User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Signal of Suicide Ideation With GLP-1 RA Semaglutide, but Experts Urge Caution
A new analysis has detected a signal of suicidal ideation associated with the glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide, especially among individuals concurrently using antidepressants or benzodiazepines.
However, the investigators and outside experts urge caution in drawing any firm conclusions based on the study’s observations.
,” study investigator Georgios Schoretsanitis, MD, PhD, Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
Nonetheless, “physicians prescribing semaglutide should inform their patients about the medications’ risks and assess the psychiatric history and evaluate the mental state of patients before starting treatment with semaglutide,” Dr. Schoretsanitis said.
“For patients with history of mental disorders or suicidal ideation/behaviors/attempts, physicians should be cautious and regularly monitor their mental state while taking semaglutide. If needed, the treating physician should involve different specialists, including a psychiatrist and/or clinical psychologists,” he added.
The study was published online on August 20 in JAMA Network Open.
Emerging Concerns
GLP-1 RAs are increasingly prescribed not only for type 2 diabetes but also for weight loss. However, concerns have emerged about a potential association with suicidality, which has prompted a closer look by regulators in the United States and Europe.
Dr. Schoretsanitis and colleagues evaluated potential signals of suicidality related to semaglutide and liraglutide using data from global World Health Organization database of suspected adverse drug reactions (ADRs).
They conducted sensitivity analyses including patients with co-reported use of antidepressants and benzodiazepines and using dapagliflozin, metformin, and orlistat as comparators.
Between November 2000 and August 2023, there were 107 cases of suicidal and/or self-injurious ADRs reported with semaglutide (median age, 48 years; 55% women) and 162 reported with liraglutide (median age 47 years; 61% women).
The researchers noted that a “significant disproportionality” signal emerged for semaglutide-associated suicidal ideation (reporting odds ratio [ROR], 1.45), when compared with comparator drugs.
This signal remained significant in sensitivity analyses that included patients on concurrent antidepressants (ROR, 4.45) and benzodiazepines (ROR, 4.07), “suggesting that people with anxiety and depressive disorders may be at higher probability of reporting suicidal ideation when medicated with semaglutide,” the authors wrote.
No significant disproportionality signal was detected for liraglutide regarding suicidal ideation (ROR, 1.04).
However, the authors noted that pooled data from previous phase 2 and 3 trials on liraglutide vs placebo for weight management identified a potential risk for suicidal ideation, with nine of 3384 participants in the liraglutide group vs two of 1941 in the placebo group reporting suicidal ideation or behavior during the trial (0.27% vs 0.10%).
More Research Needed
GLP-1 RAs “should be used cautiously until further data are available on this topic,” Dr. Schoretsanitis said.
“Further real-world studies should investigate the risk of suicidal ideation or behavior in people treated with these drugs in every-day clinical practice. We categorically discourage off-label use of GLP1-RA and without any medical supervision,” he added.
The coauthors of an invited commentary published with the study note that between 2020 and 2023, GLP-1 RA use rose 594% in younger people, particularly in women.
This “timely and well-conducted study” by Dr. Schoretsanitis and colleagues adds “an important piece to the very relevant safety issue” related to GLP-1 RAs, wrote Francesco Salvo, MD, PhD, with Université de Bordeaux, and Jean-Luc Faillie, MD, PhD, with Université de Montpellier, both in France.
Pending further studies, the position of the US Food and Drug Administration (FDA) recommending caution “continues to be reasonable. Whatever the cause, depression or suicidality are rare but extremely severe events and need to be prevented and managed as much as possible.
“Waiting for more precise data, GPL-1 receptor agonists, and appetite suppressants in general, should be prescribed with great caution in patients with a history of depression or suicidal attempts, while in patients with new onset of depression without other apparent precipitants, immediate discontinuation of GLP-1 receptor agonists should be considered,” wrote Dr. Salvo and Dr. Faillie.
Outside experts also weighed in on the study in a statement from the UK nonprofit Science Media Centre.
The paper presents, “at best, weak evidence of an association between semaglutide and suicidality,” Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, United Kingdom, said in the statement. “Signal detection studies in pharmacovigilance databases are good for generating hypotheses but are not suitable for assessing whether there is a causal association between a drug and an outcome.”
Stephen Evans, MSc, emeritus professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, cautioned that the study has “major limitations.”
“This paper is based just on spontaneous reports which are sent to regulatory authorities in the country of the person reporting a suspected adverse reaction. These are sent by health professionals and patients to authorities, but are very subject to bias, including effects of media reporting. The evidence is extremely weak for a genuine effect in this instance,” Mr. Evans said.
The study had no specific funding. Dr. Schoretsanitis reported receiving personal fees from HLS, Dexcel, Saladax, and Thermo Fisher outside the submitted work. Dr. Salvo and Dr. Faillie have no conflicts of interest. Dr. Douglas has received research grants from GSK and AstraZeneca. Mr. Evans has no conflicts of interest.
A version of this article appeared on Medscape.com.
A new analysis has detected a signal of suicidal ideation associated with the glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide, especially among individuals concurrently using antidepressants or benzodiazepines.
However, the investigators and outside experts urge caution in drawing any firm conclusions based on the study’s observations.
,” study investigator Georgios Schoretsanitis, MD, PhD, Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
Nonetheless, “physicians prescribing semaglutide should inform their patients about the medications’ risks and assess the psychiatric history and evaluate the mental state of patients before starting treatment with semaglutide,” Dr. Schoretsanitis said.
“For patients with history of mental disorders or suicidal ideation/behaviors/attempts, physicians should be cautious and regularly monitor their mental state while taking semaglutide. If needed, the treating physician should involve different specialists, including a psychiatrist and/or clinical psychologists,” he added.
The study was published online on August 20 in JAMA Network Open.
Emerging Concerns
GLP-1 RAs are increasingly prescribed not only for type 2 diabetes but also for weight loss. However, concerns have emerged about a potential association with suicidality, which has prompted a closer look by regulators in the United States and Europe.
Dr. Schoretsanitis and colleagues evaluated potential signals of suicidality related to semaglutide and liraglutide using data from global World Health Organization database of suspected adverse drug reactions (ADRs).
They conducted sensitivity analyses including patients with co-reported use of antidepressants and benzodiazepines and using dapagliflozin, metformin, and orlistat as comparators.
Between November 2000 and August 2023, there were 107 cases of suicidal and/or self-injurious ADRs reported with semaglutide (median age, 48 years; 55% women) and 162 reported with liraglutide (median age 47 years; 61% women).
The researchers noted that a “significant disproportionality” signal emerged for semaglutide-associated suicidal ideation (reporting odds ratio [ROR], 1.45), when compared with comparator drugs.
This signal remained significant in sensitivity analyses that included patients on concurrent antidepressants (ROR, 4.45) and benzodiazepines (ROR, 4.07), “suggesting that people with anxiety and depressive disorders may be at higher probability of reporting suicidal ideation when medicated with semaglutide,” the authors wrote.
No significant disproportionality signal was detected for liraglutide regarding suicidal ideation (ROR, 1.04).
However, the authors noted that pooled data from previous phase 2 and 3 trials on liraglutide vs placebo for weight management identified a potential risk for suicidal ideation, with nine of 3384 participants in the liraglutide group vs two of 1941 in the placebo group reporting suicidal ideation or behavior during the trial (0.27% vs 0.10%).
More Research Needed
GLP-1 RAs “should be used cautiously until further data are available on this topic,” Dr. Schoretsanitis said.
“Further real-world studies should investigate the risk of suicidal ideation or behavior in people treated with these drugs in every-day clinical practice. We categorically discourage off-label use of GLP1-RA and without any medical supervision,” he added.
The coauthors of an invited commentary published with the study note that between 2020 and 2023, GLP-1 RA use rose 594% in younger people, particularly in women.
This “timely and well-conducted study” by Dr. Schoretsanitis and colleagues adds “an important piece to the very relevant safety issue” related to GLP-1 RAs, wrote Francesco Salvo, MD, PhD, with Université de Bordeaux, and Jean-Luc Faillie, MD, PhD, with Université de Montpellier, both in France.
Pending further studies, the position of the US Food and Drug Administration (FDA) recommending caution “continues to be reasonable. Whatever the cause, depression or suicidality are rare but extremely severe events and need to be prevented and managed as much as possible.
“Waiting for more precise data, GPL-1 receptor agonists, and appetite suppressants in general, should be prescribed with great caution in patients with a history of depression or suicidal attempts, while in patients with new onset of depression without other apparent precipitants, immediate discontinuation of GLP-1 receptor agonists should be considered,” wrote Dr. Salvo and Dr. Faillie.
Outside experts also weighed in on the study in a statement from the UK nonprofit Science Media Centre.
The paper presents, “at best, weak evidence of an association between semaglutide and suicidality,” Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, United Kingdom, said in the statement. “Signal detection studies in pharmacovigilance databases are good for generating hypotheses but are not suitable for assessing whether there is a causal association between a drug and an outcome.”
Stephen Evans, MSc, emeritus professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, cautioned that the study has “major limitations.”
“This paper is based just on spontaneous reports which are sent to regulatory authorities in the country of the person reporting a suspected adverse reaction. These are sent by health professionals and patients to authorities, but are very subject to bias, including effects of media reporting. The evidence is extremely weak for a genuine effect in this instance,” Mr. Evans said.
The study had no specific funding. Dr. Schoretsanitis reported receiving personal fees from HLS, Dexcel, Saladax, and Thermo Fisher outside the submitted work. Dr. Salvo and Dr. Faillie have no conflicts of interest. Dr. Douglas has received research grants from GSK and AstraZeneca. Mr. Evans has no conflicts of interest.
A version of this article appeared on Medscape.com.
A new analysis has detected a signal of suicidal ideation associated with the glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide, especially among individuals concurrently using antidepressants or benzodiazepines.
However, the investigators and outside experts urge caution in drawing any firm conclusions based on the study’s observations.
,” study investigator Georgios Schoretsanitis, MD, PhD, Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York, told this news organization.
Nonetheless, “physicians prescribing semaglutide should inform their patients about the medications’ risks and assess the psychiatric history and evaluate the mental state of patients before starting treatment with semaglutide,” Dr. Schoretsanitis said.
“For patients with history of mental disorders or suicidal ideation/behaviors/attempts, physicians should be cautious and regularly monitor their mental state while taking semaglutide. If needed, the treating physician should involve different specialists, including a psychiatrist and/or clinical psychologists,” he added.
The study was published online on August 20 in JAMA Network Open.
Emerging Concerns
GLP-1 RAs are increasingly prescribed not only for type 2 diabetes but also for weight loss. However, concerns have emerged about a potential association with suicidality, which has prompted a closer look by regulators in the United States and Europe.
Dr. Schoretsanitis and colleagues evaluated potential signals of suicidality related to semaglutide and liraglutide using data from global World Health Organization database of suspected adverse drug reactions (ADRs).
They conducted sensitivity analyses including patients with co-reported use of antidepressants and benzodiazepines and using dapagliflozin, metformin, and orlistat as comparators.
Between November 2000 and August 2023, there were 107 cases of suicidal and/or self-injurious ADRs reported with semaglutide (median age, 48 years; 55% women) and 162 reported with liraglutide (median age 47 years; 61% women).
The researchers noted that a “significant disproportionality” signal emerged for semaglutide-associated suicidal ideation (reporting odds ratio [ROR], 1.45), when compared with comparator drugs.
This signal remained significant in sensitivity analyses that included patients on concurrent antidepressants (ROR, 4.45) and benzodiazepines (ROR, 4.07), “suggesting that people with anxiety and depressive disorders may be at higher probability of reporting suicidal ideation when medicated with semaglutide,” the authors wrote.
No significant disproportionality signal was detected for liraglutide regarding suicidal ideation (ROR, 1.04).
However, the authors noted that pooled data from previous phase 2 and 3 trials on liraglutide vs placebo for weight management identified a potential risk for suicidal ideation, with nine of 3384 participants in the liraglutide group vs two of 1941 in the placebo group reporting suicidal ideation or behavior during the trial (0.27% vs 0.10%).
More Research Needed
GLP-1 RAs “should be used cautiously until further data are available on this topic,” Dr. Schoretsanitis said.
“Further real-world studies should investigate the risk of suicidal ideation or behavior in people treated with these drugs in every-day clinical practice. We categorically discourage off-label use of GLP1-RA and without any medical supervision,” he added.
The coauthors of an invited commentary published with the study note that between 2020 and 2023, GLP-1 RA use rose 594% in younger people, particularly in women.
This “timely and well-conducted study” by Dr. Schoretsanitis and colleagues adds “an important piece to the very relevant safety issue” related to GLP-1 RAs, wrote Francesco Salvo, MD, PhD, with Université de Bordeaux, and Jean-Luc Faillie, MD, PhD, with Université de Montpellier, both in France.
Pending further studies, the position of the US Food and Drug Administration (FDA) recommending caution “continues to be reasonable. Whatever the cause, depression or suicidality are rare but extremely severe events and need to be prevented and managed as much as possible.
“Waiting for more precise data, GPL-1 receptor agonists, and appetite suppressants in general, should be prescribed with great caution in patients with a history of depression or suicidal attempts, while in patients with new onset of depression without other apparent precipitants, immediate discontinuation of GLP-1 receptor agonists should be considered,” wrote Dr. Salvo and Dr. Faillie.
Outside experts also weighed in on the study in a statement from the UK nonprofit Science Media Centre.
The paper presents, “at best, weak evidence of an association between semaglutide and suicidality,” Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, United Kingdom, said in the statement. “Signal detection studies in pharmacovigilance databases are good for generating hypotheses but are not suitable for assessing whether there is a causal association between a drug and an outcome.”
Stephen Evans, MSc, emeritus professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, cautioned that the study has “major limitations.”
“This paper is based just on spontaneous reports which are sent to regulatory authorities in the country of the person reporting a suspected adverse reaction. These are sent by health professionals and patients to authorities, but are very subject to bias, including effects of media reporting. The evidence is extremely weak for a genuine effect in this instance,” Mr. Evans said.
The study had no specific funding. Dr. Schoretsanitis reported receiving personal fees from HLS, Dexcel, Saladax, and Thermo Fisher outside the submitted work. Dr. Salvo and Dr. Faillie have no conflicts of interest. Dr. Douglas has received research grants from GSK and AstraZeneca. Mr. Evans has no conflicts of interest.
A version of this article appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
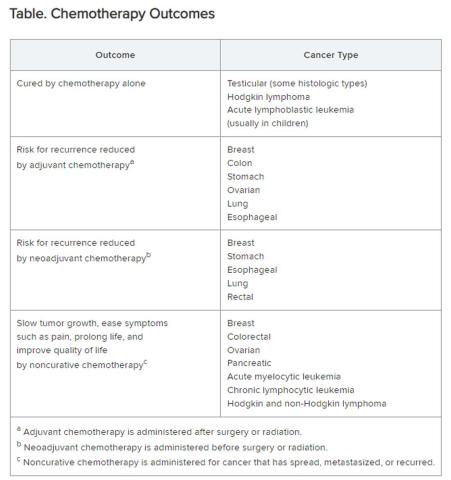
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
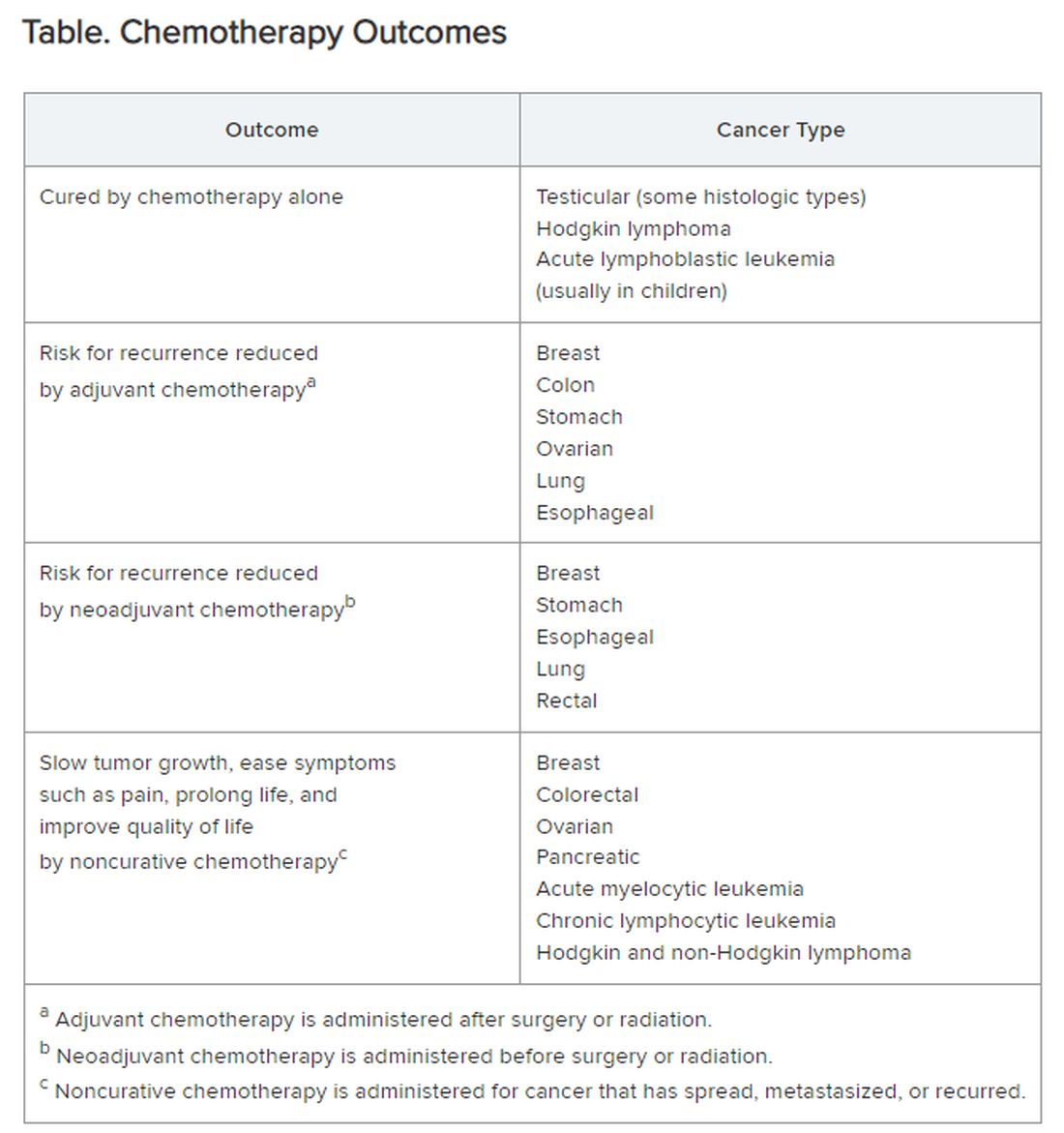
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Severe COVID-19 Tied to Increased Risk for Mental Illness
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Physicians Lament Over Reliance on Relative Value Units: Survey
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
The Most Misinterpreted Study in Medicine: Don’t be TRICCed
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA OKs First-Line Lazertinib With Amivantamab for NSCLC
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
This marks the first approval for lazertinib. Amivantamab was initially approved by the FDA in 2021 and carries a few indications for locally advanced or metastatic NSCLC. Both drugs are manufactured by Janssen Biotech Inc.
“Patients will now have the option of a potential new first-line standard of care with significant clinical benefits over osimertinib,” study investigator Alexander Spira, MD, PhD, director, Virginia Cancer Specialists Research Institute, said in a news release from Johnson & Johnson .
Lazertinib is an oral, highly selective, third-generation EGFR tyrosine kinase inhibitor that can penetrate the brain and amivantamab is a bispecific antibody targeting EGFR and MET.
The approval was based on results from the phase 3 MARIPOSA trial, which showed that the combination reduced the risk of disease progression or death by 30% compared with osimertinib.
The MARIPOSA trial randomly allocated 1074 patients with exon 19 deletion or exon 21 L858R substitution mutation-positive locally advanced or metastatic NSCLC and no prior systemic therapy for advanced disease to amivantamab plus lazertinib, osimertinib alone, or lazertinib alone.
Lazertinib plus amivantamab demonstrated a statistically significant improvement in progression-free survival compared with osimertinib (hazard ratio, 0.70; P < .001). Median progression-free survival was 23.7 months with the combination vs 16.6 months osimertinib alone and 18.5 months with lazertinib alone.
The median duration of response was 9 months longer with the combination compared with osimertinib (25.8 months vs 16.7 months).
The most common adverse reactions (≥ 20%) were rash, nail toxicity, infusion-related reactions (amivantamab), musculoskeletal pain, edema, stomatitis, venous thromboembolism, paresthesia, fatigue, diarrhea, constipation, COVID-19, hemorrhage, dry skin, decreased appetite, pruritus, nausea, and ocular toxicity.
“A serious safety signal of venous thromboembolic events was observed with lazertinib in combination with amivantamab and prophylactic anticoagulation should be administered for the first four months of therapy,” the FDA noted in a statement announcing the approval.
Results from MARIPOSA were first presented at the European Society for Medical Oncology 2023 Congress and published in The New England Journal of Medicine in June. Longer-term follow-up data from MARIPOSA will be presented at the International Association for the Study of Lung Cancer 2024 World Congress on Lung Cancer in September.
A version of this article appeared on Medscape.com.
‘Gift That Keeps Giving’: The Impact of GLP-1 in Asthma
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Alert System Could Warn of Impact of Severe Weather on Health
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
Could Adipose Tissue Be a Better Measure for Obesity Than BMI?
Take a look at any of the evidence-based US obesity treatment guidelines. The key criteria for diagnosing overweight and obesity is based on the body mass index (BMI).
The guidelines also use BMI to stratify care options to decrease cardiovascular risk. For example, persons with BMI ≥30 are classified as having obesity, and antiobesity medications are recommended. Those with BMI ≥ 40 are classified as having severe obesity, and metabolic bariatric surgery may be appropriate.
But where did these cutoff points for more and less aggressive treatments come from? These BMI cutoffs are based primarily on mortality data collected from large non-Hispanic White populations, without data on potential differences by gender and ethnicity.
For example, it is certainly true that those with BMI ≥ 30 have more cardiovascular risk factors than those with BMI < 30. But Asian American individuals have more risk factors at lower BMIs than do White or African American individuals likely because of more visceral fat accumulation at lower BMIs.
Besides the variation in gender and ethnicity, BMI does not take the type and location of body fat into consideration. Adipose tissue in visceral or ectopic areas have much higher risks for disease than subcutaneous adipose tissue because of the associated inflammation. Measures such as waist circumference, waist-to-hip ratio, and skinfold measurements aim to capture this aspect but often fall short because of variation in techniques.
BMI does not account for muscle mass either, so fit athletes and bodybuilders can be classified as having obesity by BMI alone. More accurate body fat percent measures, such as dual-energy X-ray absorptiometry or MRI specifically for ectopic fat, are labor intensive, expensive, and not feasible to perform in a busy primary care or endocrinology clinic.
Assessing Risks From Obesity Beyond BMI
Clearly, better risk measures than BMI are needed, but until they are available, supplemental clinical tools can aid diagnosis and treatment decisions at obesity medicine specialty centers, endocrinology and diabetes centers, and those centers that focus on the treatment of obesity.
For example, a seca scale can measure percent body fat by bioelectric impedance analysis. This technique also has its limitations, but for persons who are well hydrated, it can be used as a baseline to determine efficacy of behavioral interventions, such as resistance-exercise training and a high-protein diet to protect muscle mass as the patient loses weight.
A lot also can be gleaned from diet and exercise history, social history, family history, and physical exam as well as laboratory analyses. For example, an Asian American patient with a BMI of 26 who has been gaining weight mostly in the abdominal region after age 35 years is likely to have cardiometabolic risk, and a family history can solidify that. An exam can show signs of acanthosis nigricans or an enlarged liver and generous abdominal adipose tissue. This would be the patient in whom you would want to obtain a hemoglobin A1c measurement in the chance that it is elevated at > 5.7 mg/dL, suggesting high risk for type 2 diabetes.
A Fibrosis-4 score can assess the risk for liver disease from aspartate transaminase and alanine aminotransferase and platelet count and age, providing clues to cardiometabolic disease risk.
In the next 10, years there may be a better measure for cardiometabolic risk that is more accurate than BMI is. It could be the sagittal abdominal diameter, which has been purported to more accurately measure visceral abdominal fat. But this has not made it to be one of the vital signs in a busy primary care clinic, however.
Will New Body Fat Tools Change Practice?
In the next 10 years, there may be an affordable gadget to scan the body to determine visceral vs subcutaneous deposition of fat — like radiography for tissue. Now, three-dimensional (3D) total-body scanners can obtain body composition, but they are extremely expensive. The more important clinical question is: How will the use of these imaging modalities change your practice protocol for a particular patient?
Think about the FibroScan, a type of ultrasound used to determine fatty liver disease and fibrosis. We order the test for those patients in whom we already have a strong suspicion for liver disease and, in obesity practices, for fatty liver and metabolic-associated fatty liver disease or metabolic associated steatohepatitis.
The test results do much to educate the patient and help the patient understand the need for aggressive treatment for their obesity. But it doesn’t necessarily change the clinician’s practice protocols and decisions. We would still recommend weight management and medications or surgery to patients regardless of the findings.
A FibroScan is an expense, and not all primary care or endocrine practitioners may feel it necessary to purchase one for the added benefit of patient education. And I would argue that a 3D body scanner is a great tool but more for educational purposes than to really determine practice decision-making or outcomes.
In the meantime, an old-fashioned physical examination, along with a thorough medical, social, and family history should give even the busiest primary care provider enough information to decide whether their patient is a candidate for preventive measures to reduce body fat with diet, exercise, and medication as well as whether the patient is a candidate for metabolic bariatric surgery. Higher suspicion of cardiovascular risk at lower BMI ranges for various ethnicities can help primary care providers pick up on the patients with low BMI but who are at higher risk for type 2 diabetes or prediabetes and cardiovascular disease.
So the answer to whether we need a better measure than the BMI: Yes, we do. We need a physical examination on all patients.
Dr. Apovian, professor of medicine, Harvard Medical School, and codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, both in Boston, Massachusetts, disclosed ties with Altimmune, CinFina Pharma, Cowen and Company, EPG Communication Holdings, Form Health, Gelesis, L-Nutra, NeuroBo Pharm, Novo, OptumRx, Pain Script, Palatin, Pursuit by You, Roman Health, Xeno, and Riverview School.
A version of this article appeared on Medscape.com.
Take a look at any of the evidence-based US obesity treatment guidelines. The key criteria for diagnosing overweight and obesity is based on the body mass index (BMI).
The guidelines also use BMI to stratify care options to decrease cardiovascular risk. For example, persons with BMI ≥30 are classified as having obesity, and antiobesity medications are recommended. Those with BMI ≥ 40 are classified as having severe obesity, and metabolic bariatric surgery may be appropriate.
But where did these cutoff points for more and less aggressive treatments come from? These BMI cutoffs are based primarily on mortality data collected from large non-Hispanic White populations, without data on potential differences by gender and ethnicity.
For example, it is certainly true that those with BMI ≥ 30 have more cardiovascular risk factors than those with BMI < 30. But Asian American individuals have more risk factors at lower BMIs than do White or African American individuals likely because of more visceral fat accumulation at lower BMIs.
Besides the variation in gender and ethnicity, BMI does not take the type and location of body fat into consideration. Adipose tissue in visceral or ectopic areas have much higher risks for disease than subcutaneous adipose tissue because of the associated inflammation. Measures such as waist circumference, waist-to-hip ratio, and skinfold measurements aim to capture this aspect but often fall short because of variation in techniques.
BMI does not account for muscle mass either, so fit athletes and bodybuilders can be classified as having obesity by BMI alone. More accurate body fat percent measures, such as dual-energy X-ray absorptiometry or MRI specifically for ectopic fat, are labor intensive, expensive, and not feasible to perform in a busy primary care or endocrinology clinic.
Assessing Risks From Obesity Beyond BMI
Clearly, better risk measures than BMI are needed, but until they are available, supplemental clinical tools can aid diagnosis and treatment decisions at obesity medicine specialty centers, endocrinology and diabetes centers, and those centers that focus on the treatment of obesity.
For example, a seca scale can measure percent body fat by bioelectric impedance analysis. This technique also has its limitations, but for persons who are well hydrated, it can be used as a baseline to determine efficacy of behavioral interventions, such as resistance-exercise training and a high-protein diet to protect muscle mass as the patient loses weight.
A lot also can be gleaned from diet and exercise history, social history, family history, and physical exam as well as laboratory analyses. For example, an Asian American patient with a BMI of 26 who has been gaining weight mostly in the abdominal region after age 35 years is likely to have cardiometabolic risk, and a family history can solidify that. An exam can show signs of acanthosis nigricans or an enlarged liver and generous abdominal adipose tissue. This would be the patient in whom you would want to obtain a hemoglobin A1c measurement in the chance that it is elevated at > 5.7 mg/dL, suggesting high risk for type 2 diabetes.
A Fibrosis-4 score can assess the risk for liver disease from aspartate transaminase and alanine aminotransferase and platelet count and age, providing clues to cardiometabolic disease risk.
In the next 10, years there may be a better measure for cardiometabolic risk that is more accurate than BMI is. It could be the sagittal abdominal diameter, which has been purported to more accurately measure visceral abdominal fat. But this has not made it to be one of the vital signs in a busy primary care clinic, however.
Will New Body Fat Tools Change Practice?
In the next 10 years, there may be an affordable gadget to scan the body to determine visceral vs subcutaneous deposition of fat — like radiography for tissue. Now, three-dimensional (3D) total-body scanners can obtain body composition, but they are extremely expensive. The more important clinical question is: How will the use of these imaging modalities change your practice protocol for a particular patient?
Think about the FibroScan, a type of ultrasound used to determine fatty liver disease and fibrosis. We order the test for those patients in whom we already have a strong suspicion for liver disease and, in obesity practices, for fatty liver and metabolic-associated fatty liver disease or metabolic associated steatohepatitis.
The test results do much to educate the patient and help the patient understand the need for aggressive treatment for their obesity. But it doesn’t necessarily change the clinician’s practice protocols and decisions. We would still recommend weight management and medications or surgery to patients regardless of the findings.
A FibroScan is an expense, and not all primary care or endocrine practitioners may feel it necessary to purchase one for the added benefit of patient education. And I would argue that a 3D body scanner is a great tool but more for educational purposes than to really determine practice decision-making or outcomes.
In the meantime, an old-fashioned physical examination, along with a thorough medical, social, and family history should give even the busiest primary care provider enough information to decide whether their patient is a candidate for preventive measures to reduce body fat with diet, exercise, and medication as well as whether the patient is a candidate for metabolic bariatric surgery. Higher suspicion of cardiovascular risk at lower BMI ranges for various ethnicities can help primary care providers pick up on the patients with low BMI but who are at higher risk for type 2 diabetes or prediabetes and cardiovascular disease.
So the answer to whether we need a better measure than the BMI: Yes, we do. We need a physical examination on all patients.
Dr. Apovian, professor of medicine, Harvard Medical School, and codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, both in Boston, Massachusetts, disclosed ties with Altimmune, CinFina Pharma, Cowen and Company, EPG Communication Holdings, Form Health, Gelesis, L-Nutra, NeuroBo Pharm, Novo, OptumRx, Pain Script, Palatin, Pursuit by You, Roman Health, Xeno, and Riverview School.
A version of this article appeared on Medscape.com.
Take a look at any of the evidence-based US obesity treatment guidelines. The key criteria for diagnosing overweight and obesity is based on the body mass index (BMI).
The guidelines also use BMI to stratify care options to decrease cardiovascular risk. For example, persons with BMI ≥30 are classified as having obesity, and antiobesity medications are recommended. Those with BMI ≥ 40 are classified as having severe obesity, and metabolic bariatric surgery may be appropriate.
But where did these cutoff points for more and less aggressive treatments come from? These BMI cutoffs are based primarily on mortality data collected from large non-Hispanic White populations, without data on potential differences by gender and ethnicity.
For example, it is certainly true that those with BMI ≥ 30 have more cardiovascular risk factors than those with BMI < 30. But Asian American individuals have more risk factors at lower BMIs than do White or African American individuals likely because of more visceral fat accumulation at lower BMIs.
Besides the variation in gender and ethnicity, BMI does not take the type and location of body fat into consideration. Adipose tissue in visceral or ectopic areas have much higher risks for disease than subcutaneous adipose tissue because of the associated inflammation. Measures such as waist circumference, waist-to-hip ratio, and skinfold measurements aim to capture this aspect but often fall short because of variation in techniques.
BMI does not account for muscle mass either, so fit athletes and bodybuilders can be classified as having obesity by BMI alone. More accurate body fat percent measures, such as dual-energy X-ray absorptiometry or MRI specifically for ectopic fat, are labor intensive, expensive, and not feasible to perform in a busy primary care or endocrinology clinic.
Assessing Risks From Obesity Beyond BMI
Clearly, better risk measures than BMI are needed, but until they are available, supplemental clinical tools can aid diagnosis and treatment decisions at obesity medicine specialty centers, endocrinology and diabetes centers, and those centers that focus on the treatment of obesity.
For example, a seca scale can measure percent body fat by bioelectric impedance analysis. This technique also has its limitations, but for persons who are well hydrated, it can be used as a baseline to determine efficacy of behavioral interventions, such as resistance-exercise training and a high-protein diet to protect muscle mass as the patient loses weight.
A lot also can be gleaned from diet and exercise history, social history, family history, and physical exam as well as laboratory analyses. For example, an Asian American patient with a BMI of 26 who has been gaining weight mostly in the abdominal region after age 35 years is likely to have cardiometabolic risk, and a family history can solidify that. An exam can show signs of acanthosis nigricans or an enlarged liver and generous abdominal adipose tissue. This would be the patient in whom you would want to obtain a hemoglobin A1c measurement in the chance that it is elevated at > 5.7 mg/dL, suggesting high risk for type 2 diabetes.
A Fibrosis-4 score can assess the risk for liver disease from aspartate transaminase and alanine aminotransferase and platelet count and age, providing clues to cardiometabolic disease risk.
In the next 10, years there may be a better measure for cardiometabolic risk that is more accurate than BMI is. It could be the sagittal abdominal diameter, which has been purported to more accurately measure visceral abdominal fat. But this has not made it to be one of the vital signs in a busy primary care clinic, however.
Will New Body Fat Tools Change Practice?
In the next 10 years, there may be an affordable gadget to scan the body to determine visceral vs subcutaneous deposition of fat — like radiography for tissue. Now, three-dimensional (3D) total-body scanners can obtain body composition, but they are extremely expensive. The more important clinical question is: How will the use of these imaging modalities change your practice protocol for a particular patient?
Think about the FibroScan, a type of ultrasound used to determine fatty liver disease and fibrosis. We order the test for those patients in whom we already have a strong suspicion for liver disease and, in obesity practices, for fatty liver and metabolic-associated fatty liver disease or metabolic associated steatohepatitis.
The test results do much to educate the patient and help the patient understand the need for aggressive treatment for their obesity. But it doesn’t necessarily change the clinician’s practice protocols and decisions. We would still recommend weight management and medications or surgery to patients regardless of the findings.
A FibroScan is an expense, and not all primary care or endocrine practitioners may feel it necessary to purchase one for the added benefit of patient education. And I would argue that a 3D body scanner is a great tool but more for educational purposes than to really determine practice decision-making or outcomes.
In the meantime, an old-fashioned physical examination, along with a thorough medical, social, and family history should give even the busiest primary care provider enough information to decide whether their patient is a candidate for preventive measures to reduce body fat with diet, exercise, and medication as well as whether the patient is a candidate for metabolic bariatric surgery. Higher suspicion of cardiovascular risk at lower BMI ranges for various ethnicities can help primary care providers pick up on the patients with low BMI but who are at higher risk for type 2 diabetes or prediabetes and cardiovascular disease.
So the answer to whether we need a better measure than the BMI: Yes, we do. We need a physical examination on all patients.
Dr. Apovian, professor of medicine, Harvard Medical School, and codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, both in Boston, Massachusetts, disclosed ties with Altimmune, CinFina Pharma, Cowen and Company, EPG Communication Holdings, Form Health, Gelesis, L-Nutra, NeuroBo Pharm, Novo, OptumRx, Pain Script, Palatin, Pursuit by You, Roman Health, Xeno, and Riverview School.
A version of this article appeared on Medscape.com.