User login
Stage I mycosis fungoides is the general dermatologist’s bailiwick
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Oral propranolol shown safe in PHACE
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
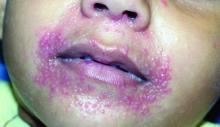
Don’t call it perioral dermatitis
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
What is seronegative rheumatoid arthritis, anyway?
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2020
New topicals coming for pediatric atopic dermatitis
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Study finds spironolactone doesn’t boost breast cancer recurrence
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
Are CRMO and SAPHO syndrome one and the same?
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
EXPERT ANALYSIS FROM RWCS 2020
Expect to encounter more rheumatology/primary immunodeficiency overlap
MAUI, HAWAII – An underlying primary immunodeficiency is far more common among patients in rheumatologists’ offices than is generally appreciated, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“Autoimmunity and immunodeficiency are often incorrectly thought to be mutually exclusive, but I hope to convey to you that they actually run together more than we think, and more than you realize. In the immunodeficiency community, we’re now realizing that autoimmunity can be the first presentation of an immunodeficiency disease,” according to Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
She cited a major retrospective study of 2,183 consecutive patients with primary immunodeficiencies (PIDs), mean age 25.8 years, enrolled in a French national PID registry. Of these patients, 26% had at least one autoimmune or inflammatory condition. The French investigators, all immunologists, categorized 12.8% of the PID patients as having rheumatologic disorders, but Dr. Postolova is convinced this figure would have been substantially higher had a rheumatologist been on the research team. That’s because vasculitis and inflammatory eye disorders weren’t included in the rheumatologic disease category, and psoriatic arthritis was probably present but undiagnosed in some psoriasis patients grouped in the skin disease category.
“Our results demonstrate that autoimmune and inflammatory diseases are much more frequent by a factor of at least 10 in patients with PIDs than in the general population,” the French investigators observed.
Indeed, the relative risks of vasculitis, inflammatory eye disease, and skin disease in patients with PID were increased 13-, 7-, and 10-fold, respectively. French children with PID were at 40-fold increased risk for rheumatoid arthritis and 80-fold elevated risk for inflammatory bowel disease.
Among the PID patients with at least one autoimmune or inflammatory condition, 31.6% had more than one. And therein lies a clue for rheumatologists.
“You might have some patients in your panel who have both arthritis and vasculitis, for example. It’s not that common for our patients to have two of our diseases, but those are the ones who might have an immune dysregulatory inborn error of metabolism mutation that’s driving their disease – and we’re now able to look into that,” Dr. Postolova explained.
At present, more than 300 identified single-gene inborn errors of immunity have been identified, some of which can manifest variously as systemic lupus erythematosus (SLE), arthritis, vasculitis, and/or cytopenias, she noted.
In the French study, onset of autoimmune or inflammatory manifestations of PID occurred across the age spectrum. The age of onset was earlier with T-cell and innate immunity deficiencies than in patients with B-cell deficiencies. The prevalence of an autoimmune or inflammatory disorder was greater than 40% in PID patients age 50 years or older.
“What I want to hammer home is these patients with PID are no longer succumbing to their infections. They’re being identified as having an immunodeficiency, they’re going on antibiotics, they’re strictly monitored, and they’re growing older. And as their aberrant immune system fights an infection, they have more and more time to dysregulate and develop one of our diseases. So in the coming years I think you’re going to see a lot more of these patients show up in your rheumatology clinic because they are getting older,” Dr. Postolova said.
Red flags for underlying PID
Recurrent infections are a hallmark of PIDs. And mutations that cause increased infections can alter central and peripheral tolerance, affecting cell growth, signaling, and survival, which in turn affects immunity.
“As we use biologics in our patients with rheumatologic diseases, I think there’s a cohort of patients we’re starting to identify who are getting very serious recurrent infections. It’s not every patient. But that patient who’s had three or four serious infections, that’s the patient who I think we’ll be able to identify in our clinics through an immunodeficiency evaluation. Likewise, the patients who are not responding to multiple different drugs, that’s where I’d stop and think about an underlying immune deficiency,” she said.
A show of hands indicated only about 25% of her audience of rheumatologists routinely ask new patients if they have a personal or family history of recurrent infections. That should be routine practice, in Dr. Postolova’s view. The 10 warning signs of PID for adults put forth by the Jeffrey Modell Foundation focus on a family or personal history of recurrent ear or sinus infections, deep skin abscesses, pneumonia, viral infections, persistent thrush, or chronic diarrhea with weight loss.
