User login
Editor’s note
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
Home oxygen therapy: What does the data show?
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Atopic dermatitis: Five things to know
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin condition that typically affects the face (cheeks), neck, arms, and legs but usually spares the groin and axillary regions. AD usually starts in early infancy but also affects some adults. AD is often associated with elevated levels of immunoglobulin E (IgE). That it is the first disease to present in a series of allergic diseases – including food allergy, asthma, and allergic rhinitis, in order – and has given rise to the “atopic march” theory, which suggests that AD is part of a progression that may lead to subsequent allergic disease at other epithelial barrier surfaces.
.
1. Essential features of AD are pruritus and eczema
The diagnosis of AD is primarily observational. It is made on the basis of patient and family history, pattern of lesions, morphology, and clinical signs. No genetic features or biomarkers are specific enough to reliably aid in diagnosis or severity assessment. Many individual findings are used to diagnose AD, as summarized by the American Academy of Dermatology based on essential, important, associated, and exclusionary features:
- Essential features (must be present for diagnosis) are pruritus and eczema (acute, subacute, or chronic) with typical morphology and age-specific patterns and chronic or relapsing history.
- Important features (usually seen in AD and support the diagnosis) are early age of onset, atopy (personal/family history, IgE reactivity), and xerosis.
- Associated features (nonspecific but suggestive) are atypical vascular response (e.g., delayed blanch response); keratosis pilaris (and some others); ocular/periorbital changes; other regional findings (e.g., perioral changes); and perifollicular accentuation, lichenification, or prurigo lesions.
- Exclusionary conditions (must be excluded to make the AD diagnosis) are scabies, seborrheic dermatitis, contact dermatitis, ichthyoses, cutaneous T-cell lymphoma, psoriasis, photosensitivity dermatoses, immune deficiency diseases, and erythroderma due to other causes.
AD should be differentiated from other red, scaly skin conditions. It is often difficult to separate AD from seborrheic dermatitis in infancy, and the two conditions may overlap in this age group. Particularly if the condition is not responding to therapy, the diagnosis of AD should be re-reviewed and other disorders considered, including more serious nutritional, metabolic, and immunologic conditions in children and cutaneous T-cell lymphoma in adults. Allergic contact dermatitis may be both an alternative diagnosis to AD and an exacerbator of AD in some individuals.
2. Associated comorbidities of AD may exacerbate the condition and lead to other atopic disorders
Reported comorbidities of AD include other atopic or allergic conditions, autoimmune diseases, infections, metabolic conditions, mental health disorders, and cardiovascular disease. Certain aspects of AD, such as chronic pruritus, psychosocial distress, and inflammation, can lead to anxiety, depression, and suicidality. AD is associated with and may predispose to higher risk for other atopic disorders, including asthma, hay fever, food allergy, and eosinophilic esophagitis.
Persons with AD also appear to be at higher risk for infectious diseases. The prevalence of cutaneous and systemic infections in patients with AD is significantly higher than those without AD. Infectious complications can include skin and soft-tissue infections, bacteremia, eczema herpeticum, osteomyelitis, endocarditis, and septic arthritis.
3. Climate change has a profound impact on AD
The incidence of AD has increased over the past several decades, and environmental factors such as climate change have been implicated as a potential mechanism. Climate change–related factors affect the skin’s capacity to maintain homeostasis, leading to various cutaneous diseases. AD, psoriasis, pemphigus, acne vulgaris, melasma, and photoaging are all associated with rising levels of air pollution. Elevated temperatures due to global warming induce disruption of the skin microbiome, thereby affecting AD.
Extreme weather events due to climate change, including floods and wildfires, are implicated in cutaneous injuries, skin infections, and acute worsening of inflammatory skin disorders.
4. The impact and appearance of AD varies in different racial groups
It was once believed that AD was just one single disease affecting people of many different races. More recently, it has been proposed that AD is in fact a group of different diseases. Both epidemiologic and genetic factors may play a role in influencing the main features of AD.
Spongiotic processes such as AD that would be pink or erythematous on white skin are often hypopigmented in individuals with darkly pigmented skin. AD has a higher prevalence and severity in Black and mixed-race populations, probably owing to a combination of environmental and intrinsic factors. Black skin has been shown to have increased transepidermal water loss and lower levels of ceramides, which are important components of the lipid barrier in the stratum corneum.
The American College of Allergy, Asthma & Immunology, along with the Allergy & Asthma Network, are partnering to create Eczema in Skin of Color, a website to aid physicians and patients in recognizing eczema in people with all skin types.
5. New and emerging therapies are poised to improve outcomes with AD treatment
Ruxolitinib cream, a topical Janus kinase (JAK)-1/JAK2 inhibitor, was approved for AD by the U.S. Food and Drug Administration in September 2021. The approval was based on results from the Topical Ruxolitinib Evaluation in AD (TRuE-AD) clinical trial program, which consisted of phase 3 studies that investigated 1,249 patients aged greater than or equal to 12 years with mild to moderate AD (Investigator’s Global Assessment score of 2-3) with a body surface area of 3%-20% (excluding scalp). The 2023 AAD guidelines for topical treatment recommend ruxolitinib cream for adults with mild to moderate AD.
Tralokinumab is a monoclonal antibody that inhibits the interleukin-13 cytokines, which prevents the release of cytokines, chemokines, and IgE. It was approved by the FDA in 2021 for treatment of moderate to severe AD. It is administered by subcutaneous injection every 2 weeks. Approval was based on the phase 3 trials ECZTRA 1, 2, and 3, which assessed the efficacy of tralokinumab in 1,934 adults.
Abrocitinib is an oral, once-daily JAK1 inhibitor for treatment of adults living with refractory, moderate to severe AD. FDA approval was based on results of five clinical trials from a large-scale trial program of more than 1,600 patients. Across the trials, abrocitinib demonstrated a consistent safety profile and profound improvements in skin clearance, extent of disease, and severity, as well as rapid improvement in itch after 2 weeks, for some people living with AD vs placebo.
Upadacitinib, another oral JAK1 inhibitor, was approved by the FDA in January 2022 for refractory moderate to severe AD. Approval was based on three double-blind phase 3 trials (Measure Up 1, Measure Up 2, AD Up) in which 2,584 patients with moderate to severe AD were randomized to receive oral upadacitinib 15 mg/d and 30 mg/d. In Measure Up 1 and Measure Up 2, upadacitinib was evaluated as monotherapy; in AD Up, upadacitinib was evaluated in combination with topical corticosteroids.
On the horizon
Baricitinib, an oral JAK1/2 inhibitor, is not yet approved by the FDA for AD. It is, however, approved for moderate to severe AD treatment in the European Union and many other countries. A 2022 review of studies evaluating baricitinib for the treatment of moderate to severe AD in adults (BREEZE-AD1, -AD2, -AD3, -AD4, -AD5, -AD6) reported that current evidence supports baricitinib, used as monotherapy or in combination with topical corticosteroids, as a safe and effective agent that can be used as an alternative to subcutaneous biologics in adults with moderate to severe AD.
Topical JAK inhibitors
A 2023 systematic review (19 studies, 3,600 participants) reported on several topical JAK inhibitors that are effective for treating AD. It suggests a stronger safety profile and better results, compared with systemic JAK inhibitors. The review focused on topical delgocitinib, tofacitinib, ruxolitinib, cerdulatinib, and ifidancitinib. All agents were effective in treating AD. All of these topical JAK inhibitors had minimal risk for mild to moderate adverse effects.
Biologics
Lebrikizumab was evaluated in a phase 2b, double-blind, placebo-controlled randomized clinical trial. After 16 weeks (280 participants), patients with moderate to severe AD showed a dose-dependent significant improvement in the primary endpoint, compared with placebo. Two phase 3 trials (ADvocate1, ADvocate2) evaluated the safety and efficacy of monotherapy with lebrikizumab in adults and adolescents with moderate to severe AD.
Nemolizumab, assessed in long-term phase 3 trials of AD-associated pruritus, resulted in clinically meaningful improvements from the beginning of treatment to week 68. Nemolizumab is being evaluated in two identical phase 3 studies (Arcadia 1, Arcadia 2) and a long-term extension study.
Dr. Kim is Professor and Vice Chair of Research in the department of dermatology, as well as Director of the Mark Lebwohl Center for Neuroinflammation and Sensation at the Icahn School of Medicine at Mount Sinai, New York. He reported conflicts of interest with 23andMe, Abrax Japan, AbbVie, Almirall, Amgen, and KiiRNA Biotech.
A version of this article first appeared on Medscape.com.
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin condition that typically affects the face (cheeks), neck, arms, and legs but usually spares the groin and axillary regions. AD usually starts in early infancy but also affects some adults. AD is often associated with elevated levels of immunoglobulin E (IgE). That it is the first disease to present in a series of allergic diseases – including food allergy, asthma, and allergic rhinitis, in order – and has given rise to the “atopic march” theory, which suggests that AD is part of a progression that may lead to subsequent allergic disease at other epithelial barrier surfaces.
.
1. Essential features of AD are pruritus and eczema
The diagnosis of AD is primarily observational. It is made on the basis of patient and family history, pattern of lesions, morphology, and clinical signs. No genetic features or biomarkers are specific enough to reliably aid in diagnosis or severity assessment. Many individual findings are used to diagnose AD, as summarized by the American Academy of Dermatology based on essential, important, associated, and exclusionary features:
- Essential features (must be present for diagnosis) are pruritus and eczema (acute, subacute, or chronic) with typical morphology and age-specific patterns and chronic or relapsing history.
- Important features (usually seen in AD and support the diagnosis) are early age of onset, atopy (personal/family history, IgE reactivity), and xerosis.
- Associated features (nonspecific but suggestive) are atypical vascular response (e.g., delayed blanch response); keratosis pilaris (and some others); ocular/periorbital changes; other regional findings (e.g., perioral changes); and perifollicular accentuation, lichenification, or prurigo lesions.
- Exclusionary conditions (must be excluded to make the AD diagnosis) are scabies, seborrheic dermatitis, contact dermatitis, ichthyoses, cutaneous T-cell lymphoma, psoriasis, photosensitivity dermatoses, immune deficiency diseases, and erythroderma due to other causes.
AD should be differentiated from other red, scaly skin conditions. It is often difficult to separate AD from seborrheic dermatitis in infancy, and the two conditions may overlap in this age group. Particularly if the condition is not responding to therapy, the diagnosis of AD should be re-reviewed and other disorders considered, including more serious nutritional, metabolic, and immunologic conditions in children and cutaneous T-cell lymphoma in adults. Allergic contact dermatitis may be both an alternative diagnosis to AD and an exacerbator of AD in some individuals.
2. Associated comorbidities of AD may exacerbate the condition and lead to other atopic disorders
Reported comorbidities of AD include other atopic or allergic conditions, autoimmune diseases, infections, metabolic conditions, mental health disorders, and cardiovascular disease. Certain aspects of AD, such as chronic pruritus, psychosocial distress, and inflammation, can lead to anxiety, depression, and suicidality. AD is associated with and may predispose to higher risk for other atopic disorders, including asthma, hay fever, food allergy, and eosinophilic esophagitis.
Persons with AD also appear to be at higher risk for infectious diseases. The prevalence of cutaneous and systemic infections in patients with AD is significantly higher than those without AD. Infectious complications can include skin and soft-tissue infections, bacteremia, eczema herpeticum, osteomyelitis, endocarditis, and septic arthritis.
3. Climate change has a profound impact on AD
The incidence of AD has increased over the past several decades, and environmental factors such as climate change have been implicated as a potential mechanism. Climate change–related factors affect the skin’s capacity to maintain homeostasis, leading to various cutaneous diseases. AD, psoriasis, pemphigus, acne vulgaris, melasma, and photoaging are all associated with rising levels of air pollution. Elevated temperatures due to global warming induce disruption of the skin microbiome, thereby affecting AD.
Extreme weather events due to climate change, including floods and wildfires, are implicated in cutaneous injuries, skin infections, and acute worsening of inflammatory skin disorders.
4. The impact and appearance of AD varies in different racial groups
It was once believed that AD was just one single disease affecting people of many different races. More recently, it has been proposed that AD is in fact a group of different diseases. Both epidemiologic and genetic factors may play a role in influencing the main features of AD.
Spongiotic processes such as AD that would be pink or erythematous on white skin are often hypopigmented in individuals with darkly pigmented skin. AD has a higher prevalence and severity in Black and mixed-race populations, probably owing to a combination of environmental and intrinsic factors. Black skin has been shown to have increased transepidermal water loss and lower levels of ceramides, which are important components of the lipid barrier in the stratum corneum.
The American College of Allergy, Asthma & Immunology, along with the Allergy & Asthma Network, are partnering to create Eczema in Skin of Color, a website to aid physicians and patients in recognizing eczema in people with all skin types.
5. New and emerging therapies are poised to improve outcomes with AD treatment
Ruxolitinib cream, a topical Janus kinase (JAK)-1/JAK2 inhibitor, was approved for AD by the U.S. Food and Drug Administration in September 2021. The approval was based on results from the Topical Ruxolitinib Evaluation in AD (TRuE-AD) clinical trial program, which consisted of phase 3 studies that investigated 1,249 patients aged greater than or equal to 12 years with mild to moderate AD (Investigator’s Global Assessment score of 2-3) with a body surface area of 3%-20% (excluding scalp). The 2023 AAD guidelines for topical treatment recommend ruxolitinib cream for adults with mild to moderate AD.
Tralokinumab is a monoclonal antibody that inhibits the interleukin-13 cytokines, which prevents the release of cytokines, chemokines, and IgE. It was approved by the FDA in 2021 for treatment of moderate to severe AD. It is administered by subcutaneous injection every 2 weeks. Approval was based on the phase 3 trials ECZTRA 1, 2, and 3, which assessed the efficacy of tralokinumab in 1,934 adults.
Abrocitinib is an oral, once-daily JAK1 inhibitor for treatment of adults living with refractory, moderate to severe AD. FDA approval was based on results of five clinical trials from a large-scale trial program of more than 1,600 patients. Across the trials, abrocitinib demonstrated a consistent safety profile and profound improvements in skin clearance, extent of disease, and severity, as well as rapid improvement in itch after 2 weeks, for some people living with AD vs placebo.
Upadacitinib, another oral JAK1 inhibitor, was approved by the FDA in January 2022 for refractory moderate to severe AD. Approval was based on three double-blind phase 3 trials (Measure Up 1, Measure Up 2, AD Up) in which 2,584 patients with moderate to severe AD were randomized to receive oral upadacitinib 15 mg/d and 30 mg/d. In Measure Up 1 and Measure Up 2, upadacitinib was evaluated as monotherapy; in AD Up, upadacitinib was evaluated in combination with topical corticosteroids.
On the horizon
Baricitinib, an oral JAK1/2 inhibitor, is not yet approved by the FDA for AD. It is, however, approved for moderate to severe AD treatment in the European Union and many other countries. A 2022 review of studies evaluating baricitinib for the treatment of moderate to severe AD in adults (BREEZE-AD1, -AD2, -AD3, -AD4, -AD5, -AD6) reported that current evidence supports baricitinib, used as monotherapy or in combination with topical corticosteroids, as a safe and effective agent that can be used as an alternative to subcutaneous biologics in adults with moderate to severe AD.
Topical JAK inhibitors
A 2023 systematic review (19 studies, 3,600 participants) reported on several topical JAK inhibitors that are effective for treating AD. It suggests a stronger safety profile and better results, compared with systemic JAK inhibitors. The review focused on topical delgocitinib, tofacitinib, ruxolitinib, cerdulatinib, and ifidancitinib. All agents were effective in treating AD. All of these topical JAK inhibitors had minimal risk for mild to moderate adverse effects.
Biologics
Lebrikizumab was evaluated in a phase 2b, double-blind, placebo-controlled randomized clinical trial. After 16 weeks (280 participants), patients with moderate to severe AD showed a dose-dependent significant improvement in the primary endpoint, compared with placebo. Two phase 3 trials (ADvocate1, ADvocate2) evaluated the safety and efficacy of monotherapy with lebrikizumab in adults and adolescents with moderate to severe AD.
Nemolizumab, assessed in long-term phase 3 trials of AD-associated pruritus, resulted in clinically meaningful improvements from the beginning of treatment to week 68. Nemolizumab is being evaluated in two identical phase 3 studies (Arcadia 1, Arcadia 2) and a long-term extension study.
Dr. Kim is Professor and Vice Chair of Research in the department of dermatology, as well as Director of the Mark Lebwohl Center for Neuroinflammation and Sensation at the Icahn School of Medicine at Mount Sinai, New York. He reported conflicts of interest with 23andMe, Abrax Japan, AbbVie, Almirall, Amgen, and KiiRNA Biotech.
A version of this article first appeared on Medscape.com.
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin condition that typically affects the face (cheeks), neck, arms, and legs but usually spares the groin and axillary regions. AD usually starts in early infancy but also affects some adults. AD is often associated with elevated levels of immunoglobulin E (IgE). That it is the first disease to present in a series of allergic diseases – including food allergy, asthma, and allergic rhinitis, in order – and has given rise to the “atopic march” theory, which suggests that AD is part of a progression that may lead to subsequent allergic disease at other epithelial barrier surfaces.
.
1. Essential features of AD are pruritus and eczema
The diagnosis of AD is primarily observational. It is made on the basis of patient and family history, pattern of lesions, morphology, and clinical signs. No genetic features or biomarkers are specific enough to reliably aid in diagnosis or severity assessment. Many individual findings are used to diagnose AD, as summarized by the American Academy of Dermatology based on essential, important, associated, and exclusionary features:
- Essential features (must be present for diagnosis) are pruritus and eczema (acute, subacute, or chronic) with typical morphology and age-specific patterns and chronic or relapsing history.
- Important features (usually seen in AD and support the diagnosis) are early age of onset, atopy (personal/family history, IgE reactivity), and xerosis.
- Associated features (nonspecific but suggestive) are atypical vascular response (e.g., delayed blanch response); keratosis pilaris (and some others); ocular/periorbital changes; other regional findings (e.g., perioral changes); and perifollicular accentuation, lichenification, or prurigo lesions.
- Exclusionary conditions (must be excluded to make the AD diagnosis) are scabies, seborrheic dermatitis, contact dermatitis, ichthyoses, cutaneous T-cell lymphoma, psoriasis, photosensitivity dermatoses, immune deficiency diseases, and erythroderma due to other causes.
AD should be differentiated from other red, scaly skin conditions. It is often difficult to separate AD from seborrheic dermatitis in infancy, and the two conditions may overlap in this age group. Particularly if the condition is not responding to therapy, the diagnosis of AD should be re-reviewed and other disorders considered, including more serious nutritional, metabolic, and immunologic conditions in children and cutaneous T-cell lymphoma in adults. Allergic contact dermatitis may be both an alternative diagnosis to AD and an exacerbator of AD in some individuals.
2. Associated comorbidities of AD may exacerbate the condition and lead to other atopic disorders
Reported comorbidities of AD include other atopic or allergic conditions, autoimmune diseases, infections, metabolic conditions, mental health disorders, and cardiovascular disease. Certain aspects of AD, such as chronic pruritus, psychosocial distress, and inflammation, can lead to anxiety, depression, and suicidality. AD is associated with and may predispose to higher risk for other atopic disorders, including asthma, hay fever, food allergy, and eosinophilic esophagitis.
Persons with AD also appear to be at higher risk for infectious diseases. The prevalence of cutaneous and systemic infections in patients with AD is significantly higher than those without AD. Infectious complications can include skin and soft-tissue infections, bacteremia, eczema herpeticum, osteomyelitis, endocarditis, and septic arthritis.
3. Climate change has a profound impact on AD
The incidence of AD has increased over the past several decades, and environmental factors such as climate change have been implicated as a potential mechanism. Climate change–related factors affect the skin’s capacity to maintain homeostasis, leading to various cutaneous diseases. AD, psoriasis, pemphigus, acne vulgaris, melasma, and photoaging are all associated with rising levels of air pollution. Elevated temperatures due to global warming induce disruption of the skin microbiome, thereby affecting AD.
Extreme weather events due to climate change, including floods and wildfires, are implicated in cutaneous injuries, skin infections, and acute worsening of inflammatory skin disorders.
4. The impact and appearance of AD varies in different racial groups
It was once believed that AD was just one single disease affecting people of many different races. More recently, it has been proposed that AD is in fact a group of different diseases. Both epidemiologic and genetic factors may play a role in influencing the main features of AD.
Spongiotic processes such as AD that would be pink or erythematous on white skin are often hypopigmented in individuals with darkly pigmented skin. AD has a higher prevalence and severity in Black and mixed-race populations, probably owing to a combination of environmental and intrinsic factors. Black skin has been shown to have increased transepidermal water loss and lower levels of ceramides, which are important components of the lipid barrier in the stratum corneum.
The American College of Allergy, Asthma & Immunology, along with the Allergy & Asthma Network, are partnering to create Eczema in Skin of Color, a website to aid physicians and patients in recognizing eczema in people with all skin types.
5. New and emerging therapies are poised to improve outcomes with AD treatment
Ruxolitinib cream, a topical Janus kinase (JAK)-1/JAK2 inhibitor, was approved for AD by the U.S. Food and Drug Administration in September 2021. The approval was based on results from the Topical Ruxolitinib Evaluation in AD (TRuE-AD) clinical trial program, which consisted of phase 3 studies that investigated 1,249 patients aged greater than or equal to 12 years with mild to moderate AD (Investigator’s Global Assessment score of 2-3) with a body surface area of 3%-20% (excluding scalp). The 2023 AAD guidelines for topical treatment recommend ruxolitinib cream for adults with mild to moderate AD.
Tralokinumab is a monoclonal antibody that inhibits the interleukin-13 cytokines, which prevents the release of cytokines, chemokines, and IgE. It was approved by the FDA in 2021 for treatment of moderate to severe AD. It is administered by subcutaneous injection every 2 weeks. Approval was based on the phase 3 trials ECZTRA 1, 2, and 3, which assessed the efficacy of tralokinumab in 1,934 adults.
Abrocitinib is an oral, once-daily JAK1 inhibitor for treatment of adults living with refractory, moderate to severe AD. FDA approval was based on results of five clinical trials from a large-scale trial program of more than 1,600 patients. Across the trials, abrocitinib demonstrated a consistent safety profile and profound improvements in skin clearance, extent of disease, and severity, as well as rapid improvement in itch after 2 weeks, for some people living with AD vs placebo.
Upadacitinib, another oral JAK1 inhibitor, was approved by the FDA in January 2022 for refractory moderate to severe AD. Approval was based on three double-blind phase 3 trials (Measure Up 1, Measure Up 2, AD Up) in which 2,584 patients with moderate to severe AD were randomized to receive oral upadacitinib 15 mg/d and 30 mg/d. In Measure Up 1 and Measure Up 2, upadacitinib was evaluated as monotherapy; in AD Up, upadacitinib was evaluated in combination with topical corticosteroids.
On the horizon
Baricitinib, an oral JAK1/2 inhibitor, is not yet approved by the FDA for AD. It is, however, approved for moderate to severe AD treatment in the European Union and many other countries. A 2022 review of studies evaluating baricitinib for the treatment of moderate to severe AD in adults (BREEZE-AD1, -AD2, -AD3, -AD4, -AD5, -AD6) reported that current evidence supports baricitinib, used as monotherapy or in combination with topical corticosteroids, as a safe and effective agent that can be used as an alternative to subcutaneous biologics in adults with moderate to severe AD.
Topical JAK inhibitors
A 2023 systematic review (19 studies, 3,600 participants) reported on several topical JAK inhibitors that are effective for treating AD. It suggests a stronger safety profile and better results, compared with systemic JAK inhibitors. The review focused on topical delgocitinib, tofacitinib, ruxolitinib, cerdulatinib, and ifidancitinib. All agents were effective in treating AD. All of these topical JAK inhibitors had minimal risk for mild to moderate adverse effects.
Biologics
Lebrikizumab was evaluated in a phase 2b, double-blind, placebo-controlled randomized clinical trial. After 16 weeks (280 participants), patients with moderate to severe AD showed a dose-dependent significant improvement in the primary endpoint, compared with placebo. Two phase 3 trials (ADvocate1, ADvocate2) evaluated the safety and efficacy of monotherapy with lebrikizumab in adults and adolescents with moderate to severe AD.
Nemolizumab, assessed in long-term phase 3 trials of AD-associated pruritus, resulted in clinically meaningful improvements from the beginning of treatment to week 68. Nemolizumab is being evaluated in two identical phase 3 studies (Arcadia 1, Arcadia 2) and a long-term extension study.
Dr. Kim is Professor and Vice Chair of Research in the department of dermatology, as well as Director of the Mark Lebwohl Center for Neuroinflammation and Sensation at the Icahn School of Medicine at Mount Sinai, New York. He reported conflicts of interest with 23andMe, Abrax Japan, AbbVie, Almirall, Amgen, and KiiRNA Biotech.
A version of this article first appeared on Medscape.com.
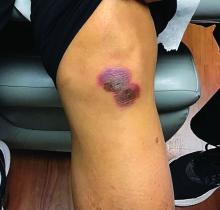
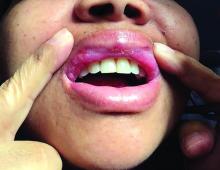
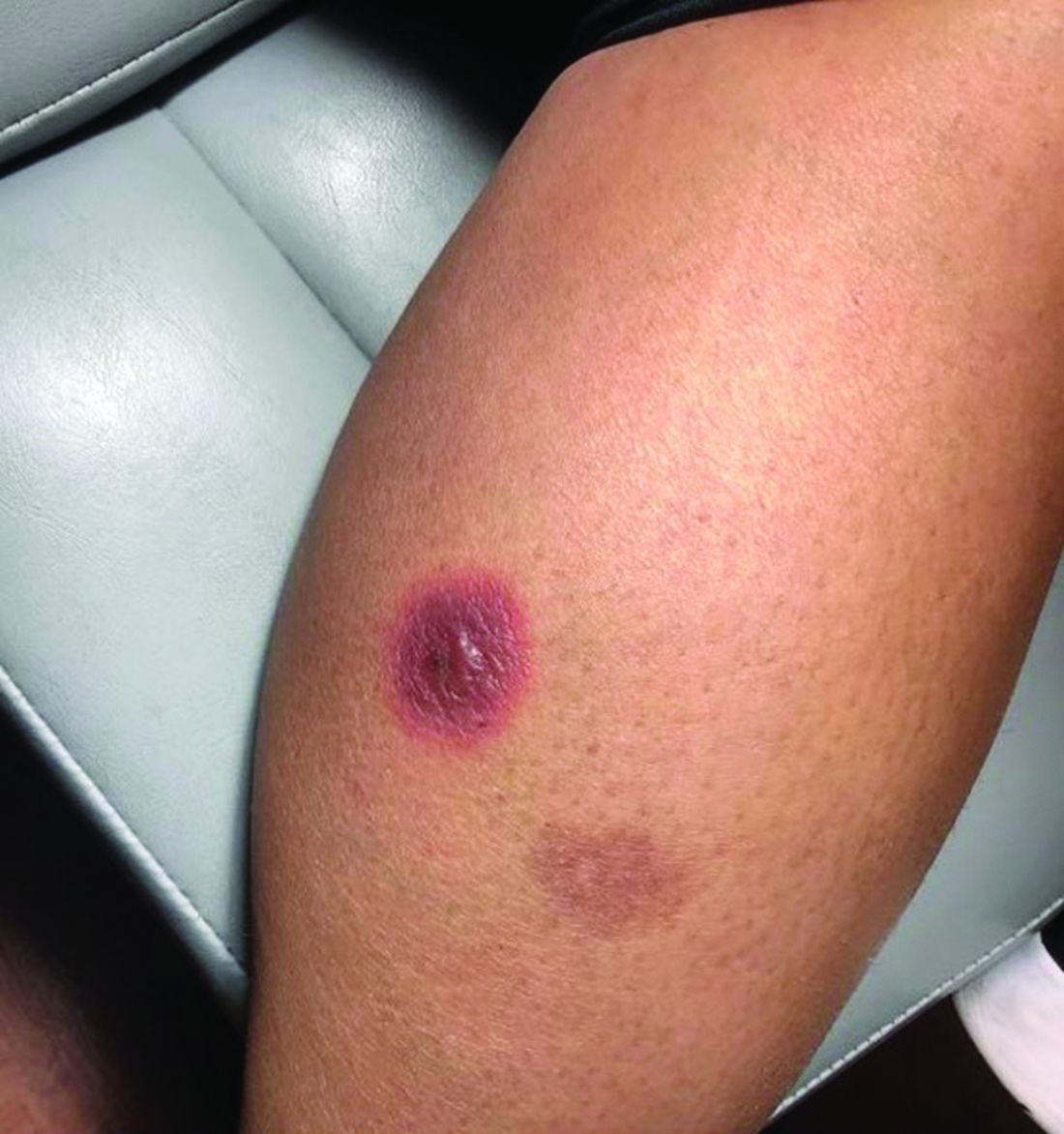
A 42-year-old woman presented with a few days of erosions on her buccal mucosa, tongue, and soft palate
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
RVUs: A fair measure of your productivity?
This transcript has been edited for clarity.
The other day, I received a flowery, elaborate email from none other than a physician recruiter: “Beautiful parks, hiking, great schools, blah blah blah, worked RVU production bonus on top of base pay.”
That last part – RVUs. I’m lost. I hear mixed reviews from physicians who work in RVU-based systems. The entire thing seems overly complex and confusing, so let’s clear it up. I did my research, and I’m going to explain RVUs.
Types of RVUs
RVUs, or relative value units, are a standard set by Medicare, used to measure physician productivity and ultimately determine compensation. There are three types:
- Work RVUs (basically everything that happens during a patient encounter).
- Practice expense RVUs.
- Professional liability insurance RVUs.
Now, envision this equation. All three of those RVUs are each multiplied by a geographic practice cost index to come up with a total number, and then that is multiplied by the Medicare conversion factor, which right now is around $33 to $34, to come up with a total dollar amount.
Work RVUs make up the bulk of total RVUs and they get their value from CPT codes. That value is determined by CMS. The AMA’s Relative Value Scale Update Committee, or RUC, which is made up of 32 people from various medical and surgical subspecialties, regularly meets and makes recommendations on the value of various CPT codes.
Is specialty representation fair and balanced?
CMS historically has accepted a high percentage of RUC’s recommendations, so this is a very influential committee. This is also why RUC has led to some controversy, with some stating that there is a lack of primary care representation, and perhaps this is why CPT codes related to procedures tend to reimburse higher.
How does one weigh the value of an hour-long palliative conversation against the quick removal of a benign skin lesion? That’s a loaded question.
This is especially important if your salary, or at least part of it, is determined by total RVUs. You want to have a sense of the pros and cons of working in an RVU system and how this relates to your specialty, your practice, and your schedule.
An RVU-based system provides an objective measure on complex patient encounters, volume, and procedures, and it’s a somewhat unified measure. The cons are pretty clear because these models favor you seeing many patients and billing a lot, and often this favors employers over physicians.
Dr. Patel is a clinical instructor, department of pediatrics, at Columbia University, New York, and a pediatric hospitalist at Morgan Stanley Children’s Hospital of New York–Presbyterian. He reported a conflict of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The other day, I received a flowery, elaborate email from none other than a physician recruiter: “Beautiful parks, hiking, great schools, blah blah blah, worked RVU production bonus on top of base pay.”
That last part – RVUs. I’m lost. I hear mixed reviews from physicians who work in RVU-based systems. The entire thing seems overly complex and confusing, so let’s clear it up. I did my research, and I’m going to explain RVUs.
Types of RVUs
RVUs, or relative value units, are a standard set by Medicare, used to measure physician productivity and ultimately determine compensation. There are three types:
- Work RVUs (basically everything that happens during a patient encounter).
- Practice expense RVUs.
- Professional liability insurance RVUs.
Now, envision this equation. All three of those RVUs are each multiplied by a geographic practice cost index to come up with a total number, and then that is multiplied by the Medicare conversion factor, which right now is around $33 to $34, to come up with a total dollar amount.
Work RVUs make up the bulk of total RVUs and they get their value from CPT codes. That value is determined by CMS. The AMA’s Relative Value Scale Update Committee, or RUC, which is made up of 32 people from various medical and surgical subspecialties, regularly meets and makes recommendations on the value of various CPT codes.
Is specialty representation fair and balanced?
CMS historically has accepted a high percentage of RUC’s recommendations, so this is a very influential committee. This is also why RUC has led to some controversy, with some stating that there is a lack of primary care representation, and perhaps this is why CPT codes related to procedures tend to reimburse higher.
How does one weigh the value of an hour-long palliative conversation against the quick removal of a benign skin lesion? That’s a loaded question.
This is especially important if your salary, or at least part of it, is determined by total RVUs. You want to have a sense of the pros and cons of working in an RVU system and how this relates to your specialty, your practice, and your schedule.
An RVU-based system provides an objective measure on complex patient encounters, volume, and procedures, and it’s a somewhat unified measure. The cons are pretty clear because these models favor you seeing many patients and billing a lot, and often this favors employers over physicians.
Dr. Patel is a clinical instructor, department of pediatrics, at Columbia University, New York, and a pediatric hospitalist at Morgan Stanley Children’s Hospital of New York–Presbyterian. He reported a conflict of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The other day, I received a flowery, elaborate email from none other than a physician recruiter: “Beautiful parks, hiking, great schools, blah blah blah, worked RVU production bonus on top of base pay.”
That last part – RVUs. I’m lost. I hear mixed reviews from physicians who work in RVU-based systems. The entire thing seems overly complex and confusing, so let’s clear it up. I did my research, and I’m going to explain RVUs.
Types of RVUs
RVUs, or relative value units, are a standard set by Medicare, used to measure physician productivity and ultimately determine compensation. There are three types:
- Work RVUs (basically everything that happens during a patient encounter).
- Practice expense RVUs.
- Professional liability insurance RVUs.
Now, envision this equation. All three of those RVUs are each multiplied by a geographic practice cost index to come up with a total number, and then that is multiplied by the Medicare conversion factor, which right now is around $33 to $34, to come up with a total dollar amount.
Work RVUs make up the bulk of total RVUs and they get their value from CPT codes. That value is determined by CMS. The AMA’s Relative Value Scale Update Committee, or RUC, which is made up of 32 people from various medical and surgical subspecialties, regularly meets and makes recommendations on the value of various CPT codes.
Is specialty representation fair and balanced?
CMS historically has accepted a high percentage of RUC’s recommendations, so this is a very influential committee. This is also why RUC has led to some controversy, with some stating that there is a lack of primary care representation, and perhaps this is why CPT codes related to procedures tend to reimburse higher.
How does one weigh the value of an hour-long palliative conversation against the quick removal of a benign skin lesion? That’s a loaded question.
This is especially important if your salary, or at least part of it, is determined by total RVUs. You want to have a sense of the pros and cons of working in an RVU system and how this relates to your specialty, your practice, and your schedule.
An RVU-based system provides an objective measure on complex patient encounters, volume, and procedures, and it’s a somewhat unified measure. The cons are pretty clear because these models favor you seeing many patients and billing a lot, and often this favors employers over physicians.
Dr. Patel is a clinical instructor, department of pediatrics, at Columbia University, New York, and a pediatric hospitalist at Morgan Stanley Children’s Hospital of New York–Presbyterian. He reported a conflict of interest with Medumo.
A version of this article first appeared on Medscape.com.
‘Vaginal dryness’ can be fatal. No, really.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
What do you mean, Dr. Rubin? How is vaginal dryness killing women? We minimize the term vaginal dryness. When women come to our offices and complain of a little vaginal dryness – or they don’t even come to our office to complain of it because the doctor can’t be bothered with a little vaginal dryness — what they don’t understand is that this “little vaginal dryness” is really something called genitourinary syndrome of menopause (GSM). They don’t know that because they’ve never heard of it, and you may have never heard of it either. In 2014, we changed the terms vaginal dryness and vulvovaginal atrophy or atrophic vaginitis to GSM to make it short and simple.
GSM – what does it mean? It’s not just a little vaginal dryness. It turns out that all of the genital and urinary symptoms from menopause just get worse over time. The bladder, the urethra, and the vagina have lots of hormone receptors, including estrogen and testosterone. When the body no longer makes those hormones, the system doesn’t work very well, and genital and urinary symptoms occur that just get worse over time without treatment. Unlike hot flashes, which tend to go away, GSM does not.
What are the symptoms of GSM? Some are sexual: a little vaginal dryness, pain with sex, and worsening orgasm. But there are also genital and urinary symptoms that get worse: itching, burning irritation, rawness, an awareness of their genitals that the patient has never had before. And as a urologist, we see frequency, urgency, and leakage.
The thing that kills women is recurrent urinary tract infections (UTIs). Did you know that UTIs account for 7 million visits and hospitalizations annually and 25% of all infections in older people? In fact, apparently one-third of the total Medicare expenditure is around UTIs. Not preventing UTIs is costing our health care system an enormous amount of money and resources.
Did you know we’ve had safe and effective treatment options for GSM since the 1970s? Vaginal hormones have existed since the 1970s, but we’re using them only for pain with sex and not for GSM. In fact, data show that by using vaginal hormones, we can prevent UTIs by more than 50%. We can save lives using safe, effective, local, low-dose vaginal hormone strategies. And they are safe and effective for all of our patients in pre- and post menopause.
There are five different treatment options: vaginal estrogen inserts, vaginal estrogen creams, vaginal dehydroepiandrosterone (DHEA), low-dose vaginal estrogen rings, and an oral pill option called ospemifene (Osphena). All are used to treat GSM and will only work if your patient actually uses them and continues to use them.
These treatments are safe. They are effective. They do not increase the level of systemic hormones in the bloodstream. I have many patients with breast cancer who use these products as well. The only patients you may want to talk to your oncology colleagues about is women on active aromatase inhibitors.
We have to understand that UTIs kill people and having GSM is debilitating, often requiring pain medication because it can hurt to sit or to wear pads and our patients’ quality of life is severely affected. So please consider learning how to treat GSM. It turns out you don’t have to do exams. You don’t have to do follow-up. You can give these therapies, and women can use them for life.
Now, if your patient has vaginal bleeding, of course they need to see their gynecologist. But this is something every primary care doctor can and should do. As a urologist, we prescribe a lot of tamsulosin (Flomax) for our male patients to help with urination. Vaginal estrogen or DHEA is basically like Flomax for women, but it prevents UTIs and actually works like sildenafil (Viagra) because it can help orgasm and reduce pain with sex.
You have access to affordable, safe, effective treatment options to treat GSM. So check them out and hopefully change the world.
Dr. Rubin is an assistant clinical professor in the department of urology at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
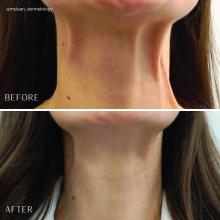
Treatment of the neck and lower face with botulinum toxin
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
Zuranolone: FAQs for clinicians and patients
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
The Food and Drug Administration approval of zuranolone for postpartum depression in August 2023 has raised many important questions (and opinions) about its future use in clinical practice.
At the UNC-Chapel Hill Center for Women’s Mood Disorders, we treat women and pregnant people throughout hormonal transitions, including pregnancy and the postpartum, and have been part of development, research, and now delivery of both brexanolone and zuranolone. While we are excited about new tools in the arsenal for alleviating maternal mental health, we also want to be clear that our work is far from complete and continued efforts to care for pregnant people and their families are imperative.
What is zuranolone?
Zuranolone (brand name Zurzuvae) is an oral medication developed by Sage Therapeutics and Biogen. It is a positive allosteric modulator of the GABAA receptor, the brain’s major inhibitory system. As a positive allosteric modulator, it increases the sensitivity of the GABAA receptor to GABA.
Zuranolone is very similar to brexanolone, a synthetic form of allopregnanolone, a neurosteroid byproduct of progesterone (see below). However, zuranolone is not an oral form of brexanolone – it was slightly modified to ensure good oral stability and bioavailability. It is metabolized by the hepatic enzyme CYP3A4 and has a half-life of 16-23 hours. Zurzuvae is currently produced in capsule form.
What does zuranolone treat?
Zuranolone is the first FDA-approved oral drug for postpartum depression (PPD). It follows brexanolone, an intravenous drug, which was the first FDA-approved medication for PPD. Though these are the first medications with specific approval for PDD, many other treatment options are currently available including therapy, SSRIs, serotonin norepinephrine reuptake inhibitors (SNRIs), and other treatments used in major depression.
How does zuranolone work?
Zuranolone is a neuroactive steroid, which means that it is a steroid that goes into and acts on the brain. Zuranolone binds to different GABA receptor subunits from those bound by other positive modulators, such as benzodiazepines (for example, lorazepam). As a synthetic form of allopregnanolone, a metabolite of progesterone which rises dramatically in pregnancy then drops during labor and delivery, zuranolone was originally thought to mitigate the response to this drop in patients that are vulnerable to it during the postpartum. An alternative proposed mechanism is that the increased GABAergic, inhibitory signaling with zuranolone may act directly to decrease depression irrespective of the exact mechanism by which the depression occurred.
How was it studied?
Zuranolone was studied in women with severe postpartum depression and had to meet criteria for major depressive disorder (MDD) no earlier than the third trimester of pregnancy (about 28 weeks’ gestation) and no later than 4 weeks post partum. Patients were excluded from these studies if they had a history of bipolar disorder, psychotic disorders, attempted suicide, or if they were at risk for suicide.
The two phase 3 clinical trials that led to FDA approval are ROBIN and SKYLARK. These studies measured the efficacy and safety of zuranolone at 30 mg and 50 mg, respectively, and met their end points of rapid improvement in depressive and anxiety symptoms in postpartum depression.
When will we be able to start using it?
It is anticipated that zuranolone will become commercially available in early 2024.
Who can prescribe it?
Those with medical licenses. Most people will likely receive treatment from their obstetric, family medicine, or psychiatric clinicians.
How much will it cost?
The manufacturers have not released this information as of August 2023.
What sort of doses and duration is recommended?
The current FDA recommended dose is 50 mg for 14 days, taken once per evening with a fatty meal. The dose can be reduced to 40 mg if there are central nervous system (CNS) depressant effects, and to 30 mg if the patient has severe hepatic or moderate-severe renal impairment. There are currently no studies on longer courses of treatment.
What happens if the patient relapses after a 14-day trial?
While there is no clear guidance, an open-label trial (The SHORELINE Study) demonstrated that a repeated 14-day administration can restore clinical response.
What are the side effects?
Common side effects include drowsiness, dizziness, lower energy, diarrhea, and symptoms similar to the common cold. Zuranolone can act like a CNS depressant and can lead to sedation and somnolence.
Are there any boxed warnings?
Because of the CNS depressant effects, zuranolone was given a boxed warning that patients should not drive or operate heavy machinery within 12 hours of taking the medication as it may lead to impairment. Similar to other antidepressants, there is also a warning that zuranolone may increase risk for suicidal thoughts in patients under 24 years old.
Can it be used with other medications?
Yes. In the original trials, women were allowed to remain on medications treating their depressive symptoms (such as SSRIs and SNRIs). According to the FDA, zuranolone can be used alone or with other antidepressants.
Are there any medicines to avoid?
We recommend caution with other medications which may increase sedation, such as benzodiazepines.
Can it be used with birth control?
Yes. In fact, because the outcomes on a fetus are not yet studied, it is recommended that patients be on concurrent birth control during treatment and for a week after cessation. This does not mean that zuranolone is known to cause issues with fetal development, but rather that we do not know at this time.
Can it be used in pregnancy?
As above, the outcomes on fetal development are not known at this time, nor are the effects of zuranolone on labor and delivery. More research will need to be done to understand if there is risk with taking zuranolone during pregnancy. It should be noted that allopregnanolone levels ordinarily reach quite high levels during pregnancy.
Long-term side effects?
Long-term side effects are unknown. The study duration of ROBIN and SKYLARK was 45 days.
Breastfeeding?
Use in lactation has not yet been studied. Continued research is needed.
Can it be used in mood changes related to other reproductive changes or diagnoses like premenstrual dysphoric disorder and perimenopause?
The mechanism by which zuranolone is thought to work – that is, during changes in reproductive hormones – is implicated in other reproductive transitions such as premenstrual dysphoric disorder and perimenopause when reproductive hormones are fluctuating, though at lower levels than in pregnancy. Research will be required to assess efficacy and safety; however, the mechanistic reasons is worth pursuing. Additionally, zuranolone has not been studied in postpartum psychosis.
Can zuranolone be used to treat other affective conditions besides postpartum depression? Bipolar disorder?
Whether it may be beneficial for patients with a depressive episode that is part of an underlying bipolar disorder or other psychiatric illness is not yet known.
Anxiety?
Along with depressive symptoms, women who received zuranolone in the clinical trials also had improvements in anxiety symptoms. These findings provide some hope that zuranolone may eventually be beneficial in patients with anxiety.
However, to date zuranolone has not been directly studied as a treatment for anxiety disorders (such as generalized anxiety disorder, panic disorder, etc.), so its efficacy for these illnesses is currently unknown.
Insomnia?
In a study of 153 postpartum women, randomized to placebo or zuranolone, scale questions for insomnia were improved in the group receiving zuranolone. This provides some hope that, if zuranolone is appropriate, concurrent polypharmacy with a sleep aid can be avoided. Additionally, future evaluation of use in insomnia outside of PPD may be warranted.
How is it different from brexanolone?
The two are slightly different molecules. Brexanolone is synthetically identical to allopregnanolone and zuranolone has been altered to be active and orally bioavailable.
Brexanolone is a 60-hour infusion that requires hospital admission at an approved health care site. Zuranolone is an oral at-home once-daily dosing treatment for 14 days. Zuranolone does not require enrollment in a risk evaluation and mitigation strategy for risk of excessive sedation and sudden loss of consciousness.
When would you consider zuranolone vs. brexanolone vs. other antidepressants?
Zuranolone and brexanolone are rapid-acting antidepressants with a response within 14 days or 60 hours, respectively. Antidepressants such as SSRIs/SNRIs are still available, well studied, and work, although take longer to reach clinical efficacy and are accompanied by potentially troubling side effects (for example, weight gain, sexual dysfunction).
Time to treatment effect should be considered when assessing severity of symptoms and functional impairment of the mother and the overall family unit. Brexanolone requires continuous monitoring which may be beneficial for women who are severely impaired and may benefit from frequent clinical monitoring. Brexanolone does not require a dose reduction with hepatic impairment, however, should be avoided in end-stage renal disease because of the potential accumulation of the solubilizing agent.
Where can I find more information?
Many states have maternal mental health consultation lines (examples include NCMATTERS here in North Carolina and MCPAP for Moms in Massachusetts) for clinicians (mental health, primary care, and obstetricians) that can be utilized for questions about prescribing. Postpartum Support International also has a clinician line for those without state services.
We plan to update this entry upon market release and access to new information.
Dr. Riddle and Dr. Nathan are assistant professors in the department of psychiatry at the University of North Carolina at Chapel Hill. Dr. Richardson is a perinatal psychiatry fellow, department of psychiatry, UNC-Chapel Hill. Dr. Rubinow is Distinguished Professor in the department of psychiatry, UNC-Chapel Hill. Dr. Meltzer-Brody is Assad Meymandi Distinguished Professor and Chair, department of psychiatry, UNC-Chapel Hill.
References
Deligiannidis KM et al. J Clin Psychiatry. 2023 Jan 30;84(1):22m14475. doi: 10.4088/JCP.22m14475.
Deligiannidis KM et al. . Obstetrics & Gynecology. 2023 May;141(5S):64S-65S. doi: 10.1097/01.AOG.0000930588.16136.3f.
Deligiannidis KM et al. Am J Psychiatry. 2023 Sep 1;180(9):668-75. doi: 10.1176/appi.ajp.20220785.
Deligiannidis KM et al. JAMA Psychiatry. 2021 Sep 1;78(9):951-59. doi: 10.1001/jamapsychiatry.2021.1559.
FDA Approves First Oral Treatment for Postpartum Depression. 2023 Aug 4. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
ZURZUVAE – HIGHLIGHTS OF PRESCRIBING INFORMATION. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
Every click you make, the EHR is watching you
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Take a closer look at sleep’s role in GERD
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
The ongoing longitudinal Nurses’ Health Study has served as an incredible database for evaluating disease states prospectively over decades, thanks to the robust input of its participants. Most recently, this allowed for an important analysis of the association between gastroesophageal reflux (GER) symptoms and sleep quality, the results of which were published in JAMA Network Open.
Approximately 49,000 women with a median age of 59 years (range, 48-69 years) provided data for this analysis. Starting in 2005, they were asked about their experience of GER symptoms. In 2017, they were also asked to respond to a questionnaire, a modified Pittsburgh Sleep Quality Index (PSQI). This is a tool we’ve used a lot in prospective studies looking at gastrointestinal diseases and sleep-related abnormalities. It’s unique in that it looks not only at sleep but also at next-day function and daytime sleepiness, which is important here for its implications related to reflux disease and sleep fragmentation.
For those with GER symptoms occurring once a week and more than once a week, the approximate relative risk increased by 30% and 53%, respectively. Clearly, the association of GER symptoms and relative sleep quality was really important.
It should be noted that the PSQI is a disease-independent, validated instrument. It’s not specific to GER disease or any diseases. It’s cross validated across 17 different languages. I think what’s most important about its use in the assessment here is the incorporation of next-day function and asking participants about daytime sleepiness, which we’ll discuss in more detail shortly.
The many causes of interrupted sleep
We’ve all experienced sleep fragmentation, whether in the form of having been on call during our medical training or common experiences like hearing a child cry in the night, a noisy truck pass by, or a dog barking. You may or may not remember that these happened the next day, but they’ve nonetheless interrupted your sleep efficiency.
When you transition laterally across the stages of sleep, that’s what establishes the circadian rhythm and ensures sleep hygiene. Typically, we require approximately 7 hours of restful sleep to do that. But if you fragment or interrupt this process, you more or less move your way erratically through the night, disrupting sleep hygiene and efficiency.
If you have a cognitive awakening during those disruptions, you may recall those events the next day. Or, you may not remember it at all, and such amnestic events are normal for some people with sleep disruptions.
You may also have a sensory arousal, whether it’s due to GER symptoms, auditory stimuli, bumping your toe, or whatever disruptive event. Any of these can cause you to lose that laterality of smooth transition through sleep.
Approximately 20% of the U.S. population have reported GER symptoms at least once a week. Incident data indicate that number may be increasing by as much as 5% a year. Much of that increase is tied to obesity. But nonetheless, it’s a problem on the rise.
It’s important to know this as we start to look at sleep. If GER is acting as a trigger to sleep disruption, you need to ask your patients with this condition about next-day function.
In particular, the next-day function questions to ask are, “How do you feel when you get up? Are you awake and refreshed? Do you have early fatigue? Do you drag yourself out of bed, have daytime somnolence, loss of concentration, or irritability?”
Those are key parameters we can use for looking back to the night before and gauging sleep efficiency. If you’re not asking those questions, you may miss out on identifying a patient having sleep fragmentation.
Sleep’s role in inflammatory disease processes
I now perform an interval assessment of this type not just in my patients with GER disease but across all my patients. I do so because sleep is physiologically important in so many ways.
In patients who have nonalcoholic fatty liver disease and a variety of other liver diseases, we’re finding an increased association with sleep fragmentation outside of sleep apnea.
The same is true with irritable bowel and other functional diseases.
When you have sleep fragmentation in inflammatory bowel disease, you turn on a variety of inflammatory proteins (e.g., C-reactive protein) and cytokines, such as interleukins and tumor necrosis factor alpha. These processes may actually tip somebody over to a pro-inflammatory state.
When it comes to what might be considered a relatively simpler condition like GER disease, Ronnie Fass and colleagues showed a number of years ago via Bernstein testing performed in patients with both fragmented and normal sleep that the sensory thresholds all get lowered in the former group. This is irrespective of whether you have a functional symptom or you’re awakened by bumping your toe, a headache, or having heartburn; your sensory thresholds are lower. As a result, the same stimulus provides a higher sense of awareness. By ramping up that awareness, you increase the interference with the next-day function.
We’ve shown that sleep fragmentation affects a variety of things, including immune function. This may be why many people get sick when they travel in between time zones.
There are also implications relating to things like obesity. When you have sleep dysfunction, you have effects on leptin and ghrelin, contrary to what you would normally want to have. This, in turn, causes adverse effects on stimulation or suppression of satiety or appetite. These are things that I counsel my patients about when I talk about reflux as well as those trying to lose weight.
Sleep disruption affects cortisol stimulation and has a significant correlation with type 2 diabetes, cardiovascular diseases, and even mortality statistics.
Advice for counseling patients
This latest analysis from the Nurses’ Health Study reminds us that a lot of people have reflux and a lot of people have sleep fragmentation. We need to do better in asking our patients if they have symptoms specific not only to reflux but also to potentially sleep-related complications.
The more we do that, the more we individualize patient treatment rather than treating them as a disease state. This, in turn, will allow us to practice personalized medicine. The more we can engage our patients with reflux disease by asking the right questions about next-day function, the better we can do in improving their outcomes.
It’s time for us all to open our eyes to the value of closing them. Let’s talk to our patients with reflux disease in a little bit of a different light, providing a new perspective on strategies we can use to mitigate and deal with those symptoms, thereby preventing the consequences of sleep fragmentation.
Hopefully, this overview gives you some guidance the next time you have a conversation with your patients. It will apply across many, many disease states, and in almost everything we do in gastroenterology.
David A. Johnson, MD, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, Va., and a past president of the American College of Gastroenterology. He reported advising with ISOTHRIVE and Johnson & Johnson.
A version of this article first appeared on Medscape.com.