with you.
I participated in the male breast cancer SCAR Project by David Jay, a famous photographer. One of the high points of my life has been appearing in The New York Times topless, right after my radiation treatment, showing my scar. There are quite a few of us out there who’ve done that.
I’ll just mention in passing the Male Breast Cancer Global Alliance, which is a patient support supergroup, if you will. We’ve got a symposium coming up in November. That’s a great place for men who are early in the stage of their disease, or at any stage, to connect to others who are facing this issue.
Dr. Cardoso: They can also find specific information. This is a really good website where you can find information. One of the most important topics that I’ve heard from my patients is, “I never thought that I could have this disease. I never heard that men could have breast cancer as well.” Information is very crucial.
I believe that if you are well informed, you will also be less scared of the disease. Sources of reliable information are really crucial for patients. Since you mentioned the SCAR Project, we have a similar project here in Portugal that really called attention to the disease. It was very visual and really interesting.
Discussions during and after treatment
Dr. Cardoso: I wanted to say something, and I don’t know if both of you would agree. I think only recently surgeons have started to pay attention to the way they operate on men with breast cancer, and even in considering techniques of breast conservation and oncoplastic surgery. I had the feeling, looking at those photos, that some years ago, it wouldn’t have mattered how they do with the mastectomy scar just because it was a man. This was biased, right?
Just because it was a man, there was no need to pay attention to the aesthetic outcome. That is wrong, in my perspective. I’m very happy to see that now there are surgeons considering other types of breast surgery to conserve as much as possible the aesthetic outcome.
Dr. Bogler: I have to say that I was offered reconstruction at MD Anderson. I declined it. It wasn’t that big a part of my body image. When I raised this issue at home, my kids, who were quite young at the time, just suggested, “Well, Dad, why don’t you just wear a swim shirt?” They came up with a very practical solution for this issue.
I agree with you that it should be an option. I was also offered a nipple tattoo. I have yet to take that up, but maybe one day.
Dr. Cardoso: I’m not sure that we need to go into reconstruction. It also depends on whether a man has gynecomastia, if it’s going to be very asymmetric. There are other techniques to do, and depending on the size of the tumor, we can also do breast conservation, which we have done here in a couple of patients.
It’s quite an interesting approach where, for example, a skin-sparing mastectomy would be less aggressive, let’s say. Sharon?
Dr. Giordano: I completely agree. I’ve noticed increasing attention to the issue over the years that I’ve been in practice. I do think that it’s more front-and-center when the surgeons are having discussions with the patients now.
Also, although it’s still a minority, some do choose to have reconstructive surgery; some have more extensive surgeries, and some maybe have nipple reconstruction or a nipple tattoo. In a few men, like you mentioned, who are somewhat asymmetric, it actually can make a difference even when they’re dressed.
For many men, it’s more that they want to take off their shirt to play basketball or go swimming, and to decrease the feeling of awkwardness or like they have to make an explanation for why they have a nipple missing and a scar across their chest.
Biological aspects of male patients
Dr. Cardoso: Let’s switch gears now to the management, and before that, the biology. Oliver, with your other hat of biology, speak a little bit on what we know so far – whether it is exactly the same disease or there are biological specific characteristics of breast cancer in men.
Dr. Bogler: I should preface this by saying that I spent my career studying brain tumors. That was clearly a mistake.
Dr. Cardoso: It starts with a B. ...
Dr. Bogler: It starts with a B, but it’s the wrong part of the body. The reality is that we don’t really know that much fundamental biology yet, though the picture is changing and it has changed in recent years. Part of the reason is we don’t have many of the tools that we’ve had for the female disease for many years, particularly laboratory models.
On the genetic and transcriptomics front, there has been some really good activity. There was a comprehensive systematic review by Professor Val Speirs from the University of Aberdeen earlier this year that summarized much of the recent data. It showed that there are a handful of molecular hallmarks of the male disease, compared with the female disease, that are worth exploring.
Interestingly enough, one of them is the androgen receptor. It does beg the question of whether hormone-driven disease might not show up quite differently in males and females, where the hormone picture is a little different. I think there’s increasing evidence that there’s information out there to go after.
I will say that I was treated by Dr. Giordano and her colleagues very much like a woman would have been with my disease, and actually, very similarly to my wife. I’ve done well with it, so I would say, in most cases, the current standard of care is very effective but it falls a little short of personalized medicine, particularly when it comes to the hormone component.
Dr. Cardoso: Sharon?
Dr. Giordano: I would add that when I think about it as a clinician, although there’s a large amount of overlap and many similarities, when we’re treating men with breast cancer, almost all of the men have hormone receptor–positive disease, which I think Oliver mentioned earlier. We’re really thinking about endocrine therapy as one of the mainstays of treatment.
Obviously, as he also mentioned, it’s a different biologic background of hormones in a male vs. a female patient. There’s reason to think that some of those treatments could differ. In general, the subtypes are a little bit different. We see very, very few cases of triple-negative breast cancer in men. I think I’ve seen only one or two in my career. The ones I remember were probably radiation induced. They were cancer survivors who’d had chest-wall radiation for previous diseases. Those patients are very uncommon.
We also tend to see that the histology patterns are a little bit different. We tend to see more ductal cancers in men than we do in women as a relative proportion.
One thing that I always try to remember is that the risk for BRCA mutations or underlying germline genetic mutations is higher in men than in women. Just having a diagnosis of male breast cancer is an indication to consider genetic testing or meet with a genetic counselor to look for a BRCA1 or BRCA2 mutation.
Now, most men will not have that. Only roughly 10% of male patients, or maybe a little less, will have a BRCA2 mutation; for BRCA1, it’s more like only 1% or 2%. They’re not that common. Certainly, male breast cancer is recognized as being associated with the BRCA mutations.
Dr. Cardoso: If I have to give a take-home message in terms of biology, it would be that if there is a diagnosis of hormone receptor–negative or HER2-positive disease in a male patient, I would ask for a confirmation of the diagnosis. It’s not that it cannot exist, but it’s so rare that it’s worthwhile to confirm.
You mentioned that triple-negative disease is less than 1%, at about 0.5%, and HER2-positive disease is about 9%-10%. I think it will be very important to keep this in mind and confirm the biology if you have a different diagnosis than ER-positive, HER2-negative. Unfortunately, I received some cases where this was not done, and in fact, it ended up being a technical problem. People can receive the wrong treatment based on that.
Dr. Giordano: I’ve also seen that happen when it’s a metastasis to the breast rather than a primary breast cancer. I completely agree. That’s an excellent point.
Management approaches
Dr. Cardoso: Let’s go now to management and focus on early breast cancer first. Sharon, what are your main take-home messages for a professional who doesn’t see this very often? What does someone need to remember when they manage a male patient who has early breast cancer?
Dr. Giordano: In general, in terms of chemotherapy, we essentially use the same guidelines as we do for women. Most of the male patients will have tumors that are hormone receptor positive. For endocrine therapy, we typically rely on tamoxifen as the standard of care for adjuvant endocrine treatment for breast cancer.
There are some data suggesting that there can be some efficacy of aromatase inhibitors as single agents. In general, and extrapolated from some population-based registry data, the outcomes for men treated with single-agent aromatase inhibitors don’t tend to be as good as for those treated with tamoxifen.
I know that these are not randomized data so there are all the caveats of that, but the best information we have suggests that tamoxifen appears to likely be more effective. Typically, we stay with tamoxifen. If, for some reason, a man cannot tolerate tamoxifen or has a contraindication, then we could use a GnRH agonist along with an aromatase inhibitor.
Dr. Cardoso: I would like to mention that, because it’s ER-positive, HER2-negative disease most of the time, there will be the question as to whether we can use genomic tests. I think it is important that people know that we have much less data regarding the use of Oncotype DX, MammaPrint, or any of the genomic tests in male patients.
We have some data on the distribution of, for example, Oncotype DX or MammaPrint scores. Whether we can use these tests for the decision of chemotherapy, we don’t have much data on that. I’ve seen many people making exactly the same decisions as with female patients, but that’s not really based on very strong evidence.
Dr. Giordano: It’s hard to know what to do with that. There are prognostic data on Oncotype, so the higher-risk tumors do seem to have a worse outcome than the lower-score tumors. You’re right, though; I don’t think we have any predictive information to really show that the Oncotype DX score predicts benefit to chemotherapy.
Having said that, I will sometimes order the test in my practice. If somebody comes back with a score of 5 or a very low-risk score, I will use that in my decision-making.
Dr. Cardoso: There is something we didn’t exactly mention in the diagnosis that may be important. We discussed most men not knowing that they can have breast cancer, and Oliver, you mentioned that sometimes the first-line physicians can think that very often. Usually, we have late diagnosis and that means a higher tumor burden.
Sometimes we have to go to chemotherapy because of locally advanced or very positive axillas and not really because of the biology. That’s one of the reasons to go for chemotherapy in this setting, right?
Dr. Bogler: Yes. I remember that conversation with you, Dr. Giordano. I asked you whether I should do one of these tests. You said, “Don’t worry about it. At stage III, you’re going to have chemo anyway.”
Dr. Cardoso: The problem of these rare diagnoses is the not thinking about it, even from the health professional side, and then having the diagnosis quite late that will demand chemotherapy use.
To clarify to everybody, in terms of distinguishing luminal A–like, luminal B–like, and what that implies in a male patient, we really don’t know if it’s the same as in a female. There have been some very interesting studies from our Nordic country colleagues showing that maybe the subtyping is different. There is likely a male-specific subtype that does not exist in female breast cancer and that probably behaves differently. We still have a large amount of research to do to understand that.
Is there anything else you would like to mention about early breast cancer management?
Dr. Bogler: One of the things that’s probably underexplored is adherence to tamoxifen therapy in men. I do know anecdotally that this is the discussion among men because of the impact on quality of life. I do worry that sometimes men perhaps make the wrong choice, and I think that’s an opportunity for more research. Again, if there were alternative therapies that were perhaps a little less impactful on things like libido, that might be an advance in the field.
Dr. Cardoso: We have been seeing more studies on the issue of quality of life. Noncompliance is also an issue in female patients. We have to acknowledge that. Not everybody is able to keep taking the treatments. Interestingly, when there is a relapse and people had stopped taking the tamoxifen, most of them say, “I stopped because I had not understood exactly how important it is.”
We come back to the importance of explaining that it is the most crucial treatment for this subtype of breast cancer. Again, information is really key.
Sometimes I also use the argument with my patients that the alternative is even worse because if you use an aromatase inhibitor, and you have to use an LHRH agonist, then the implications for your sexual life are even worse. That’s how I try to convince them to stay on tamoxifen.
Let’s finalize with a couple of words on metastatic breast cancer in male patients. Sharon, I’ll start with you again. Is there any difference in the management if you have a patient with metastatic, ER-positive, HER2-negative disease? How do you treat? How do you sequence the available therapies? Is it different from the female patient?
Dr. Giordano: I’d say that, big picture, it’s quite similar. Again, most of the men have hormone receptor–positive disease, so really, the mainstay of treatment and the first treatments are going to be endocrine therapies. We’ll sequence through the endocrine therapies like we do in women. When using aromatase inhibitors, I typically would add a GnRH agonist to that, and I have had that be a very successful therapy, along now with the CDK inhibitors that are also approved.
I don’t think the studies of CDK inhibitors included male patients, but at least palbociclib actually was approved in the United States, based on some real-world evidence of its efficacy. Anecdotally, again, in my clinical practice, that tends to be a really powerful combination of leuprolide, an aromatase inhibitor, and a CDK inhibitor.
I think there’s less information about drugs like fulvestrant, whether that would benefit from combination with a GnRH agonist or whether those should be given as single agents. We just don’t really know. We have a few case series out there.
Similar to the early breast cancer setting, I think it’s really important to remember to check for BRCA1 and BRCA2 mutations. PARP inhibitors could be a part of the treatment plan if those underlying germline mutations are found. Generally, we’re following a similar sequence of endocrine therapies and then, eventually, chemotherapy.
Dr. Cardoso: Maybe, Oliver, you’re also seeing that one consistent finding in the biology study is the importance of the AKT/PI3K/mTOR pathway in male patients with breast cancer, because we now have at least two classes of agents to tackle this pathway. Again, anecdotally – we’re not talking about trials – I’ve been seeing quite interesting responses, for example, to everolimus combined with endocrine therapy.
We have a little less experience with the PI3K inhibitor, but that’s just because of accessibility to the drug. I think this combination is also something to keep in mind that can be quite effective in these patients.
Dr. Bogler: I agree. Those findings are exciting in the context of dealing with something as difficult as metastatic breast cancer. It’s good to know that there’s some information coming and opportunities and options, hopefully, down the road for men facing that problem.
Dr. Cardoso: Sharon, although small numbers, in these cases where there is HER2-positive disease, you would also use the new anti-HER2 agents and more or less the same sequence, right?
Dr. Giordano: Absolutely. It’s not particularly data driven, but yes, I would. If it’s a HER2-positive tumor, I would use the same HER2-targeted therapies that are used for women with breast cancer.
Working toward a balance in patient care
Dr. Cardoso: I would like to add something for all of us to be united in the fight. I don’t know if it happens in the U.S., but in many countries, access to these new agents for male patients is very difficult because of the approval and the labeling. This is why I’m always fighting with those who are proposing that the labeling, again, says “women with breast cancer.”
It is really important that we keep on lobbying and pushing for the labeling to say “patients with breast cancer” so that nobody can withhold access to these new therapies because of gender. In the U.S., maybe you don’t have this problem. There are many European countries where men cannot access, for example, fulvestrant because it has been approved for women with breast cancer.
Dr. Giordano: Thankfully, I have not faced that issue very often. I’ve had occasional issues with getting GnRH agonists approved. Generally, in the U.S., if I provide, for example, the NCCN guideline recommendations, most insurers will cover it. I think it’s often just lack of knowledge.
Dr. Cardoso: It’s something to keep working hard on because for the old drugs that were approved with the wording that still said “women,” we have to keep fighting for accessibility.
I think we had a really nice discussion. I’m going to give you an opportunity for any last words that you want to say on this topic. Perhaps we’ll start with you, Sharon, and we’ll leave the very last word to Oliver.
Dr. Giordano: I would just emphasize the importance of doing research in this area. Hopefully, we will be able to get clinical trials. There are reasons to think that endocrine therapies may behave differently in men and women. We need to continue to work together as a community to collect the data so that we can ultimately improve the outcomes for our patients.
Dr. Bogler: I would echo what you just said, Dr. Giordano. I would like to express my gratitude to both of you. Dr. Giordano, you have a huge practice of men at MD Anderson. You took care of me and many other people I know.
Dr. Cardoso, you are a pioneer of a big registry trial that I am privileged to be working on, trying to gather data on men. You’re both pioneers in this field of working on behalf of people like me. I’m just very grateful for what you do.
Dr. Giordano: Thank you.
Dr. Cardoso: Thank you both for accepting this invitation. We hope that everybody takes more interest in this field. Who knows? Maybe we can find enough funds to run a specific trial for male patients with breast cancer.
Dr. Cardoso is director of the breast unit at Champalimaud Clinical Centre/Champalimaud Foundation, Lisbon. Dr. Giordano is professor of breast medical oncology and chair of health services research at the University of Texas MD Anderson Cancer Center, Houston. Dr. Bogler is a cancer biologist at the Randolph (Vt.) Center. Dr. Cardoso reported conflicts of interest with numerous pharmaceutical companies; Dr. Giordano and Dr. Bogler reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A note from NORD
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer