User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Few Severe Toxicities After SBRT in Oligometastatic Cancer
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
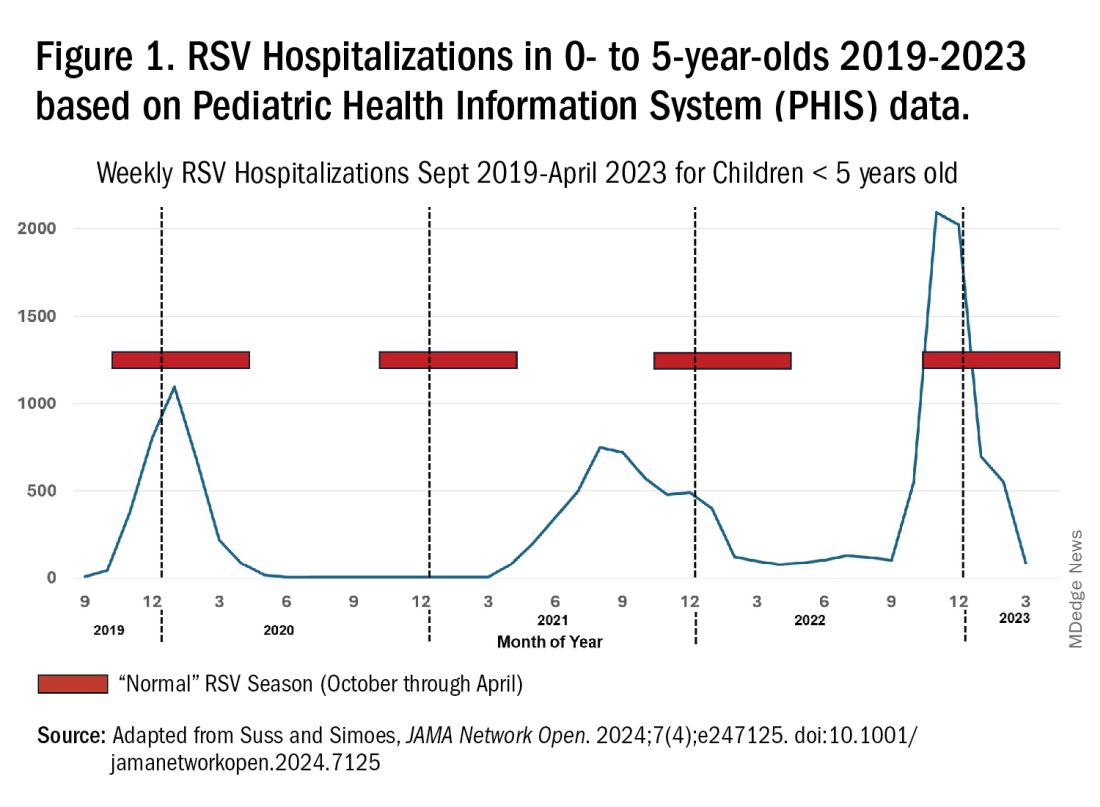
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
It’s Never Too Late to Convince Patients to Quit Smoking
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors Are Seeking Professional Coaches More Often. Here’s Why
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
Whooping Cough Likely on Pace for a 5-Year High
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
FDA Approves Neoadjuvant/Adjuvant Durvalumab for NSCLC
The agency approved durvalumab alongside platinum-containing chemotherapy in the neoadjuvant setting and as monotherapy in the adjuvant setting.
The approval comes shortly after a meeting of FDA’s Oncology Drug Advisory Committee, where agency personnel took AstraZeneca to task for not following its request to include an arm in the approval study, AEGEAN, to clarify whether or not treatment after surgery was necessary.
Even so, advisers at the July 25 meeting voted “yes” to approving the neoadjuvant/adjuvant indication to give patients another immunotherapy option in NSCLC. However, the committee voted unanimously that, going forward, the agency should require — instead of simply request — that companies seeking combined neoadjuvant/adjuvant NSCLC indications show that patients actually need treatment after surgery.
The new approval is durvalumab’s first indication for resectable NSCLC. The agent has been previously approved for unresectable or metastatic disease as well as extensive-stage small cell lung cancer, locally advanced or metastatic biliary tract cancer, unresectable hepatocellular carcinoma, and advanced or recurrent endometrial cancer.
AEGEAN included 802 patients with previously untreated and resectable stage IIA-IIIB squamous or nonsquamous NSCLC. Patients were randomly assigned to receive either durvalumab (400 patients) or placebo (402 patients) on a background of platinum-based chemotherapy every 3 weeks for four cycles then, following surgery, durvalumab or placebo once a month for a year.
The pathologic complete response rate was 17% in the durvalumab arm vs 4.3% in the placebo arm. At 12 months, event-free survival was 73.4% with durvalumab vs 64.5% with placebo. Overall survival differences have not been tested for statistical significance, but there was “no clear detriment” with durvalumab, FDA said in a press release.
Adverse reactions in 20% or more of durvalumab recipients included anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash; 1.7% of durvalumab recipients and 1% of placebo recipients could not have surgery because of side effects during neoadjuvant treatment.
The dosage for patients weighing > 30 kg is 1500 mg every 3 weeks before surgery and every 4 weeks afterward. For patients who weigh less than that, the recommended dosage is 20 mg/kg.
Durvalumab costs around $1,053 for 120 mg, according to drugs.com.
A version of this article appeared on Medscape.com.
The agency approved durvalumab alongside platinum-containing chemotherapy in the neoadjuvant setting and as monotherapy in the adjuvant setting.
The approval comes shortly after a meeting of FDA’s Oncology Drug Advisory Committee, where agency personnel took AstraZeneca to task for not following its request to include an arm in the approval study, AEGEAN, to clarify whether or not treatment after surgery was necessary.
Even so, advisers at the July 25 meeting voted “yes” to approving the neoadjuvant/adjuvant indication to give patients another immunotherapy option in NSCLC. However, the committee voted unanimously that, going forward, the agency should require — instead of simply request — that companies seeking combined neoadjuvant/adjuvant NSCLC indications show that patients actually need treatment after surgery.
The new approval is durvalumab’s first indication for resectable NSCLC. The agent has been previously approved for unresectable or metastatic disease as well as extensive-stage small cell lung cancer, locally advanced or metastatic biliary tract cancer, unresectable hepatocellular carcinoma, and advanced or recurrent endometrial cancer.
AEGEAN included 802 patients with previously untreated and resectable stage IIA-IIIB squamous or nonsquamous NSCLC. Patients were randomly assigned to receive either durvalumab (400 patients) or placebo (402 patients) on a background of platinum-based chemotherapy every 3 weeks for four cycles then, following surgery, durvalumab or placebo once a month for a year.
The pathologic complete response rate was 17% in the durvalumab arm vs 4.3% in the placebo arm. At 12 months, event-free survival was 73.4% with durvalumab vs 64.5% with placebo. Overall survival differences have not been tested for statistical significance, but there was “no clear detriment” with durvalumab, FDA said in a press release.
Adverse reactions in 20% or more of durvalumab recipients included anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash; 1.7% of durvalumab recipients and 1% of placebo recipients could not have surgery because of side effects during neoadjuvant treatment.
The dosage for patients weighing > 30 kg is 1500 mg every 3 weeks before surgery and every 4 weeks afterward. For patients who weigh less than that, the recommended dosage is 20 mg/kg.
Durvalumab costs around $1,053 for 120 mg, according to drugs.com.
A version of this article appeared on Medscape.com.
The agency approved durvalumab alongside platinum-containing chemotherapy in the neoadjuvant setting and as monotherapy in the adjuvant setting.
The approval comes shortly after a meeting of FDA’s Oncology Drug Advisory Committee, where agency personnel took AstraZeneca to task for not following its request to include an arm in the approval study, AEGEAN, to clarify whether or not treatment after surgery was necessary.
Even so, advisers at the July 25 meeting voted “yes” to approving the neoadjuvant/adjuvant indication to give patients another immunotherapy option in NSCLC. However, the committee voted unanimously that, going forward, the agency should require — instead of simply request — that companies seeking combined neoadjuvant/adjuvant NSCLC indications show that patients actually need treatment after surgery.
The new approval is durvalumab’s first indication for resectable NSCLC. The agent has been previously approved for unresectable or metastatic disease as well as extensive-stage small cell lung cancer, locally advanced or metastatic biliary tract cancer, unresectable hepatocellular carcinoma, and advanced or recurrent endometrial cancer.
AEGEAN included 802 patients with previously untreated and resectable stage IIA-IIIB squamous or nonsquamous NSCLC. Patients were randomly assigned to receive either durvalumab (400 patients) or placebo (402 patients) on a background of platinum-based chemotherapy every 3 weeks for four cycles then, following surgery, durvalumab or placebo once a month for a year.
The pathologic complete response rate was 17% in the durvalumab arm vs 4.3% in the placebo arm. At 12 months, event-free survival was 73.4% with durvalumab vs 64.5% with placebo. Overall survival differences have not been tested for statistical significance, but there was “no clear detriment” with durvalumab, FDA said in a press release.
Adverse reactions in 20% or more of durvalumab recipients included anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash; 1.7% of durvalumab recipients and 1% of placebo recipients could not have surgery because of side effects during neoadjuvant treatment.
The dosage for patients weighing > 30 kg is 1500 mg every 3 weeks before surgery and every 4 weeks afterward. For patients who weigh less than that, the recommended dosage is 20 mg/kg.
Durvalumab costs around $1,053 for 120 mg, according to drugs.com.
A version of this article appeared on Medscape.com.
What Every Provider Should Know About Type 1 Diabetes
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
FDA ‘Recalls’ Often Leave Targeted Medical Devices in Use
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
After Rapid Weight Loss, Monitor Antiobesity Drug Dosing
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
FTC Interim Report on Pharmacy Middlemen Is First Step of Many Needed in Addressing Drug Costs, Access
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.
Rising consolidation among pharmacy benefit managers (PBMs) allows the companies to profit at the expense of patients and independent pharmacists. That’s the conclusion of a recent Federal Trade Commission (FTC) report on interim findings from the agency’s ongoing investigation of PBMs.
Lawmakers are increasingly scrutinizing the industry amid growing concern among physicians and consumers about how PBMs exploit their market dominance. The top six PBMs managed 94% of US drug claims in 2023, with the majority handled by the industry’s three giants: CVS Caremark, Cigna’s Express Scripts, and United Healthcare’s OptumRx.
PBMs manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. They act as middlemen between health insurers and pharmacies, developing formularies of covered drugs and promising savings from the discounts and rebates they negotiate with drugmakers.
The FTC’s interim report found that the giant PBMs often exercise significant control over what drugs are available and at what price and which pharmacies patients can use to access their prescribed medications. Consumers suffer as a result, the report concluded.
Madelaine A. Feldman, MD, vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations, shared her perspective on the FTC report in an email Q&A with this news organization. She is affiliated with The Rheumatology Group, based in Metairie, Louisiana.
Dr. Feldman has long tracked the PBM industry and appeared as a witness before influential government panels, including the House Energy and Commerce Committee. She has highlighted for lawmakers the challenges physicians face in helping patients get needed medicines.
For example, she shared cases of PBMs steering patients toward the more expensive of three widely used rheumatoid arthritis medicines that have a similar mechanism of action, the Janus kinase (JAK) inhibitors, Dr. Feldman said.
One of the drugs cost roughly half of the other two — about $30,000 per year vs $65,000-$70,000. Yet only the two expensive drugs were included in the PBM formulary. As a result, the cheapest drug holds only a sliver of market share; the remainder is dominated by the two expensive products, she told the House Oversight and Accountability Committee in 2021.
This Q&A has been edited for length and clarity.
What would you want federal and state policymakers to do in response to the FTC’s report?
I think Congress needs to clearly delineate the differences between anticompetitive pharmacy issues, drug pricing issues, and their effect on formulary construction issues.
Lawmakers should demand more transparency and consider legislation that would remove perverse incentives that prompt PBMs to choose higher priced drugs for their formularies.
That may require other regulatory or legislative actions to ensure lower prices (not higher kickbacks) are incentivized. Ultimately, in order to gain true competition within the health insurance business, these oligopolies of multiple businesses need to be broken up. Anything less seems to be nibbling around the edges and allows the Big Three to continue their “whack-a mole” in circumventing piecemeal regulatory and legislative policies.
You’ve followed PBM practices closely for many years. Was there anything in this interim FTC report that surprised you?
Though not surprised, I am glad that it was released because it had been a year in investigation and there were many requests for some type of substantive report.
Two things that are missing that I feel are paramount are investigating how the three big PBMs are causing physical harm to patients as a result of the profit component in formulary construction and the profound financial impact of hidden PBM profit centers in self-insured employer health plans.
What we have seen over the years is the result of the perverse incentives for the PBMs to prefer the most profitable medications on their formularies.
They use utilization management tools such as step therapy, nonmedical switching, and exclusions to maintain their formularies’ profitability. These tools have been shown to delay and deny the proper care of patients, resulting in not just monetary but physical harm as well.
I would think the physical harm done to patients in manipulating the formularies should be addressed in this report as well and, in fact, may be the most important aspect of consumer protection of this issue.
In terms of the FTC’s mission to not “unduly burden” legitimate business, I would like to see the sector of self-insured employers addressed.
The report details how PBMs steer prescriptions to their affiliated pharmacies. The FTC says that can push smaller pharmacies out of the market, ultimately leading to higher costs and lower quality services for people. What’s your perspective?
Having more community pharmacies is better than having less. We are seeing more “pharmacy deserts” in rural areas as a result of many community pharmacies having to close.
The FTC voted 4-1 to allow staff to issue the interim report, with Commissioner Melissa Holyoak voting no. And some FTC commissioners seem divided on the usefulness of the report. Why?
Commissioner Holyoak states the “the Report leaves us without a better understanding of the competition concerns surrounding PBMs or how consumers are impacted by PBM practices.”
I do agree with her that the harm to patients’ medical status was not even addressed as far as I could tell in this report. There are multiple news articles and reports on the harms inflicted upon patients by the UM tools that drive the construction of ever changing formularies, all based on contracting with manufacturers that result in the highest profit for the PBM.
Holyoak also states, “Among other critical conclusions, the Report does not address the seemingly contradictory conclusions in the 2005 Report that PBMs, including vertically owned PBMs, generated cost savings for consumers.”
That may be true, but in 2005, the rise of PBMs was just beginning and the huge vertical and horizontal integration had yet to begin. Also, 2005 was still in the beginning of the biologic drug deluge, which did create competition to get on the formulary. Since then, PBMs have done nothing to control the rise in prices but instead, apparently have used the competition to get higher price concessions from manufacturers based on a percentage of the list price to line their pockets.
Commissioner Ferguson agreed with releasing the report but he had many issues with this report including the lack of PBM response.
I do agree with him that the FTC should have used some type of “force” to get the information they needed from the PBMs. The Big Three are known for obfuscation and delaying providing information to legislative and regulatory agencies.
A version of this article appeared on Medscape.com.