Testing for PID
It’s “absolutely appropriate” to start a work-up for suspected immunodeficiency in a rheumatology clinic, according to Dr. Postolova.
“I think every allergist/immunologist would be grateful if you can just order a quantitative immunoglobulin panel as well as specific antibody titer responses to tetanus, diphtheria, pneumococcal vaccine, and an IgG subclass analysis. That’s half of an immunologist’s initial assessment,” she said.
Corticosteroids and other disease-modifying antirheumatic drugs (DMARDs) affect flow cytometry test results. It’s best to hold off on testing until after a patient has been off therapy for 2-3 months. Similarly, treatment with intravenous immunoglobulin (IVIg) will confound measurement of vaccine antibody titers, so it’s recommended to wait for 3-6 months off IVIg before testing.
Genetic testing for PID has become a lot simpler and more affordable. Numerous companies have developed test kits featuring relatively small panels of selected genes of interest. Dr. Postolova often uses Invitae, a Bay Area company that has a 207-gene panel covered by most insurers with an out-of-pocket cost to the patient of $250.
“Upfront when I have a complicated patient where I am concerned about the possibility of immunodeficiency, I will ask if that’s something they can afford and if they want to move forward with it. Also, Invitae has a patient assistance program. I’ve found this helpful in some of my very complicated patients,” she said.
Get to know your local allergist/immunologist
Many allergist/immunologists have overcome their traditional reservations about immunosuppressing an immunosuppressed individual and are now treating PID/autoimmune disease–overlap patients with the very drugs rheumatologists use every day, including standard DMARDs, rituximab, abatacept, anakinra, and tumor necrosis factor inhibitors.
“I encourage you to work with your allergist/immunologist for these patients because they’re going to need help,” according to Dr. Postolova. “The people in this room are the most equipped to treat the overlap patients because allergists and immunologists don’t really have the training to use these drugs. A lot of them do use them, but you have a better handle on these drugs than other people.”
Dr. Postolova reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – An underlying primary immunodeficiency is far more common among patients in rheumatologists’ offices than is generally appreciated, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“Autoimmunity and immunodeficiency are often incorrectly thought to be mutually exclusive, but I hope to convey to you that they actually run together more than we think, and more than you realize. In the immunodeficiency community, we’re now realizing that autoimmunity can be the first presentation of an immunodeficiency disease,” according to Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
She cited a major retrospective study of 2,183 consecutive patients with primary immunodeficiencies (PIDs), mean age 25.8 years, enrolled in a French national PID registry. Of these patients, 26% had at least one autoimmune or inflammatory condition. The French investigators, all immunologists, categorized 12.8% of the PID patients as having rheumatologic disorders, but Dr. Postolova is convinced this figure would have been substantially higher had a rheumatologist been on the research team. That’s because vasculitis and inflammatory eye disorders weren’t included in the rheumatologic disease category, and psoriatic arthritis was probably present but undiagnosed in some psoriasis patients grouped in the skin disease category.
“Our results demonstrate that autoimmune and inflammatory diseases are much more frequent by a factor of at least 10 in patients with PIDs than in the general population,” the French investigators observed.
Indeed, the relative risks of vasculitis, inflammatory eye disease, and skin disease in patients with PID were increased 13-, 7-, and 10-fold, respectively. French children with PID were at 40-fold increased risk for rheumatoid arthritis and 80-fold elevated risk for inflammatory bowel disease.
Among the PID patients with at least one autoimmune or inflammatory condition, 31.6% had more than one. And therein lies a clue for rheumatologists.
“You might have some patients in your panel who have both arthritis and vasculitis, for example. It’s not that common for our patients to have two of our diseases, but those are the ones who might have an immune dysregulatory inborn error of metabolism mutation that’s driving their disease – and we’re now able to look into that,” Dr. Postolova explained.
At present, more than 300 identified single-gene inborn errors of immunity have been identified, some of which can manifest variously as systemic lupus erythematosus (SLE), arthritis, vasculitis, and/or cytopenias, she noted.
In the French study, onset of autoimmune or inflammatory manifestations of PID occurred across the age spectrum. The age of onset was earlier with T-cell and innate immunity deficiencies than in patients with B-cell deficiencies. The prevalence of an autoimmune or inflammatory disorder was greater than 40% in PID patients age 50 years or older.
“What I want to hammer home is these patients with PID are no longer succumbing to their infections. They’re being identified as having an immunodeficiency, they’re going on antibiotics, they’re strictly monitored, and they’re growing older. And as their aberrant immune system fights an infection, they have more and more time to dysregulate and develop one of our diseases. So in the coming years I think you’re going to see a lot more of these patients show up in your rheumatology clinic because they are getting older,” Dr. Postolova said.
Red flags for underlying PID
Recurrent infections are a hallmark of PIDs. And mutations that cause increased infections can alter central and peripheral tolerance, affecting cell growth, signaling, and survival, which in turn affects immunity.
“As we use biologics in our patients with rheumatologic diseases, I think there’s a cohort of patients we’re starting to identify who are getting very serious recurrent infections. It’s not every patient. But that patient who’s had three or four serious infections, that’s the patient who I think we’ll be able to identify in our clinics through an immunodeficiency evaluation. Likewise, the patients who are not responding to multiple different drugs, that’s where I’d stop and think about an underlying immune deficiency,” she said.
A show of hands indicated only about 25% of her audience of rheumatologists routinely ask new patients if they have a personal or family history of recurrent infections. That should be routine practice, in Dr. Postolova’s view. The 10 warning signs of PID for adults put forth by the Jeffrey Modell Foundation focus on a family or personal history of recurrent ear or sinus infections, deep skin abscesses, pneumonia, viral infections, persistent thrush, or chronic diarrhea with weight loss.
Testing for PID
It’s “absolutely appropriate” to start a work-up for suspected immunodeficiency in a rheumatology clinic, according to Dr. Postolova.
“I think every allergist/immunologist would be grateful if you can just order a quantitative immunoglobulin panel as well as specific antibody titer responses to tetanus, diphtheria, pneumococcal vaccine, and an IgG subclass analysis. That’s half of an immunologist’s initial assessment,” she said.
Corticosteroids and other disease-modifying antirheumatic drugs (DMARDs) affect flow cytometry test results. It’s best to hold off on testing until after a patient has been off therapy for 2-3 months. Similarly, treatment with intravenous immunoglobulin (IVIg) will confound measurement of vaccine antibody titers, so it’s recommended to wait for 3-6 months off IVIg before testing.
Genetic testing for PID has become a lot simpler and more affordable. Numerous companies have developed test kits featuring relatively small panels of selected genes of interest. Dr. Postolova often uses Invitae, a Bay Area company that has a 207-gene panel covered by most insurers with an out-of-pocket cost to the patient of $250.
“Upfront when I have a complicated patient where I am concerned about the possibility of immunodeficiency, I will ask if that’s something they can afford and if they want to move forward with it. Also, Invitae has a patient assistance program. I’ve found this helpful in some of my very complicated patients,” she said.
Get to know your local allergist/immunologist
Many allergist/immunologists have overcome their traditional reservations about immunosuppressing an immunosuppressed individual and are now treating PID/autoimmune disease–overlap patients with the very drugs rheumatologists use every day, including standard DMARDs, rituximab, abatacept, anakinra, and tumor necrosis factor inhibitors.
“I encourage you to work with your allergist/immunologist for these patients because they’re going to need help,” according to Dr. Postolova. “The people in this room are the most equipped to treat the overlap patients because allergists and immunologists don’t really have the training to use these drugs. A lot of them do use them, but you have a better handle on these drugs than other people.”
Dr. Postolova reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – An underlying primary immunodeficiency is far more common among patients in rheumatologists’ offices than is generally appreciated, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“Autoimmunity and immunodeficiency are often incorrectly thought to be mutually exclusive, but I hope to convey to you that they actually run together more than we think, and more than you realize. In the immunodeficiency community, we’re now realizing that autoimmunity can be the first presentation of an immunodeficiency disease,” according to Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
She cited a major retrospective study of 2,183 consecutive patients with primary immunodeficiencies (PIDs), mean age 25.8 years, enrolled in a French national PID registry. Of these patients, 26% had at least one autoimmune or inflammatory condition. The French investigators, all immunologists, categorized 12.8% of the PID patients as having rheumatologic disorders, but Dr. Postolova is convinced this figure would have been substantially higher had a rheumatologist been on the research team. That’s because vasculitis and inflammatory eye disorders weren’t included in the rheumatologic disease category, and psoriatic arthritis was probably present but undiagnosed in some psoriasis patients grouped in the skin disease category.
“Our results demonstrate that autoimmune and inflammatory diseases are much more frequent by a factor of at least 10 in patients with PIDs than in the general population,” the French investigators observed.
Indeed, the relative risks of vasculitis, inflammatory eye disease, and skin disease in patients with PID were increased 13-, 7-, and 10-fold, respectively. French children with PID were at 40-fold increased risk for rheumatoid arthritis and 80-fold elevated risk for inflammatory bowel disease.
Among the PID patients with at least one autoimmune or inflammatory condition, 31.6% had more than one. And therein lies a clue for rheumatologists.
“You might have some patients in your panel who have both arthritis and vasculitis, for example. It’s not that common for our patients to have two of our diseases, but those are the ones who might have an immune dysregulatory inborn error of metabolism mutation that’s driving their disease – and we’re now able to look into that,” Dr. Postolova explained.
At present, more than 300 identified single-gene inborn errors of immunity have been identified, some of which can manifest variously as systemic lupus erythematosus (SLE), arthritis, vasculitis, and/or cytopenias, she noted.
In the French study, onset of autoimmune or inflammatory manifestations of PID occurred across the age spectrum. The age of onset was earlier with T-cell and innate immunity deficiencies than in patients with B-cell deficiencies. The prevalence of an autoimmune or inflammatory disorder was greater than 40% in PID patients age 50 years or older.
“What I want to hammer home is these patients with PID are no longer succumbing to their infections. They’re being identified as having an immunodeficiency, they’re going on antibiotics, they’re strictly monitored, and they’re growing older. And as their aberrant immune system fights an infection, they have more and more time to dysregulate and develop one of our diseases. So in the coming years I think you’re going to see a lot more of these patients show up in your rheumatology clinic because they are getting older,” Dr. Postolova said.
Red flags for underlying PID
Recurrent infections are a hallmark of PIDs. And mutations that cause increased infections can alter central and peripheral tolerance, affecting cell growth, signaling, and survival, which in turn affects immunity.
“As we use biologics in our patients with rheumatologic diseases, I think there’s a cohort of patients we’re starting to identify who are getting very serious recurrent infections. It’s not every patient. But that patient who’s had three or four serious infections, that’s the patient who I think we’ll be able to identify in our clinics through an immunodeficiency evaluation. Likewise, the patients who are not responding to multiple different drugs, that’s where I’d stop and think about an underlying immune deficiency,” she said.
A show of hands indicated only about 25% of her audience of rheumatologists routinely ask new patients if they have a personal or family history of recurrent infections. That should be routine practice, in Dr. Postolova’s view. The 10 warning signs of PID for adults put forth by the Jeffrey Modell Foundation focus on a family or personal history of recurrent ear or sinus infections, deep skin abscesses, pneumonia, viral infections, persistent thrush, or chronic diarrhea with weight loss.
Testing for PID
It’s “absolutely appropriate” to start a work-up for suspected immunodeficiency in a rheumatology clinic, according to Dr. Postolova.
“I think every allergist/immunologist would be grateful if you can just order a quantitative immunoglobulin panel as well as specific antibody titer responses to tetanus, diphtheria, pneumococcal vaccine, and an IgG subclass analysis. That’s half of an immunologist’s initial assessment,” she said.
Corticosteroids and other disease-modifying antirheumatic drugs (DMARDs) affect flow cytometry test results. It’s best to hold off on testing until after a patient has been off therapy for 2-3 months. Similarly, treatment with intravenous immunoglobulin (IVIg) will confound measurement of vaccine antibody titers, so it’s recommended to wait for 3-6 months off IVIg before testing.
Genetic testing for PID has become a lot simpler and more affordable. Numerous companies have developed test kits featuring relatively small panels of selected genes of interest. Dr. Postolova often uses Invitae, a Bay Area company that has a 207-gene panel covered by most insurers with an out-of-pocket cost to the patient of $250.
“Upfront when I have a complicated patient where I am concerned about the possibility of immunodeficiency, I will ask if that’s something they can afford and if they want to move forward with it. Also, Invitae has a patient assistance program. I’ve found this helpful in some of my very complicated patients,” she said.
Get to know your local allergist/immunologist
Many allergist/immunologists have overcome their traditional reservations about immunosuppressing an immunosuppressed individual and are now treating PID/autoimmune disease–overlap patients with the very drugs rheumatologists use every day, including standard DMARDs, rituximab, abatacept, anakinra, and tumor necrosis factor inhibitors.
“I encourage you to work with your allergist/immunologist for these patients because they’re going to need help,” according to Dr. Postolova. “The people in this room are the most equipped to treat the overlap patients because allergists and immunologists don’t really have the training to use these drugs. A lot of them do use them, but you have a better handle on these drugs than other people.”
Dr. Postolova reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2020
PARAGON-HF: Optimal systolic pressure in HFpEF is 120-129 mm Hg
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
FROM ACC 2020
Dupilumab approval sought for AD under age 12
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR