User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Two COVID-19 outpatient antibody drugs show encouraging results
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
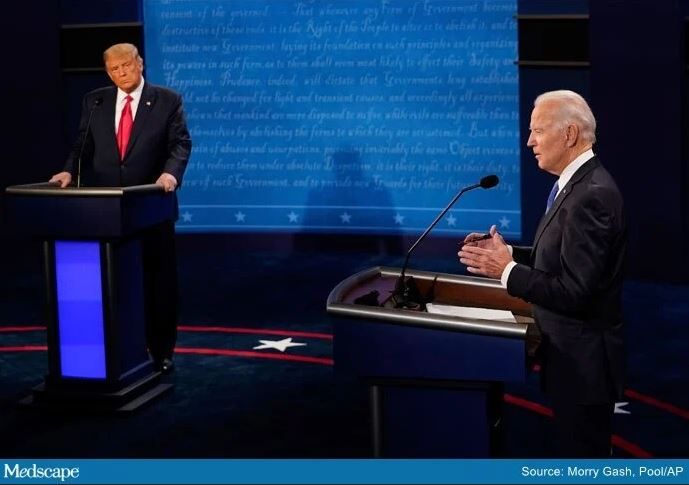
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Tocilizumab stumbles as COVID-19 treatment, narrow role possible
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Tocilizumab (Actemra/RoActemra) was not found to have any clear role as a treatment for COVID-19 in four new studies.
Three randomized controlled trials showed that the drug either had no benefit or only a modest one, contradicting a large retrospective study that had hinted at a more robust effect.
“This is not a blockbuster,” said David Cennimo, MD, an infectious disease expert at Rutgers New Jersey Medical School, Newark, New Jersey. “This is not something that’s going to revolutionize our treatment of COVID-19.”
But some researchers still regard these studies as showing evidence that the drug benefits certain patients with severe inflammation.
The immune response to SARS-CoV-2 includes elevated levels of the cytokine interleukin-6 (IL-6). In some patients, this response becomes a nonspecific inflammation, a “cytokine storm,” involving edema and inflammatory cell infiltration in the lungs. These cases are among the most severe.
Dexamethasone has proved effective in controlling this inflammation in some patients. Researchers have theorized that a more targeted suppression of IL-6 could be even more effective or work in cases that don’t respond to dexamethasone.
A recombinant monoclonal antibody, tocilizumab blocks IL-6 receptors. It is approved by the US Food and Drug Administration for use in patients with rheumatologic disorders and cytokine release syndrome induced by chimeric antigen receptor T-cell therapy.
Current National Institutes of Health (NIH) guidelines recommend against the use of tocilizumab as a treatment for COVID-19, despite earlier observational studies that suggested the drug might help patients with moderate to severe disease. Controlled trials were lacking until now.
The most hopeful results in this batch came from the CORIMUNO-19 platform of open-label, randomized controlled trials of immune modulatory treatments for moderate or severe COVID-19 in France.
Published in JAMA Internal Medicine , the trial recruited patients from nine French hospitals. Patients were eligible if they required at least 3 L/min of oxygen without ventilation or admission to the intensive care unit.
The investigators randomly assigned 64 patients to receive tocilizumab 8 mg/kg body weight intravenously plus usual care and 67 patients to usual care alone. Usual care included antibiotic agents, antiviral agents, corticosteroids, vasopressor support, and anticoagulants.
After 4 days, the investigators scored patients on the World Health Organization 10-point Clinical Progression Scale. Twelve of the patients who received tocilizumab scored higher than 5 vs 19 of the patients in the usual care group, with higher scores indicating clinical deterioration.
After 14 days, 24% of the patients taking tocilizumab required either noninvasive ventilation or mechanical ventilation or had died, vs 36% in the usual care group (median posterior hazard ratio [HR], 0.58; 90% credible interval, 0.33 – 1.00).
“We reduced the risk of dying or requiring mechanical ventilation, so for me, the study was positive,” said Olivier Hermine, MD, PhD, a professor of hematology at Paris Descartes University in Paris, France.
However, there was no difference in mortality at 28 days. Hermine hopes to have longer-term outcomes soon, he told Medscape Medical News.
A second randomized controlled trial, also published in JAMA Internal Medicine , provided less hope. In this RCT-TCZ-COVID-19 Study Group trial, conducted at 24 Italian centers, patients were enrolled if their partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratios were between 200 and 300 mm Hg and if their inflammatory phenotypes were defined by fever and elevated C-reactive protein level.
The investigators randomly assigned 60 patients to receive tocilizumab 8 mg/kg up to a maximum of 800 mg within 8 hours of randomization, followed by a second dose after 12 hours. They assigned 66 patients to a control group that received supportive care until clinical worsening, at which point patients could receive tocilizumab as a rescue therapy.
Of the patients who received tocilizumab, 28.3% showed clinical worsening within 14 days, compared to 27.0% in the control group (rate ratio, 1.05; 95% CI, 0.59 – 1.86). There was no significant difference between the groups in terms of the proportion admitted to intensive care. The researchers stopped the trial prematurely because tocilizumab did not seem to be making a difference.
The BACC Bay Tocilizumab Trial was conducted at seven Boston hospitals. The results, which were published in The New England Journal of Medicine, were also discouraging.
In that trial, enrolled patients met two sets of parameters. First, the patients had at least one of the following signs: C-reactive protein level higher than 50 mg/L, ferritin level higher than 500 ng/mL, D-dimer level higher than 1000 ng/mL, or a lactate dehydrogenase level higher than 250 U/L. Second, the patients had to have at least two of the following signs: body temperature >38° C, pulmonary infiltrates, or the need for supplemental oxygen to maintain an oxygen saturation greater than 92%.
The investigators randomly assigned 161 patients to receive intravenous tocilizumab 8 mg/kg up to 800 mg and 81 to receive a placebo.
They didn’t find a statistically significant difference between the groups. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% CI, 0.38 – 1.81; P = .64). The hazard ratio for disease worsening was 1.11 (95% CI, 0.59 – 2.10; P = .73). At 14 days, the conditions of 18.0% of the patients who received tocilizumab and 14.9% of the patients who received the placebo worsened.
In contrast to these randomized trials, STOP-COVID, a retrospective analysis of 3924 patients, also published in JAMA Internal Medicine, found that the risk for death was lower for patients treated with tocilizumab compared with those not treated with tocilizumab (HR, 0.71; 95% CI, 0.56 – 0.92) over a median follow-up period of 27 days.
Also on the bright side, none of the new studies showed significant adverse reactions to tocilizumab.
