User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Analysis of Internal Dermatology Matches Following the COVID-19 Pandemic
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
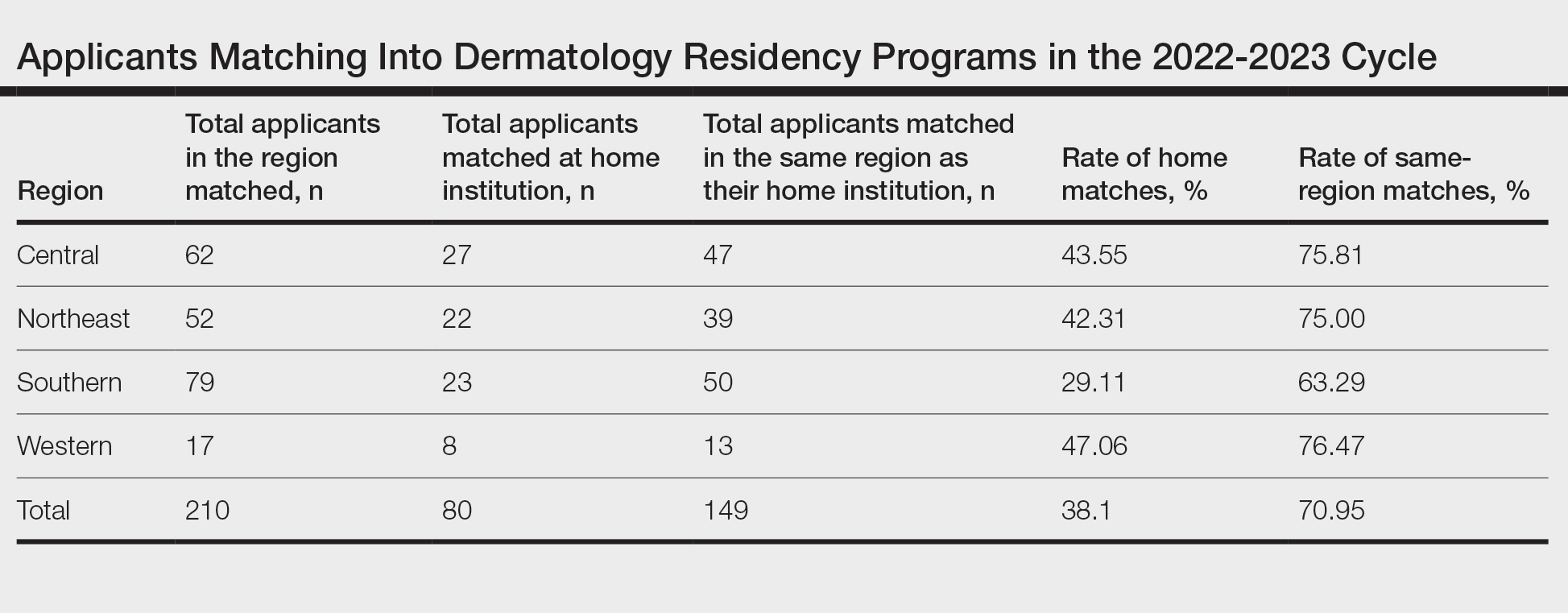
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.
Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.

Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.

Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
PRACTICE POINTS
- Following the COVID-19 pandemic, affiliation with a home program is even more impactful in successful application to dermatology residency. Applicants from institutions without dermatology programs should consider completing additional externships.
- The high rate of applicants matching to the same regions as their home programs is due to several factors. Applicants may have a larger social support system near their home institution. Additionally, programs are more comfortable matching applicants within their own regions.
Hospital Dermatology: Review of Research in 2022-2023
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Practice Points
- A severe hypersensitivity reaction to trimethoprim-sulfamethoxazole—sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes (SCoRCH)—has been described.
- Patients experiencing cutaneous reactions to immune checkpoint inhibitors have improved progression-free and overall survival rates if evaluated by a dermatologist who can optimize skin-directed and targeted therapies.
- Interventions, including shorter time to dermatology outpatient follow-up, are needed to reduce emergency department utilization by patients with hidradenitis suppurativa.
- Asynchronous store-and-forward dermatology e-consultation is effective for immunobullous diseases, vasculitis, herpes zoster, and cellulitis, demonstrating the utility of teledermatology in the inpatient setting, particularly when standardized data capture tools are used.
Botanical Briefs: Australian Stinging Tree (Dendrocnide moroides)
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6
The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Practice Points
- Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.
- Clinical observations after contact reveal immediate piloerection and local swelling, which may disappear after 1 hour or last as long as 24 hours, but subjective pain, pruritus, and burning can persist for months.
- The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.
Suture Selection to Minimize Postoperative Postinflammatory Hyperpigmentation in Patients With Skin of Color During Mohs Micrographic Surgery
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.
Teledermatology: A Postpandemic Update
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2
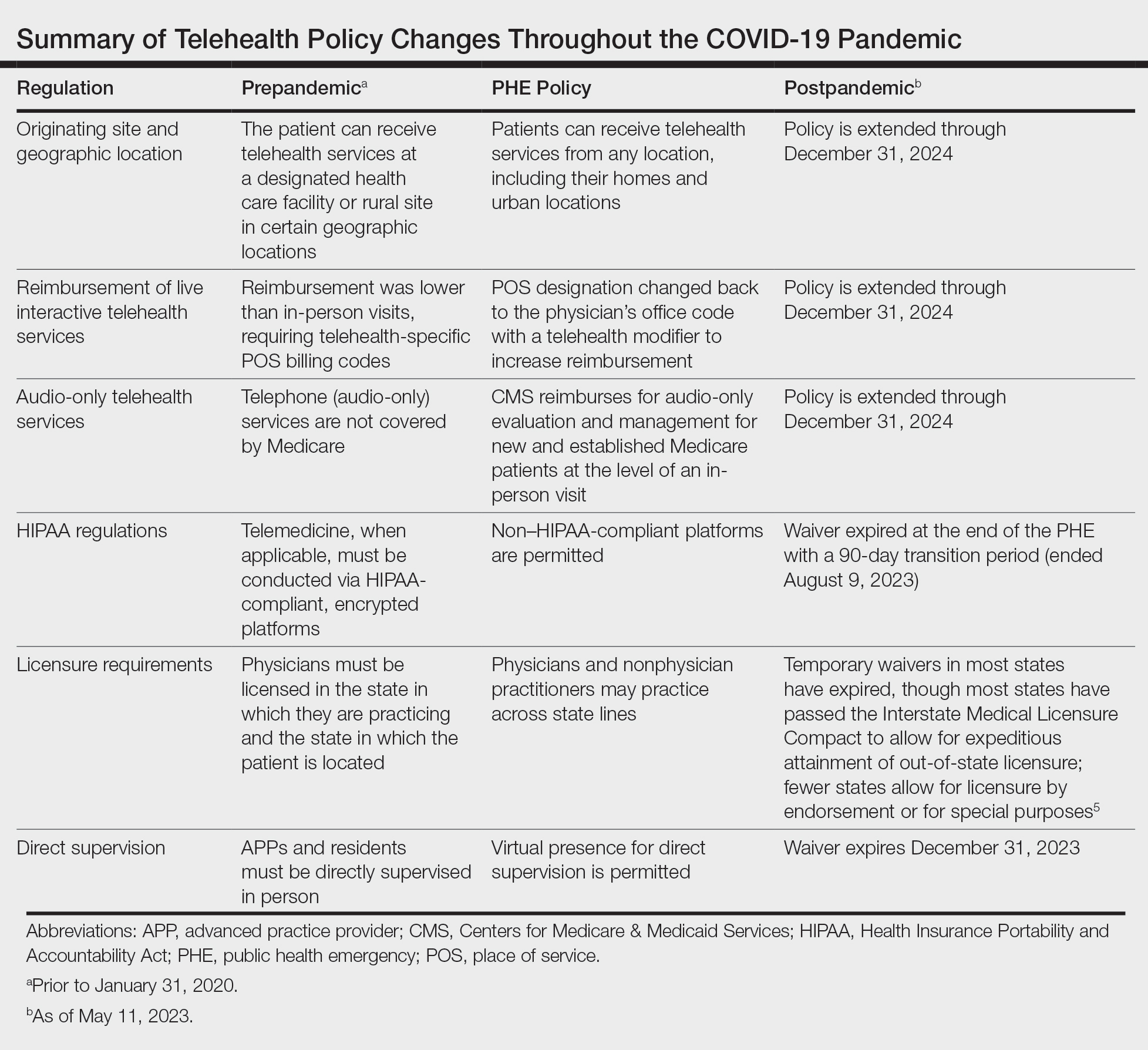
What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
Clustered Vesicles on the Neck
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.
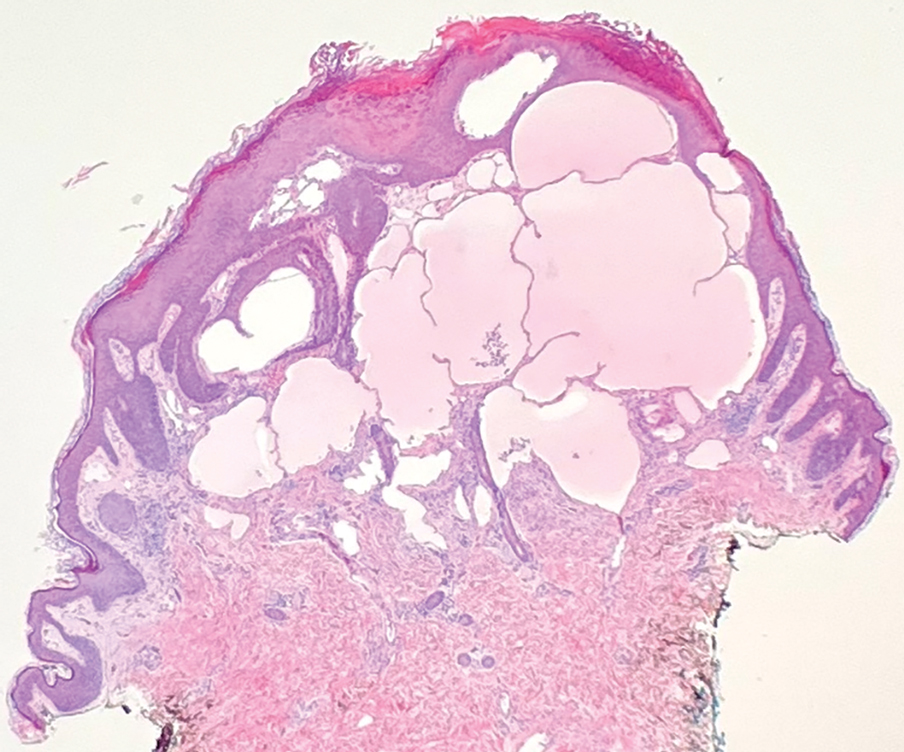
The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
The Diagnosis: Microcystic Lymphatic Malformation
A punch biopsy demonstrated anastomosing fluidfilled spaces within the papillary and reticular dermal layers (Figure), confirming the diagnosis of microcystic lymphatic malformation (LM)(formerly known as lymphangioma circumscriptum), a congenital vascular malformation composed of slow-flow lymphatic channels.1 The patient underwent serial excisions with improvement of the LM, though the treatment course was complicated by hypertrophic scar formation.

The classic clinical presentation of microcystic LM includes a crop of vesicles containing clear or hemorrhagic fluid with associated oozing or bleeding.2 When cutaneous lesions resembling microcystic LM develop in response to lymphatic damage and resulting stasis, such as from prior radiotherapy or surgery, the term lymphangiectasia is used to distinguish this entity from congenital microcystic LM.3
Microcystic LMs are histologically indistinguishable from macrocystic LMs; however, macrocystic LMs typically are clinically evident at birth as ill-defined subcutaneous masses.2,4-6 Dermatitis herpetiformis, a dermatologic manifestation of gluten sensitivity, causes intensely pruritic vesicles in a symmetric distribution on the elbows, knees, and buttocks. Histopathology shows neutrophilic microabscesses in the dermal papillae with subepidermal blistering. Direct immunofluorescence demonstrates the deposition of IgA along the basement membrane with dermal papillae aggregates.6 The underlying dermis also may contain a lymphohistiocytic infiltrate rich in neutrophils. The vesicles of herpes zoster virus are painful and present in a dermatomal distribution. A viral cytopathic effect often is observed in keratinocytes, specifically with multinucleation, molding, and margination of chromatin material. The lesions are accompanied by variable lymphocytic inflammation and epithelial necrosis resulting in intraepidermal blistering.7 Extragenital lichen sclerosus presents as polygonal white papules merging to form plaques and may include hemorrhagic blisters in some instances. Histopathology shows hyperkeratosis, epidermal atrophy with flattened rete ridges, vacuolar interface changes, loss of elastic fibers, and hyalinization of the lamina propria with lymphocytic infiltrate.8
Endothelial cells in LM exhibit activating mutations in the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PIK3CA, which may lead to proliferation and overgrowth of the lymphatic vasculature, as well as increased production of cyclic guanosine monophosphate.9,10 Phosphodiesterase 5 (PDE5) is expressed in the perivascular smooth muscle adjacent to lymphatic spaces in LMs but not in the their vasculature. 10 This pattern of PDE5 expression may cause perilesional vasculature to constrict, preventing lymphatic fluid from draining into the veins.11 It is theorized that the PDE5 inhibitor sildenafil leads to relaxation of the vasculature adjacent to LMs, allowing the outflow of the accumulated lymphatic fluid and thus decompression.11-13
Management of LM should not only take into account the depth and location of involvement but also any associated symptoms or complications, such as pruritus, pain, bleeding, or secondary infections. Magnetic resonance imaging (MRI) typically has been considered the gold standard for determining the size and depth of involvement of the malformation.1,3,4 However, ultrasonography with Doppler flow may be considered an initial diagnostic and screening test, as it can distinguish between macrocystic and microcystic components and provide superior images of microcystic lesions, which are below the resolution capacity of MRI.4 Notably, our patient’s LM was undetectable on ultrasonography and was found to be largely superficial in nature on MRI.
Serial excision of the microcystic LM was conducted in our patient, but there currently is no consensus on optimal treatment of LM, and many treatment options are complicated by high recurrence rates or complications.5 Procedural approaches may include excision, cryotherapy, radiotherapy, sclerotherapy, or laser therapy, while pharmacologic approaches may include sildenafil for its inhibition of PDE5 or sirolimus (oral or topical) for its inhibition of mammalian target of rapamycin.5,12-14 Because recurrence is highly likely, patients may require repeat treatments or a combination approach to therapy.1,5 The development of targeted therapies may lead to a shift in management of LMs in the future, as successful use of the PIK3CA inhibitor alpelisib recently has been reported to lead to clinical improvement of PIK3CA-related LMs, including in patients with PIK3CA-related overgrowth syndromes.15
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
- Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353-374. doi:10.1016/j.jaad.2006.05.069
- Alrashdan MS, Hammad HM, Alzumaili BAI, et al. Lymphangioma circumscriptum of the tongue: a case with marked hemorrhagic component. J Cutan Pathol. 2018;45:278-281. doi:10.1111/cup.13101
- Osborne GE, Chinn RJ, Francis ND, et al. Magnetic resonance imaging in the investigation of penile lymphangioma circumscriptum. Br J Dermatol. 2000;143:467-468. doi:10.1046/j.1365-2133.2000.03695.x
- Davies D, Rogers M, Lam A, et al. Localized microcystic lymphatic malformations—ultrasound diagnosis. Pediatr Dermatol. 1999;16: 423-429. doi:10.1046/j.1525-1470.1999.00110.x
- García-Montero P, Del Boz J, Baselga-Torres E, et al. Use of topical rapamycin in the treatment of superficial lymphatic malformations. J Am Acad Dermatol. 2019;80:508-515. doi:10.1016/j.jaad.2018.09.050
- Clarindo MV, Possebon AT, Soligo EM, et al. Dermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatment. An Bras Dermatol. 2014;89:865-875; quiz 876-877. doi:10.1590/abd1806-4841.20142966
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Shiver M, Papasakelariou C, Brown JA, et al. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31:383-385. doi:10.1111 /pde.12025
- Blesinger H, Kaulfuß S, Aung T, et al. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations [published online July 9, 2018]. PLoS One. 2018;13:E0200343. doi:10.1371/journal.pone.0200343
- Green JS, Prok L, Bruckner AL. Expression of phosphodiesterase-5 in lymphatic malformation tissue. JAMA Dermatol. 2014;150:455-456. doi:10.1001/jamadermatol.2013.7002
- Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366:384-386. doi:10.1056 /NEJMc1112482
- Tu JH, Tafoya E, Jeng M, et al. Long-term follow-up of lymphatic malformations in children treated with sildenafil. Pediatr Dermatol. 2017;34:559-565. doi:10.1111/pde.13237
- Maruani A, Tavernier E, Boccara O, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021;157:1289-1298. doi:10.1001/jamadermatol.2021.3459
- Delestre F, Venot Q, Bayard C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. 2021;13:eabg0809. doi:10.1126/scitranslmed .abg0809
- Garreta Fontelles G, Pardo Pastor J, Grande Moreillo C. Alpelisib to treat CLOVES syndrome, a member of the PIK3CA-related overgrowth syndrome spectrum [published online February 21, 2022]. Br J Clin Pharmacol. 2022;88:3891-3895. doi:10.1111/bcp.15270
A 6-year-old girl presented to the dermatology clinic with a rash on the right side of the neck that was noted at birth as a small raised lesion but slowly increased over time in size and number of lesions. She reported pruritus and irritation, particularly when rubbed or scratched. There was no family history of similar skin abnormalities. Her medical history was notable for a left-sided cholesteatoma on tympanomastoidectomy. Physical examination revealed clustered vesicles on the right side of the neck with underlying erythema. The vesicles contained mostly clear fluid with a few focal areas of hemorrhagic fluid. Ultrasonography was unremarkable, and magnetic resonance imaging revealed superficial T2 hyperintense nonenhancing cutaneous and subcutaneous lesions overlying the right lateral neck with minimal extension into the superficial right supraclavicular soft tissues.
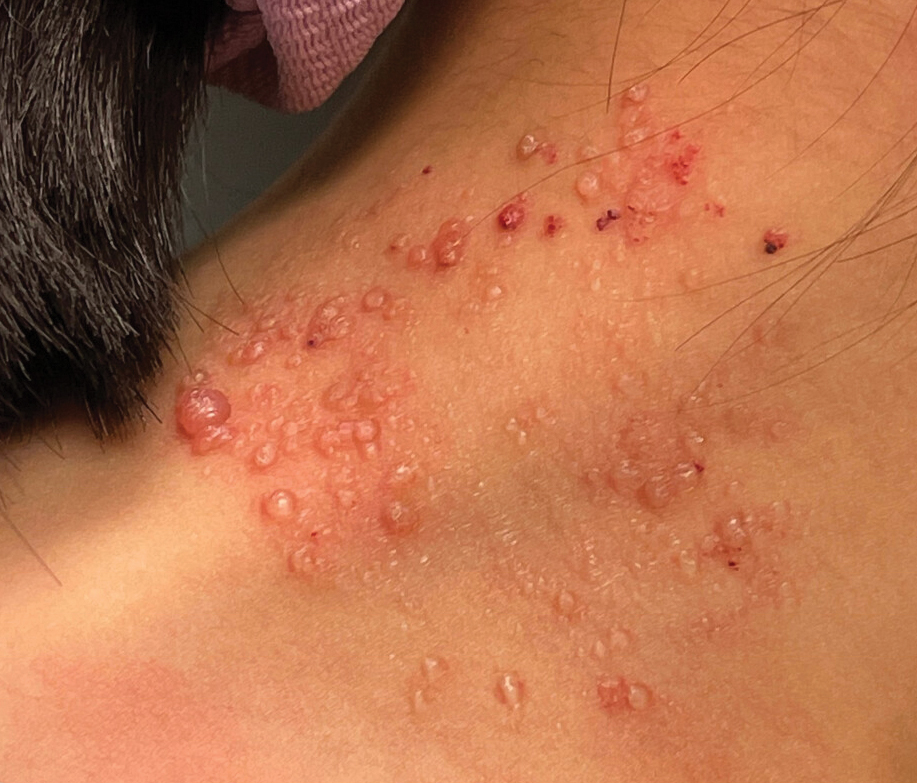
Cutaneous Collagenous Vasculopathy With Ocular Involvement
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.
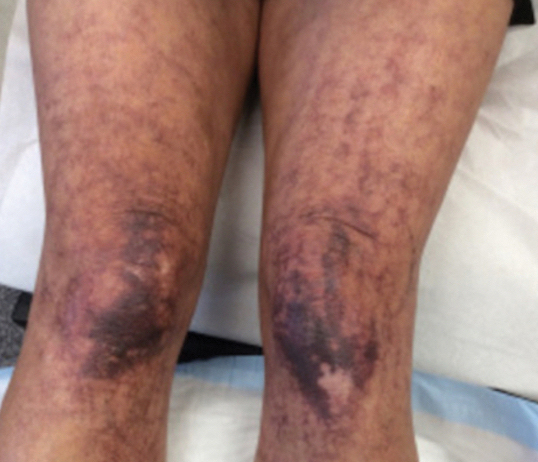
Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.
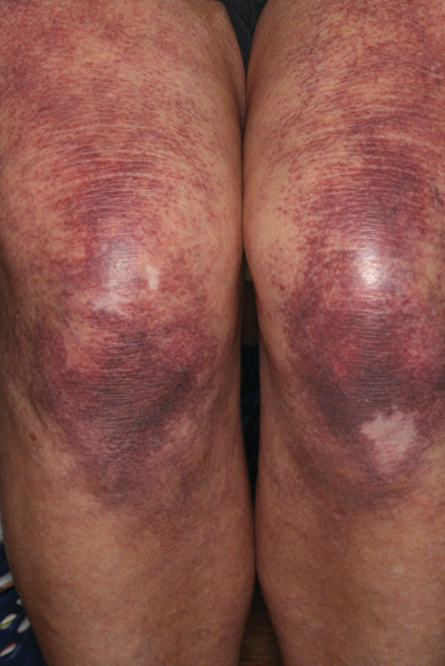
Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2
The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9
Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is an uncommon microangiopathy that presents with progressive telangiectases on the lower extremities that can eventually spread to involve the upper extremities and trunk. Systemic involvement is uncommon. The diagnosis is confirmed by biopsy, which demonstrates dilated capillaries and postcapillary venules with eosinophilic hyalinized walls. Treatment generally has focused on the use of vascular lasers.1 We report a patient with advanced CCV and ocular involvement that responded to a combination of pulsed dye laser (PDL) therapy and sclerotherapy for cutaneous lesions.
A 63-year-old woman presented with partially blanchable, purple-black patches on the lower extremities (Figure 1). The upper extremities had minimal involvement at the time of presentation. A medical history revealed the lesions presented on the legs 10 years prior but were beginning to form on the arms. She had a history of hypertension and bleeding in the retina.

Histopathology revealed prominent dilation of postcapillary venules with eosinophilic collagenous materials in the vessel walls that was positive on periodic acid–Schiff stain, confirming the diagnosis of CCV. The perivascular collagenous material failed to stain with Congo red. Laboratory testing for serum protein electrophoresis, antinuclear antibodies, and baseline hematologic and metabolic panels revealed no abnormalities.
Over 3 years of treatment with PDL, most of the black patches resolved, but prominent telangiectatic vessels remained (Figure 2). Sclerotherapy with polidocanol (10 mg/mL) resulted in clearance of the majority of telangiectatic vessels. After each sclerotherapy treatment, Unna boots were applied for a minimum of 24 hours. The patient had no adverse effects from either PDL or sclerotherapy and was pleased with the results (Figure 3). An ophthalmologist had attributed the retinal bleeding to central serous chorioretinopathy, but tortuosity of superficial scleral and episcleral vessels progressed, suggesting CCV as the more likely cause (Figure 4). Currently, she is being followed for visual changes and further retinal bleeding.

Early CCV typically appears as blanchable pink or red macules, telangiectases, or petechiae on the lower extremities, progressing to involve the trunk and upper extremity.1-3 In rare cases, CCV presents in a papular or annular variant instead of the typical telangiectatic form.4,5 As the lesions progress, they often darken in appearance. Bleeding can occur, and the progressive patches are disfiguring.6,7 Middle-aged to older adults typically present with CCV (range, 16–83 years), with a mean age of 62 years.1,2,6 This disease affects both males and females, predominantly in White individuals.1 Extracutaneous manifestations are rare.1,2,6 One case of mucosal involvement was described in a patient with glossitis and oral erosions.8 We found no prior reports of nail or eye changes.1,2

The etiology of CCV is unknown, but different theories have been proposed. One is that CCV is due to a genetic defect that changes collagen synthesis in the cutaneous microvasculature. Another more widely held belief is that CCV originates from an injury that occurs to the microvasculature endothelial cells. Regardless of the cause of the triggering injury, the result is induced intravascular occlusive microthrombi that cause perivascular fibrosis and endothelial hyperplasia.2,6,7,9

Cutaneous collagenous vasculopathy may be influenced by systemic diseases. The most common comorbidities are hypertension, cardiovascular disease, diabetes mellitus, and hyperlipidemia.1,3,6-8 The presentation of CCV with a malignancy is rare; 1 patient was diagnosed with multiple myeloma 18 months after CCV, and another patient’s cutaneous presentation led to discovery of pancreatic cancer with metastasis.8,10 In this setting, the increased growth factors or hypercoagulability of malignancy may play a role in endothelial cell damage and hyperplasia. Autoimmune vascular injury also has been suggested to trigger CCV; 1 case involved antiribonucleoprotein antibodies, while another case involved anti–endothelial cell antibody assays.11 In addition, CCV has been reported in hypercoagulable patients, demonstrating another route for endothelial damage, with 1 patient being heterozygous for prothrombin G20210A, a report of CCV in a patient with cryofibrinogenemia, and another patient being found positive for lupus anticoagulant.11,12 Drugs also have been thought to influence CCV, including corticosteroids, lithium, thiothixene, interferon, isotretinoin, calcium channel blockers, antibiotics, hydroxyurea, and antidepressants.7,11
The diagnosis of CCV is confirmed using light microscopy and collagen-specific immunostaining. Examination shows hyaline eosinophilic deposition of type IV collagen around the affected vessels, with the postcapillary venules showing characteristic duplication of the basal lamina.3,9 The material stains positive with periodic acid-Schiff and Masson trichrome.3
Underreporting may contribute to the low incidence of CCV. The clinical presentation of CCV is similar to generalized essential telangiectasia, with biopsy distinguishing the two. Other diagnoses in the differential include hereditary hemorrhagic telangiectasia, which typically would have mucosal involvement; radiating telangiectatic mats and a strong family history; and hereditary benign telangiectasia, which typically presents in younger patients aged 1 year to adolescence.1
Treatment with vascular lasers has been the main focus, using either the 595-nm PDL or the 1064-nm Nd:YAG laser.6,13 Pulsed dye laser or intense pulsed light devices can improve patient well-being1,2; intense pulsed light allows for a larger spot size and may be preferred in patients with a larger body surface area involved.13 However, a few other treatments have been proposed. One case report noted poor response to sclerotherapy.1 In another case, a patient treated with a chemotherapy agent, bortezomib, for their concurrent multiple myeloma showed notable CCV cutaneous improvement. The proposed mechanism for bortezomib improving CCV is through its antiproliferative effect on endothelial cells of the superficial dermal vessels.8 Our patient did not achieve an adequate response with PDL, but the addition of sclerotherapy with polidocanol induced a successful response.
Patients should be examined for evidence of ocular involvement and referred to an ophthalmologist for appropriate care. Although there is no definite association with systemic illnesses or mediation, recent associations with an autoimmune disorder or underlying malignancy have been noted.8,10,11 Age-appropriate cancer screening and attention to associated signs and symptoms are recommended.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52. https://doi.org/10.1097/dad.0000000000000194
- Castiñeiras-Mato I, Rodríguez-Lojo R, Fernández-Díaz ML, et al. Cutaneous collagenous vasculopathy: a case report and review of the literature. Actas Dermosifiliogr. 2016;107:444-447. https://doi.org/10.1016/j.ad.2015.11.006
- Rambhia KD, Hadawale SD, Khopkar US. Cutaneous collagenous vasculopathy: a rare case report. Indian Dermatol Online J. 2016;7:40-42. https://doi.org/10.4103/2229-5178.174327
- Conde-Ferreirós A, Roncero-Riesco M, Cañueto J, et al. Cutaneous collagenous vasculopathy: papular form [published online August 15, 2019]. Dermatol Online J. https://doi.org/10.5070/d3258045128
- García-Martínez P, Gomez-Martin I, Lloreta J, et al. Multiple progressive annular telangiectasias: a clinicopathological variant of cutaneous collagenous vasculopathy? J Cutan Pathol. 2017;44:982-985. https://doi.org/10.1111/cup.13029
- Sartori DS, de Almeida Jr HL, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213. https://doi.org/10.1590/abd1806-4841.20198166
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111. https://doi.org/10.1159/000439126
- Dura M, Pock L, Cetkovska P, et al. A case of cutaneous collagenous vasculopathy associated with multiple myeloma and with a pathogenic variant of the glucocerebrosidase gene. J Cutan Pathol. 2022;49:717-721. https://doi.org/10.1111/cup.14227
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393. https://doi.org/10.1111/cup.12285
- Holder E, Schreckenberg C, Lipsker D. Cutaneous collagenous vasculopathy leading to the diagnosis of an advanced pancreatic cancer. J Eur Acad Dermatol Venereol. 2022;36:E699-E701. https://doi.org/10.1111/jdv.18152
- Grossman ME, Cohen M, Ravits M, et al. Cutaneous collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2022;49:491-495. https://doi.org/10.1111/cup.14192
- Eldik H, Leisenring NH, Al-Rohil RN, et al. Cutaneous collagenous vasculopathy in a middle-aged woman with a history of prothrombin G20210A thrombophilia. J Cutan Pathol. 2022;49:679-682. https://doi.org/10.1111/cup.13895
- Weiss E, Lazzara DR. Commentary on clinical improvement of cutaneous collagenous vasculopathy with intense pulsed light therapy. Dermatol Surg. 2021;47:1412. https://doi.org/10.1097/DSS.0000000000003209
Practice Points
- Collagenous vasculopathy is an underrecognized entity.
- Although most patients exhibit only cutaneous disease, systemic involvement also should be assessed.