More randomized clinical trials are underway. In press releases announcing topline data, Roche reported mostly negative results in its phase 3 COVACTA trial but noted a 44% reduction in the risk for progression to death or ventilation in its phase 3 IMPACTA trial. Roche did not comment on the ethnicity of its COVACTA patients; it said IMPACTA enrolled a majority of Hispanic patients and included large representations of Native American and Black patients.
Results don’t support routine use
Commenting on the new studies, editorialists in both JAMA Internal Medicine and The New England Journal of Medicine concluded that the tocilizumab results were not strong enough to support routine use.
“My take-home point from looking at all of these together is that, even if it does help, it’s most likely in a small subset of the population and/or a small effect,” Cennimo told Medscape Medical News.
But the NIH recommendation against tocilizumab goes too far, argued Cristina Mussini, MD, a professor of infectious diseases at the University of Modena and Reggio Emilia in Italy, who is a coauthor of a cohort study of tocilizumab and served on the CORIMUNO-19 Data Safety and Monitoring Board.
“I really think it’s too early to recommend against it because at least two clinical trials showed protection against mechanical ventilation and death,” she said.
She prescribes tocilizumab for patients who have not been helped by dexamethasone. “It’s just a rescue drug,” she told Medscape Medical News. “It’s not something you use for everybody, but it’s the only weapon we have now when the patient is really going to the intensive care unit.”
The BACC Bay Tocilizumab Trial was funded by Genentech/Roche. Genentech/Roche provided the drug for the CORIMUNO and RCT-TCZ-COVID-19 trials. The STOP-COVID study was supported by grants from the NIH and by the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor. Cennimo, Hermine, and Mussini have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lilly stops antibody trial in hospitalized COVID-19 patients, other trials continue
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
Eli Lilly announced it will halt its ACTIV-3 trial evaluating the antibody bamlanivimab in combination with remdesivir for people hospitalized with COVID-19, after new evidence regarding efficacy emerged.
The new data from the National Institutes of Health suggest that the experimental neutralizing antibody therapy does not offer significant clinical benefit for people with more advanced COVID-19 illness, according to a company statement.
Eli Lilly also announced it plans to continue its other trials evaluating the antibody, including those assessing a potential role in treating people in the earlier stages of COVID-19.
“While there was insufficient evidence that bamlanivimab improved clinical outcomes when added to other treatments in hospitalized patients with COVID-19, we remain confident based on data from Lilly’s BLAZE-1 study that bamlanivimab monotherapy may prevent progression of disease for those earlier in the course of COVID-19,” the statement reads.
The ACTIV-3 trial was paused on October 13 after a data and safety monitoring board cited safety concerns.
The most recent data update that triggered an end to the trial did not reveal any significant differences in safety, though.
This article first appeared on Medscape.com.
COVID-19: Immunity from antibodies may decline rapidly
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.
Antibody response to the SARS-CoV-2 virus wanes over time, latest research has suggested.
An ongoing study led by Imperial College London (ICL) found that the proportion of people testing positive for COVID-19 antibodies dropped by 26.5% over a 3-month period between June and September.
The findings from a non–peer reviewed preprint suggested that infection with SARS-CoV-2 confers only limited protection against reinfection.
Professor Paul Elliott, director of the REACT-2 programme at ICL, said: “Testing positive for antibodies does not mean you are immune to COVID-19.
“It remains unclear what level of immunity antibodies provide, or for how long this immunity lasts.”
Experts said that, while the findings suggested that immunity might fade over time, the severity of illness from further infections could be reduced.
Antibody prevalence declined in all adults
Results from cross-sectional studies over the 3-month period involved 365,104 adults who self-administered a lateral flow immunoassay test.
There were 17,576 positive tests over the three rounds.
Antibody prevalence, adjusted for test characteristics and weighted to the adult population of England, declined from 6.0% to 4.4%, a reduction of 26.5% over the 3 months.
The decline was seen in all age groups. However, the lowest prevalence of a positive test, and the largest fall, was seen in those aged 75 years and older.
No change was seen in positive antibody tests in health care workers over the 3 months.
The results suggested that people who did not show symptoms of COVID-19 were more likely to lose detectable antibodies sooner than those who did show symptoms.
Prof Helen Ward, one of the lead authors of the report said that, while it was clear that the proportion of people with antibodies was falling over time, “We don’t yet know whether this will leave these people at risk of reinfection with the virus that causes COVID-19, but it is essential that everyone continues to follow guidance to reduce the risk to themselves and others.”
Results ‘weaken argument for herd immunity’
Commenting on the results to the Science Media Centre, Rowland Kao, professor of veterinary epidemiology and data science at the University of Edinburgh, warned that, if the results were correct, “any strategy that relies on ‘herd immunity’ lacks credibility.”
However, he added that, “while the decline is substantial, nevertheless substantial proportions of the population do retain some immune response, over 4 months after the peak of the epidemic”.
Eleanor Riley, professor of immunology and infectious disease, also from the University of Edinburgh, said it was too early to assume that immunity to SARS-CoV-2 did not last because “the study does not look at antibody concentrations, antibody function, or other aspects of immunity such as T-cell immunity and does not look at the trajectory of antibody levels in the same individuals over time”.
However, she said the findings did not mean that a vaccine would be ineffective because vaccines contained adjuvants that could induce durable immune responses, particularly with multiple immunizations.
“What is not clear is how quickly antibody levels would rise again if a person encounters the SARS-CoV-2 virus a second time. It is possible they will still rapidly respond, and either have a milder illness, or remain protected through immune memory,” commented Dr. Alexander Edwards, associate professor in biomedical technology at the University of Reading.
Health Minister Lord Bethell said: “Regardless of the result of an antibody test, everyone must continue to comply with government guidelines including social distancing, self-isolating, and getting a test if you have symptoms, and always remember: hands, face, space.”
This article first appeared on Medscape.com.
Health care workers implore OSHA for more oversight on COVID-19 safety
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
Last spring, when Cliff Willmeng, RN, was working at United Hospital in St. Paul, Minnesota, he’d take off his personal protective equipment (PPE) in the same hallway where children were transported from ambulances to the neighboring Children’s Hospital emergency department. Stretchers would roll across red tape on the floor that designated the area as a “hot zone.” The door from a break room was about 10 feet away.
Willmeng has been a union activist all his life, but he’d never filed a complaint with the Occupational Safety and Health Administration (OSHA) until the COVID-19 pandemic hit.
Concerned about the inadequate space for doffing PPE and other situations in which the spread of SARS-CoV-2 seemed possible, Willmeng and other colleagues filed multiple OSHA complaints with the Minnesota Department of Labor in March and April. Willmeng was also worried about bringing SARS-CoV-2 on his scrubs home to his wife and kids, and he started wearing hospital-supplied scrubs that were meant for doctors and that were washed on site, which was against hospital policy. The hospital fired Willmeng on May 8, citing code of conduct and respectful workplace violations arising from the uniform dispute.
In August, the state agency issued Willmeng’s hospital a $2,100 fine for failure to comply with guidance regarding “respiratory protection” in response to worker complaints over the fact that they were instructed to restaple elastic bands on N95 masks early in the pandemic. In a statement, United Hospital said it contested the citation, and it is in discussions with Minnesota OSHA. “We have and continue to instruct employees not to alter N95 respirators or reuse damaged or soiled N95 respirators,” such as when the straps are broken, the statement says.
Minnesota OSHA has received three times as many emails and phone calls from workers and employers requesting information and assistance during the pandemic, compared with last year, said spokesperson James Honerman. “If Minnesota OSHA is made aware of a workplace safety or health issue, it assesses the situation and determines how best to respond, including conducting a workplace investigation.”
But Willmeng, who has been out of work since he was fired, says that without a receipt or confirmation from OSHA, he has no way of knowing whether there has been any follow-up regarding his complaints. Minnesota OSHA said workers should receive a letter once a case is resolved.
Like Willmeng’s case, none of the more than 10,000 COVID-related complaints the federal OSHA office has received from across the country have resulted in meaningful sanctions. Unions have picketed local OSHA offices and publicized complaints on behalf of their members to protest what they see as a lack of oversight. Legislators have called on US Department of Labor Secretary Eugene Scalia to step up enforcement.
For many health care workers, complaining to OSHA is a last resort after failing to get satisfactory responses from supervisors and appealing to unions for help. But with such minimal oversight from OSHA, some union leaders and legislators say it’s actually more dangerous than not having workplace safety enforcement at all. Lack of directives from the Trump administration has left the agency without the teeth it has cut under previous administrations, and recent changes to the agency’s rules raise questions about whether companies are ever required to report workers’ hospitalizations due to COVID-19.
“It’s so ineffective that it’s more dangerous to workers,” said Kim Cordova, president of United Food and Commercial Workers (UFCW) Local 7, which represents 22,000 health care and other workers in Colorado and Wyoming. “Employers only do what they’re forced to do.” Instead of deterring a multi-billion-dollar company, she said, such low fines signal that a company doesn’t need to worry about COVID-related safety.
“OSHA is doing a lamentably poor job protecting workers during the pandemic,” said James Brudney, JD, a professor at Fordham Law School, in New York, and former chief counsel of the U.S. Senate Subcommittee on Labor. “I’m not alone in saying that the agency has performed so badly.”
Former government officials writing in JAMA were similarly critical: “In the face of the greatest worker health crisis in recent history, OSHA, the lead government agency responsible for worker health and safety, has not fulfilled its responsibilities.”
What could have been
There were early signs that the agency wouldn’t be heavy-handed about COVID-19 safety concerns, Brudney said.
The agency could have issued Emergency Temporary Standards, rules it can put in place during pandemics that address specific short-term concerns. These rules could have required employers to take infection-control measures to protect workers, including mask wearing, providing proper PPE, and screening for COVID-19 symptoms. “That’s what the agency is supposed to do. They’re supposed to respond to an emergency with emergency measures,” Brudney said.
But despite legislative pressure and a court case, Secretary of Labor Eugene Scalia has declined to do so, saying that the agency would instead rely on its regular general duty clause, which is always in place to keep workplaces free from hazards that “cause death or serious physical harm.” The agency invoked the general duty clause for COVID-19–related violations for the first time in September to levy modest fines.
In response to a request for an interview, a Department of Labor spokesperson said that preexisting OSHA requirements apply to workers during the pandemic, including providing PPE for workers and assessing sanitation and cleanliness standards. The agency has issued specific guidance to companies on pandemic preparedness, she said, and that it responds to all complaints. Additionally, she cited whistleblower laws that make it illegal for employers to retaliate against employees for making safety and health complaints.
The federal OSHA office received 10,868 COVID-related complaints from Feb. 1 through Oct. 20, citing issues ranging from failure to provide proper PPE to not informing workers about exposures. As of Oct. 22, a total of 2,349 of the complaints involved healthcare workers. This count doesn’t include the untold number of “informal” complaints handled by state OSHA offices.
In a recent JAMA opinion piece, two former government officials agreed that “the federal government has not fully utilized OSHA’s public safety authority” and called the issuing of an Emergency Temporary Standard that would require employers to develop and implement infection control plans “the most important action the federal government could take” to protect workers.
“Employers are more likely to implement these controls if they are mandated by a government agency that has adequate enforcement tools to ensure compliance,” wrote former Assistant Secretary of Labor David Michaels, PhD, MPH, now at the Milken Institute School of Public Health of the George Washington University, Washington, and Gregory Wagner, MD, a former senior adviser at the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention, now at the Harvard T.H. Chan School of Public Health, Boston.
They cited the success of a standard that OSHA issued in 1991 in response to the HIV/AIDS crisis. “The bloodborne pathogens standard has contributed to a substantial decline in health care worker risk for bloodborne diseases like HIV and hepatitis B and C,” they wrote. In a new report for the Century Foundation, the pair offered recommendations to the federal government for controlling the spread of the disease by ramping up OSHA’s role.
OSHA did issue a response plan that requires employers to report in regard to employees who experienced workplace exposures to SARS-CoV-2 and who were hospitalized with COVID-19 or died of the disease within certain time frames, but recent changes to these rules make experts question whether companies are in fact required to report hospitalizations.
In its second revision of guidelines, added to its FAQ page on Sept. 30, the agency said that, in order to be reportable, “an in-patient hospitalization due to COVID-19 must occur within 24 hours of an exposure to SARS-CoV-2 at work” and that the employer must report the hospitalization within 24 hours of learning both that the employee has been hospitalized and that the reason for the hospitalization was a work-related case of COVID-19. Previously, the 24-hour hospitalization window started at the time of diagnosis of the disease, rather than the work-related exposure.
The agency subsequently dropped the first citation it had issued for a COVID-related violation, even though the company, a nursing home, had already agreed to pay $3,904 for reporting employee hospitalizations late.
“It’s a step backwards from an important workplace and public health function that OSHA should be doing,” said Wagner, coauthor of the JAMA opinion piece.
Even without issuing Emergency Temporary Standards, critics say OSHA could have acted much earlier. OSHA issued its first COVID-related federal citation, the one against the nursing home that was dropped, in May for events that occurred in mid-April. The second COVID-related federal citation came in July.
The agency could also charge much more substantial fines for the citations it has issued. If a medical facility was cited for a PPE violation, such as the Minnesota hospital where workers were told to restaple the elastic bands on N95s, the agency could have cited the hospital for one violation per employee. Such fines based on multiple violations could add up to the hundreds of thousands to millions of dollars.
“It would send a signal to the highest-risk employers that these are violations that need to be addressed immediately,” Brudney said.
Many of the 22 state OSHA offices appear to be more responsive to COVID-related complaints than the federal agency, creating a system in which health care workers have substantially different rights from one state to the next. The governor of California, for example, recently authorized California’s OSHA division to consider COVID-19 an imminent hazard, to prohibit workers from entering areas where the hazard exists, and to require employers to disclose exposures. The state also recently issued large fines for COVID safety issues: $222,075 to frozen food manufacturer Overhill Farms and $214,080 to employment agency Jobsource North America.
Elsewhere, state laws such as New Jersey’s Conscientious Employee Protection Act give workers the right to refuse to work in unsafe situations, Brudney said. “A lot more action is going on at the state level because so little is being done at the federal level,” he said. “Some of it is governors committed to protecting essential workers and their families.”
Unions call for sanctions
Unions are both decrying the lack of enforcement thus far and seeking more oversight going forward.
In August, the National Nurses’ United (NNU) union filed a complaint to implore OSHA to investigate the country’s biggest hospital systems, HCA Healthcare, which operates 184 hospitals and about 2,000 other care sites in 21 states and the United Kingdom. The union describes how, throughout HCA hospitals, there is an environment conducive to the spread of coronavirus. Nurses share space and equipment, such as computers, desks, phones, bathrooms, and break rooms, where staff take off masks to eat and drink. The complaint also describes how there is resistance to testing nurses and a lack of communication about infections among colleagues.
“When they have total disregard for safety, they should be punished to the utmost,” said Markowitz, noting that HCA Healthcare is worth $40 billion. “They can penalize them, but if it’s unsafe conditions for RNs and healthcare workers, we know it’s unsafe for the patients. There needs to be drastic measures to prevent hospital corporations from behaving that way.”
In a statement, HCA spokesman Harlow Sumerford said the company has followed CDC guidance for protecting frontline caregivers. “We’re proud of our response and the significant resources we’ve deployed to help protect our colleagues. Meanwhile, the NNU has chosen to use this pandemic as an opportunity to gain publicity by attacking hospitals across the country,” Sumerford said.
Members of the union recently protested in front of the federal OSHA offices in Denver.
After several months, OSHA finally penalized a meat packing plant where eight workers (six union members) had died of COVID-19 last spring. But the amount – $15,615 – was so low that Cordova worries it will actually have a worse impact than no fine.
“It’s more dangerous to workers because now employers know [they won’t be punished meaningfully],” she said. “During the pandemic, OSHA has been absolutely absent.”
Thus, the recent picketing outside the offices in Denver. But, Cordova noted, it’s unlikely OSHA employees saw them. Their own offices were deemed too risky to stay open during the pandemic. They were vacant.
A version of this article originally appeared on Medscape.com.
Around the world in 24 hours: A snapshot of COVID’s global havoc
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
Some medical societies feature sessions at their annual meetings that feel like they’re 24 hours long, yet few have the courage to schedule a session that actually runs all day and all night. But the five societies sponsoring the IDWeek conference had that courage. The first 24 hours of the meeting was devoted to the most pressing infectious-disease crisis of the last 100 years: the COVID-19 pandemic. They called it “COVID-19: Chasing the Sun.”
Dr. Fauci predicts a vaccine answer in mid-November
In the first segment, at 10 am Eastern time, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and the nation’s top infectious-disease expert, began the day by noting that five of the six companies the US invested in to develop a vaccine are conducting phase 3 trials. He said, “we feel confident that we will have an answer likely in mid-November to the beginning of December as to whether we have a safe and effective vaccine”. He added he was “cautiously optimistic” that “we will have a safe and effective vaccine by the end of the year, which we can begin to distribute as we go into 2021.” He highlighted the COVID-19 Prevention Network website for more information on the trials.
Glaring racial health disparities in U.S.
Some of the most glaring health disparities surrounding COVID-19 in the United States were described by Carlos del Rio, MD, professor of medicine at Emory University in Atlanta, Georgia. He pointed out that while white people have about 23 cases per 10,000 population, Blacks have about 62 cases per 10,000, and Latinos have 73 cases per 10,000. While whites don’t see a huge jump in cases until age 80, he said, “among Blacks and Latinos you start seeing that huge increase at a younger age. In fact, starting at age 20, you start seeing a major, major change.”
COVID-19 diagnostics
Audrey Odom John, MD, PhD, chief of pediatric infectious diseases at Children’s Hospital of Philadelphia, is working on a new way of diagnosing COVID-19 infection in children by testing their breath. “We’re really taking advantage of a fundamental biological fact, which is that people stink,” she said. Breath shows the health of the body as a whole, “and it’s easy to see how breath volatiles might arise from a respiratory infection.” Testing breath is easy and inexpensive, which makes it particularly attractive as a potential test globally, she said.
Long-term effects of COVID-19
Post-COVID illness threatens to overwhelm the health system in the United States, even if only 1% of the 8 million people who have been infected have some sort of long-term deficit, “which would be a very conservative estimate,” said John O’Horo, MD, MPH, with the Mayo Clinic in Rochester, Minn. Neurologic dysfunction is going to be a “fairly significant thing to keep an eye on,” he added. Preeti Malani, MD, chief health officer in infectious diseases at the University of Michigan, Ann Arbor, said the emotional aspects of the illness are “striking” and may be the major long-term effect for most patients.
Challenging cases in COVID-19: Through fire and water
In a case presented to panelists during an afternoon session, a Mexican-born woman, 42, presents to urgent care with fever, dyspnea, dry cough, and pleuritic pain, for over a week. Multiple family members have had recent respiratory illness as well. She is obese, on no medications, was not traveling. She’s a nonsmoker and lives in a multigenerational household in the Mission District of San Francisco. Her heart rate is 116, respiratory rate is 36, and her oxygen saturation on room air is 77%. She is admitted to a local hospital and quickly declines, is intubated and started on hydroxychloroquine (HCQ). One day later she is transferred to a hospital for consideration of extracorporeal membrane oxygenation (ECMO).
Panelists were asked a variety of questions about how they would treat this patient. For example, would they continue HCQ? Ravina Kullar, PharmD, MPH, an infectious disease expert from Newport Beach, Calif., answered that she would not continue the HCQ because of lack of evidence and potential harms. Asked whether she would start remdesivir, Dr. Kullar said she would steer her away from that if the patient developed renal failure. Co-moderator Peter Chin-Hong, MD, a medical educator with the University of California, San Francisco, noted that contact tracing will be important as the patient returns to her housing-dense community.
In-hospital infection prevention
The CDC acknowledged aerosol spread of COVID-19 this month, but David Weber, MD, MPH, professor in infectious diseases at the University of North Carolina at Chapel Hill, said, “this does not change anything we need to do in the hospital,” as long as protective pandemic protocols continue to be followed.
There is no evidence, he noted, that SARS-CoV-2 is transmitted far enough that a hospitalized patient could infect people in other rooms or corridors or floors. Opening windows in COVID-19 patients’ rooms is “not an option,” he said, and could be harmful as fungal elements in outside air may introduce new pathogens. The degree to which improved ventilation systems reduce transmission has not been identified and studies are needed to look at that, he said.
Preventing COVID transmission in the community
Mary-Margaret Fill, MD, deputy state epidemiologist in Tennessee, highlighted COVID-19’s spread in prisons. As of mid-October, she said, there are more than 147,000 cases among the U.S. prison population and there have been 1,246 deaths. This translates to a case rate of about 9800 cases per 100,000 people, she said, “double the highest case rate for any state in the country and over three times greater than our national case rate of about 2,500 cases per 100,000 persons.”
Testing varies widely, she noted. For instance, some states test only new prisoners, and some test only when they are symptomatic. One of the strategies to fight this spread is having staff, who go in and out of the community, be assigned to work with only certain groups at a prison. Another is widespread testing of all prisoners. And when prisoners have to leave the prison for care or court dates, a third strategy would be quarantining them upon their return.
COVID-19 vaccines
As the session stretched into the evening in the United States, Mary Marovich, MD, director of vaccine research, AIDS division, with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health, said while each of the government-funded vaccine studies has its own trial, there are standardized objectives for direct comparisons. The studies are being conducted within the same clinical trial networks, and collaborative laboratories apply the same immunoassays and define the infections in the same way. They are all randomized, placebo-controlled trials and all but one have a 30,000-volunteer sample size. She said that while a vaccine is the goal to end the pandemic, monoclonal antibodies, such as those in convalescent plasma, “may serve as a critical bridge.”
The good, the bad, and the ugly during COVID-19 in Latin America
Latin America and the Caribbean are currently the regions hardest hit by COVID-19. Gustavo D. Lopardo, of the Asociacion Panamericana de Infectologia, noted that even before the pandemic Latin America suffered from widespread poverty and inequality. While overcrowding and poverty are determining factors in the spread of the virus, diabetes and obesity – both highly prevalent – are worsening COVID outcomes.
The countries of the region have dealt with asynchronous waves of transmission within their borders by implementing different containment strategies, with dissimilar results. The presenters covered the spectrum of the pandemic, from the “ugly” in Peru, which has the highest mortality rate in the region, to the “good” in Uruguay, where testing is “winning against COVID-19.” Paradoxically, Chile has both the highest cumulative incidence and the lowest case fatality rate of COVID-19 in the region.
In the social and political turmoil imposed by COVID-19, Clóvis Arns da Cunha, MD, president of the Brazilian Society of Infectious Diseases and professor at the Federal University of Paraná, pointed out that “fake news [has become] a public health problem in Brazil” and elsewhere.
Diagnostics and therapeutics in Latin America
Eleven of the 15 countries with the highest death rate in the world are located in Latin America or the Caribbean. Dr. Arns de Cunha pointed out that tests are hard to come by and inadequate diagnostic testing is a major problem. Latin American countries have not been able to compete with the United States and Europe in purchasing polymerase chain reaction test kits from China and South Korea. The test is the best diagnostic tool in the first week of symptoms, but its scale-up has proved to be a challenge in Latin America.
Furthermore, the most sensitive serological markers, CLIA and ECLIA, which perform best after 2 weeks of symptom onset, are not widely available in Latin America where many patients do not have access to the public health system. The detection of silent hypoxemia in symptomatic patients with COVID-19 can save lives; hence, Arns da Cunha praised the program that distributed 100,000 digital oximeters to hundreds of cities in Brazil, targeting vulnerable populations.
The COVID-19 experience in Japan
Takuya Yamagishi, MD, PhD, chief of the Antimicrobial Resistance Research Center at the National Institute of Infectious Diseases in Japan, played an instrumental role in the epidemiological investigation that took place on the Diamond Princess Cruise Ship in February 2020. That COVID-19 outbreak is the largest disease outbreak involving a cruise ship to date, with 712 confirmed COVID-19 cases and 13 deaths.
The ship-based quarantine prompted a massive public health response with unique challenges. In those early days, investigators uncovered important facts about COVID-19 epidemiology, generating hot debates regarding the public health strategy at the time. Notably, the majority of asymptomatically infected persons remained asymptomatic throughout the course of the infection, transmission from asymptomatic cases was almost as likely as transmission from symptomatic cases, and isolation of passengers in their cabins prevented inter-cabin transmission but not intra-cabin transmission.
Swift response in Asia Pacific region
Infectious-disease experts from Taiwan, Singapore, and Australia, who have been at the forefront of clinical care, research, and policy-making, spoke about their experiences.
Taiwan was one of the first countries to adopt a swift response to COVID-19, shortly after they recognized an outbreak of pneumonia of unknown etiology in China and long before the WHO declared a public health emergency, said Ping-Ing Lee, MD, PhD, from the National Taiwan University Children’s Hospital.
The country began onboard health checks on flights from Wuhan as early as Dec. 31, 2019. Dr. Lee attributed Taiwan’s success in prevention and control of COVID-19 to the rigorous use of face masks and environmental disinfection procedures. Regarding the country’s antilockdown stance, he said, “Lockdown may be effective; however, it is associated with a tremendous economic loss.”
In his presentation on remdesivir vs corticosteroids, David Lye, MBBS, said, “I think remdesivir as an antiviral seems to work well given early, but steroids will need to be studied further in terms of its conflicting evidence in multiple well-designed RCTs as well as [their] potential side effects.” He is director of the Infectious Disease Research and Training Office, National Centre for Infectious Diseases, Singapore.
Allen C. Cheng, MBBS, PhD, of Monash University in Melbourne, noted that “control is possible. We seemed to have controlled this twice at the moment with fairly draconian action, but every day does matter.”
China past the first wave
China has already passed the first wave, explained Lei Zhou, MD, of the Chinese Center for Disease Control and Prevention, but there are still some small-scale resurgences. So far a total of four waves have been identified. She also mentioned that contact tracing is intense and highlighted the case of Xinfadi Market in Beijing, the site of an outbreak in June 2020.
Gui-Qiang Wang, MD, from the Department of Infectious Disease, Peking University First Hospital, emphasized the importance of a chest CT for the diagnosis of COVID-19. “In the early stage of the disease, patients may not show any symptoms; however, on CT scan you can see pneumonia. Also, early intervention of high-risk groups and monitoring of warning indicators for disease progression is extremely important,” he said.
“Early antiviral therapy is expected to stop progression, but still needs evaluation,” he said. “Convalescent plasma is safe and effective, but its source is limited; steroid therapy needs to explore appropriate population and timing; and thymosin α is safe, and its effect on outcomes needs large-sample clinical trial.”
Time to Call for an ‘Arab CDC?’
The eastern Mediterranean is geographically, politically, economically, and religiously a very distinct and sensitive region, and “COVID-19 is an added insult to this already frail region of the world,” said Zaid Haddadin, MD, Vanderbilt University Medical Center, Nashville, Tenn.
Poor healthcare and poor public health services are a consequence of weak and fragile governments and infrastructure, the result of war and regional conflicts in many countries. Millions of war refugees live in camps with high population densities and shared facilities, which makes social distancing and community mitigation very challenging. Moreover, the culture includes frequent large social gatherings. Millions of pilgrims visit holy sites in different cities in these countries. There is also movement due to trade and tourism. Travel restrictions are challenging, and there is limited comprehension of precautionary measures.
Najwa Khuri-Bulos, professor of pediatrics and infectious diseases at the University of Jordan, was part of a task force headed by the country’s Ministry of Health. A lockdown was implemented, which helped flatten the curve, but the loosening of restrictions has led to a recent increase in cases. She said, “No country can succeed in controlling spread without the regional collaboration. Perhaps it is time to adopt the call for an Arab CDC.”
Africa is “not out of the woods yet”
The Africa CDC has three key pillars as the foundation for their COVID-19 strategy: preventing transmission, preventing deaths, and preventing social harm, according to Raji Tajudeen, MBBS, FWACP, MPH, head of the agency’s Public Health Institutes and Research Division. Africa, with 1.5 million cases of COVID-19, accounts for 5% of global cases. With a recovery rate of 83% and a case fatality rate of 2.4%, the African continent has fared much better than the rest of the world. “Significant improvements have been made, but we are not out of the woods yet,” he cautioned.
Richard Lessells, PhD, from the University of KwaZulu-Natal, agreed. “Unfortunately, South Africa has not been spared from the worst effects of this pandemic despite what you might read in the press and scientific coverage.” He added, “Over 50% of cases and up to two thirds of the deaths in the African region are coming from South Africa.” A bigger challenge for South Africa has been maintaining essential health services during the COVID-19 pandemic, especially since it is also at the heart of the HIV pandemic. On the brighter side, HIV itself has not emerged as a risk factor for COVID-19 infection or severe disease in South Africa.
Dimie Ogoina, MBBS, FWACP, president of the Nigerian Infectious Diseases Society, stated that COVID-19 has significantly affected access to healthcare in Nigeria, particularly immunizations and antenatal care. Immunization uptake is likely to have dropped by 50% in the country.
Diagnostic pitfalls in COVID-19
Technical errors associated with the SARS-CoV-2 diagnostic pipeline are a major source of variations in diagnosis, explained Jim Huggett, PhD, senior lecturer, analytical microbiology, University of Surrey, Guildford, England. He believes that PCR assays are currently too biased for a single cutoff to be broadly used, and false-positive signals are most likely because of contamination.
Dana Wolf, MD, Clinical Virology Unit, Hadassah Hebrew University Medical Center in Israel, presented a large-scale data analysis of more than 133,000 pooled samples. Such a pooling strategy appeared to be highly efficient for a wide range of prevalence rates (<1% to 6%). “Our empirical evidence strongly projects on the feasibility and benefits of pooling in the current pandemic setting, to enhance continued surveillance, control, and community reopening,” she said.
Corine Geurts van Kessel, MD, PhD, Department of Virology, Erasmus University Rotterdam (the Netherlands), discussing antibodies testing for SARS-CoV-2, pointed out that disease severity can affect testing accuracy. “Reinfection cases tell us that we cannot rely on immunity acquired by natural infection to confer herd immunity,” she said.
Misinformation in the first digital pandemic
The world is not only facing a devastating pandemic, but also an alarming “infodemic” of misinformation. Between January and March 2020, a new COVID-19–related tweet appeared on Twitter every 45 milliseconds. Müge Çevik, MD, MSc, MRCP, an infectious disease clinician, scientist, and science communicator, said that “the greatest challenge for science communication is reaching the audience.”
People have always been skeptical of science reporting by journalists and would rather have scientists communicate with them directly, she noted. Science communication plays a dual role. “On one hand is the need to promote science to a wide audience in order to inform and educate and inspire the next generation of scientists, and on the other hand there is also a need to engage effectively in public dialogue,” she added. Dr. Çevik and colleagues think that “The responsibility of academics should not end with finding the truth. It should end after communicating it.”
Treatment in the ICU
Matteo Bassetti, MD, with the University of Genoa (Italy), who was asked about when to use remdesivir in the intensive care unit and for how long, said, “In the majority of cases, 5 days is probably enough.” However, if there is high viremia, he said, physicians may choose to continue the regimen beyond 5 days. Data show it is important to prescribe this drug for patients with oxygen support in an early phase, within 10 days of the first symptoms, he added. “In the late phase, there is a very limited role for remdesivir, as we know that we are already out of the viremic phase.” He also emphasized that there is no role for hydroxychloroquine or lopinavir-ritonavir.
Breaking the chains of transmission
During the wrap-up session, former US CDC Director Tom Frieden, MD, said, “We’re not even halfway through it” about the pandemic trajectory. “And we have to be very clear that the risk of explosive spread will not end with a vaccine.” He is now president and CEO of Resolve to Save Lives.
Different parts of the world will have very different experiences, Dr. Frieden said, noting that Africa, where 4% of the population is older than 65, has a very different risk level than Europe and the United States, where 10%-20% of people are in older age groups.
“We need a one-two punch,” he noted, first preventing spread, and when it does happen, boxing it in. Mask wearing is essential. “States in the US that mandated universal mask-wearing experienced much more rapid declines (in cases) for every 5 days the mandate was in place.”
Michael Ryan, MD, executive director for the WHO’s Health Emergencies Programme, added, “We need to collectively recommit to winning this game. We know how to break the chains of transmission. We need recommitment to a scientific, societal, and political strategy, and an alliance – a contract – between those entities to try to move us forward.”
This article first appeared on Medscape.com.
FROM IDWEEK 2020
Trump and Biden face off over COVID-19, ACA in final debate
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
The COVID-19 pandemic figured prominently in the final debate between President Donald Trump and former Vice President Joe Biden when they met on stage for a 90-minute debate in Nashville, Tennessee, Thursday evening.
The adequacy of the COVID-19 response to date, the likely timeline for vaccine availability, and how to reopen businesses while keeping Americans safe were among the points on which the two candidates disagreed. The two candidates also sparred over the value of the Affordable Care Act (ACA) and the future of healthcare in the United States.
Trump and Biden also differed on whether or not the country is facing a “dark winter” because of the pandemic.
Moderator Kristen Welker, NBC News White House correspondent, asked Trump to comment on the fact that 40,000 people are in the hospital on debate night with COVID-19 and that 16,000 have died since the last presidential debate.
Trump said, “2.2 million people modeled out were expected to die.” He said COVID-19 is a worldwide disease that does not only affect the United States.
“The mortality rate is down 85%, and the excess mortality is also down,” he added. He pointed out that previous spikes in Florida, Texas, and Arizona are now gone, and “spikes and surges in other places will soon be gone.
“It will go away, we are rounding the corner,” Trump said. “From personal experience, I was in the hospital, I had it, and they gave me a therapeutic, some would call it a cure...and now they say I’m immune. Whether it’s for a month or lifetime, nobody has been able to say that, but I’m immune.”
Biden countered by saying that “220,000 people are dead. If you hear nothing else I say tonight, hear this: Anyone who’s responsible for that many deaths should not remain president of the United States of America.”
Biden said there are a thousand deaths a day now and that there are over 70K new cases per day. “The expectation is we will have another 200,000 people down before the end of this year. If we just all wore these masks, we could save 100,000.”
“The New England Journal of Medicine said the way the president has handled this is absolutely tragic,” Biden added.
Vaccine timeline
Welker asked Trump if he could guarantee that there will be a COVID-19 vaccine within weeks.
“I can’t guarantee that, but it will be by end of the year. It will be distributed very quickly,” Trump said. He added that three leading vaccine developers, Johnson & Johnson, Moderna, and Pfizer, “are doing very well.”
“We’re about to go into a dark winter and he has no clear plan,” Biden said. “There is no prospect there will be a vaccine for most Americans by middle of next year.”
“It will not be a dark winter,” Trump responded.
Reopening the economy
Trump and Biden disagreed on how aggressively the economy should be reopened in light of the pandemic.
“I want to open the schools. We can’t keep this country closed,” Trump said. “This is a massive country with a massive economy.” He pointed out that rates of depression and suicide have risen because of the economic shutdown. “The cure cannot be worse than the problem.
“His Democrat governors...shut down so tight, and they’re dying,” the president added, gesturing toward Biden. “We are not going to shut down. We are going to open the schools.” As an example of the resiliency of young people, he mentioned that his son Barron tested positive for COVID-19 and recovered.
“I would shut down the virus, not the country,” Biden said. “It’s his ineptitude that caused so many schools and businesses to close in large part. Instead of being in a sand trap playing golf, he should have been negotiating with Nancy Pelosi.”
“He says we’re learning to live with it,” the former vice president said, but instead, “people are learning to die with it.”
Biden added that reopening the economy and minimizing transmission of COVID-19 are not mutually exclusive. “We can walk and chew gum at the same time.”
Divergence over the ACA
The fate of the ACA also garnered considerable attention. The discussion underlined a vast difference of opinion between the two candidates on the US healthcare system.
The moderator asked Trump what he would do for the 20 million Americans who get their healthcare through the ACA if it’s taken away.
“Through the legislature, I terminated the individual mandate, the worst part of Obamacare,” Trump said. “And now it’s in court because Obamacare is no good.
“Preexisting conditions will stay,” Trump added.
“I want to terminate Obamacare, and I want to come up with a beautiful healthcare [plan],” Trump added, turning the discussion toward private health insurance. “One thing that is very important is we have 180 million out there who have great private healthcare. Joe Biden will terminate all of their healthcare.”
Trump described Biden’s plan as “socialized medicine.” He also emphasized that protections for people with preexisting conditions “will stay.”
The Trump administration is supporting a lawsuit to overturn the ACA. The suit was filed by 18 Republican-led states. Arguments before the US Supreme Court on the constitutionality of the ACA are scheduled for November 10.
The moderator asked what Biden plans to do if the ACA is struck down. “I will pass Obamacare with a public option ― that will be ‘Bidencare.’ “ He said his plan will reduce premiums and drug prices. “I support private insurance. No one lost their private insurance under Obamacare.
“There is no way he can protect preexisting conditions,” Biden said. He added that 10 million people have already lost their private healthcare through unemployment during the pandemic.
Muting the mic
Following what many described as a chaotic first debate at the Cleveland Clinic in Ohio on September 29, the Commission on Presidential Debate opted to allow the muting of the microphone during the first 2 minutes of remarks made by each candidate during each debate segment.
The muting of the microphones appeared to prevent crosstalk during the beginning of each segment of the debate. The candidates did manage to talk over and interrupt each other, as well as the moderator, during portions of the debate.
This article first appeared on Medscape.com.
COVID-19 a new opportunity for suicide prevention
The ongoing COVID-19 pandemic poses clear threats to mental well-being, but an increase in suicide is not inevitable if appropriate action is taken, one expert says.
“Increases in suicide rates should not be a foregone conclusion, even with the negative effects of the pandemic. If the lessons of suicide prevention research are heeded during and after the pandemic, this potential for increased risk could be substantially mitigated,” writes Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, in an invited communication in JAMA Psychiatry.
“This is a moment in history when suicide prevention must be prioritized as a serious public health concern,” she writes.
Mitigating suicide risk
Although evidence from the first 6 months of the pandemic reveal specific effects on suicide risk, real-time data on suicide deaths are not available in most regions of the world. From emerging data from several countries, there is no evidence of increased suicide rates during the pandemic thus far, Moutier notes.
Still, a number of pandemic-related risk factors could increase individual and population suicide risk.
They include deterioration or recurrence of serious mental illness; increased isolation, loneliness, and bereavement; increased use of drugs and alcohol; job loss and other financial stressors; and increases in domestic violence.
There are mitigating strategies for each of these “threats to suicide risk.” The science is “very clear,” Moutier told Medscape Medical News.
“Suicide risk is never a situation of inevitability. It’s dynamic, with multiple forces at play in each individual and in the population. Lives can be saved simply by making people feel more connected to each other, that they are part of a larger community,” she added.
The political will
Moutier notes that prior to the pandemic, four countries ― Finland, Norway, Sweden, and Australia ― had fully implemented national suicide prevention plans and had achieved reductions in their national suicide rates. However, in the United States, the suicide rate has been steadily increasing since 1999.
A Centers for Disease Control and Prevention survey released in August 2020 found that 40% of US adults reported symptoms of depression, anxiety, or increased substance use during COVID-19 and that about 11% reported suicidal ideation in the past month, all increases from prior surveys.
COVID-19 presents a “new and urgent opportunity” to focus political will, federal investments, and the global community on suicide prevention, Moutier writes.
“The political will to address suicide has actually moved in the right direction during COVID, as evidenced by a number of pieces of legislation that have suddenly found their way to passing that we’ve been working on for years,” she said in an interview.
One example, she said, is the National Suicide Hotline Designation Act, signed into law earlier this month by President Donald Trump.
As previously reported, under the law, beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
Moutier reports no relevant financial relationships.
This article first appeared on Medscape.com.
The ongoing COVID-19 pandemic poses clear threats to mental well-being, but an increase in suicide is not inevitable if appropriate action is taken, one expert says.
“Increases in suicide rates should not be a foregone conclusion, even with the negative effects of the pandemic. If the lessons of suicide prevention research are heeded during and after the pandemic, this potential for increased risk could be substantially mitigated,” writes Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, in an invited communication in JAMA Psychiatry.
“This is a moment in history when suicide prevention must be prioritized as a serious public health concern,” she writes.
Mitigating suicide risk
Although evidence from the first 6 months of the pandemic reveal specific effects on suicide risk, real-time data on suicide deaths are not available in most regions of the world. From emerging data from several countries, there is no evidence of increased suicide rates during the pandemic thus far, Moutier notes.
Still, a number of pandemic-related risk factors could increase individual and population suicide risk.
They include deterioration or recurrence of serious mental illness; increased isolation, loneliness, and bereavement; increased use of drugs and alcohol; job loss and other financial stressors; and increases in domestic violence.
There are mitigating strategies for each of these “threats to suicide risk.” The science is “very clear,” Moutier told Medscape Medical News.
“Suicide risk is never a situation of inevitability. It’s dynamic, with multiple forces at play in each individual and in the population. Lives can be saved simply by making people feel more connected to each other, that they are part of a larger community,” she added.
The political will
Moutier notes that prior to the pandemic, four countries ― Finland, Norway, Sweden, and Australia ― had fully implemented national suicide prevention plans and had achieved reductions in their national suicide rates. However, in the United States, the suicide rate has been steadily increasing since 1999.
A Centers for Disease Control and Prevention survey released in August 2020 found that 40% of US adults reported symptoms of depression, anxiety, or increased substance use during COVID-19 and that about 11% reported suicidal ideation in the past month, all increases from prior surveys.
COVID-19 presents a “new and urgent opportunity” to focus political will, federal investments, and the global community on suicide prevention, Moutier writes.
“The political will to address suicide has actually moved in the right direction during COVID, as evidenced by a number of pieces of legislation that have suddenly found their way to passing that we’ve been working on for years,” she said in an interview.
One example, she said, is the National Suicide Hotline Designation Act, signed into law earlier this month by President Donald Trump.
As previously reported, under the law, beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
Moutier reports no relevant financial relationships.
This article first appeared on Medscape.com.
The ongoing COVID-19 pandemic poses clear threats to mental well-being, but an increase in suicide is not inevitable if appropriate action is taken, one expert says.
“Increases in suicide rates should not be a foregone conclusion, even with the negative effects of the pandemic. If the lessons of suicide prevention research are heeded during and after the pandemic, this potential for increased risk could be substantially mitigated,” writes Christine Moutier, MD, chief medical officer of the American Foundation for Suicide Prevention, in an invited communication in JAMA Psychiatry.
“This is a moment in history when suicide prevention must be prioritized as a serious public health concern,” she writes.
Mitigating suicide risk
Although evidence from the first 6 months of the pandemic reveal specific effects on suicide risk, real-time data on suicide deaths are not available in most regions of the world. From emerging data from several countries, there is no evidence of increased suicide rates during the pandemic thus far, Moutier notes.
Still, a number of pandemic-related risk factors could increase individual and population suicide risk.
They include deterioration or recurrence of serious mental illness; increased isolation, loneliness, and bereavement; increased use of drugs and alcohol; job loss and other financial stressors; and increases in domestic violence.
There are mitigating strategies for each of these “threats to suicide risk.” The science is “very clear,” Moutier told Medscape Medical News.
“Suicide risk is never a situation of inevitability. It’s dynamic, with multiple forces at play in each individual and in the population. Lives can be saved simply by making people feel more connected to each other, that they are part of a larger community,” she added.
The political will
Moutier notes that prior to the pandemic, four countries ― Finland, Norway, Sweden, and Australia ― had fully implemented national suicide prevention plans and had achieved reductions in their national suicide rates. However, in the United States, the suicide rate has been steadily increasing since 1999.
A Centers for Disease Control and Prevention survey released in August 2020 found that 40% of US adults reported symptoms of depression, anxiety, or increased substance use during COVID-19 and that about 11% reported suicidal ideation in the past month, all increases from prior surveys.
COVID-19 presents a “new and urgent opportunity” to focus political will, federal investments, and the global community on suicide prevention, Moutier writes.
“The political will to address suicide has actually moved in the right direction during COVID, as evidenced by a number of pieces of legislation that have suddenly found their way to passing that we’ve been working on for years,” she said in an interview.
One example, she said, is the National Suicide Hotline Designation Act, signed into law earlier this month by President Donald Trump.
As previously reported, under the law, beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
Moutier reports no relevant financial relationships.
This article first appeared on Medscape.com.
Florida will investigate all COVID-19 deaths
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE