User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
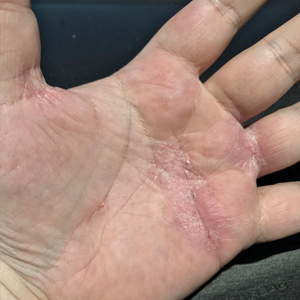
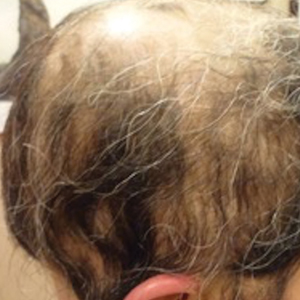
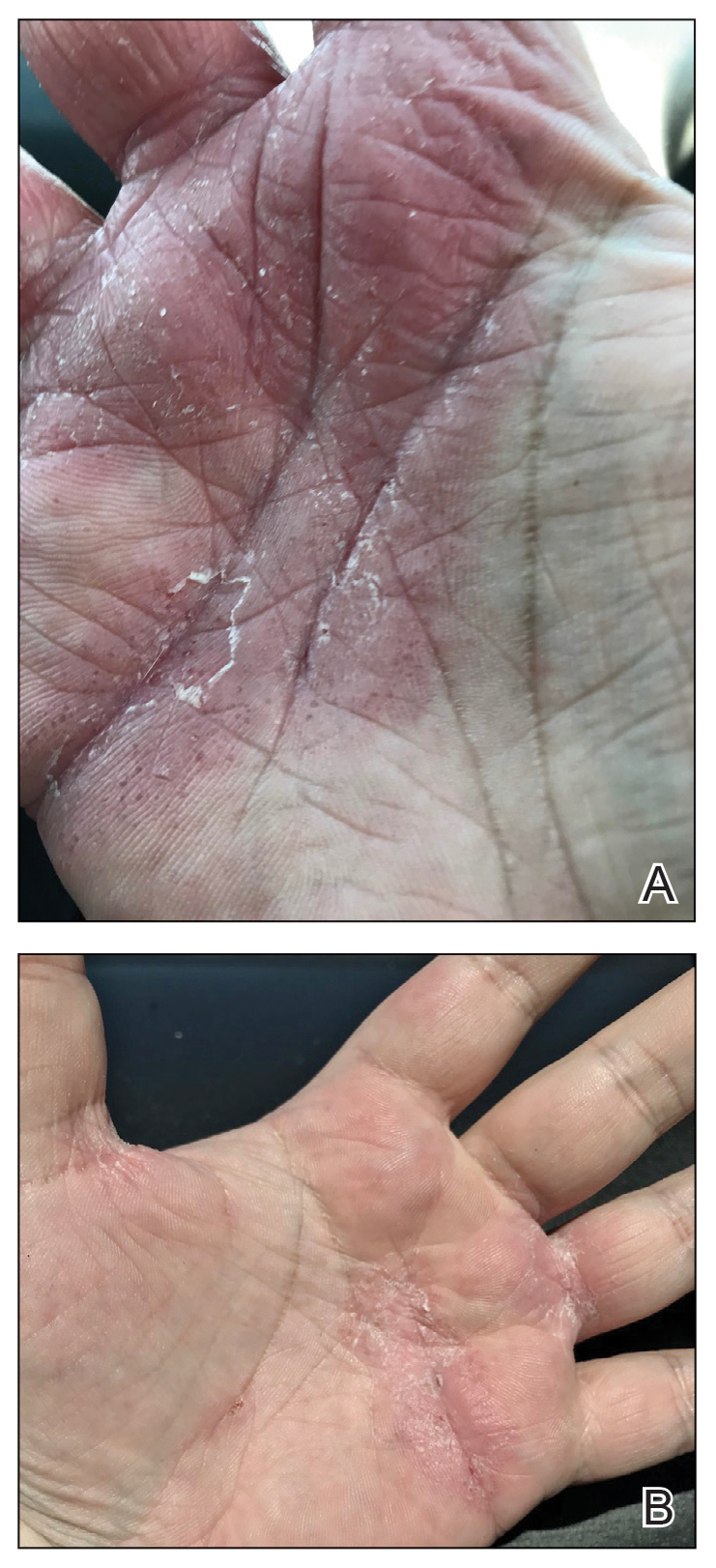
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
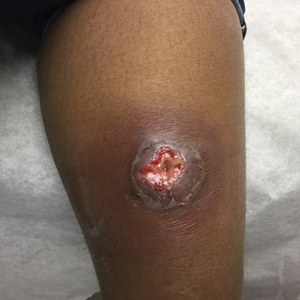
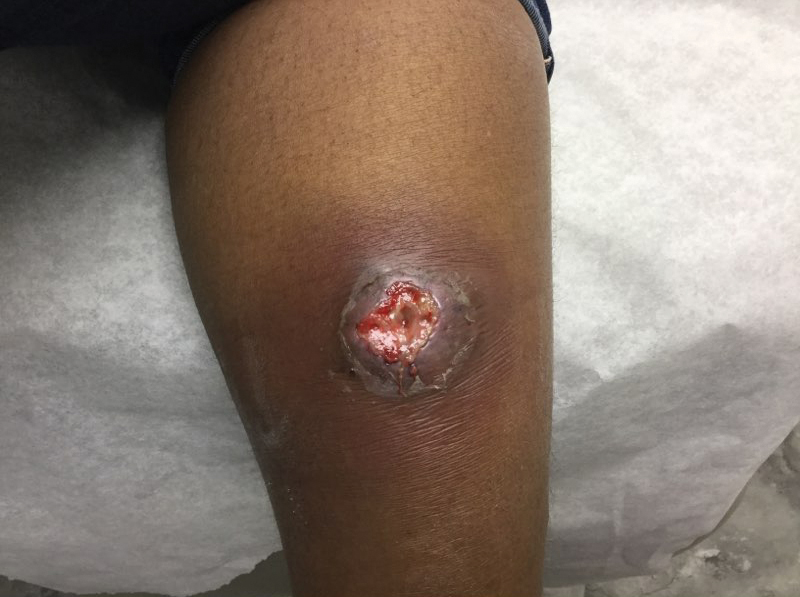
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
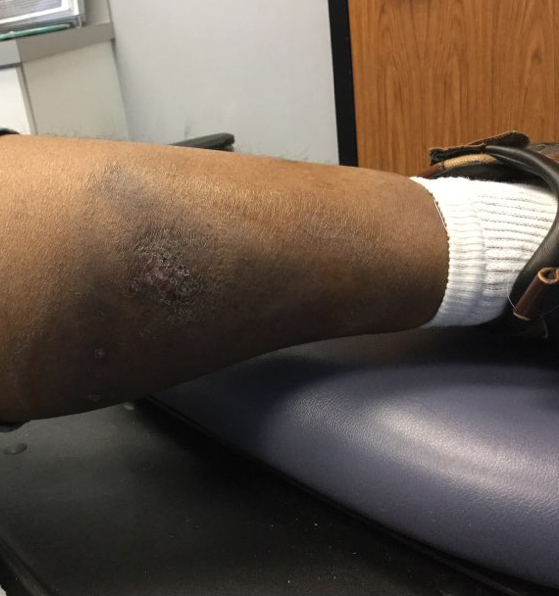
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
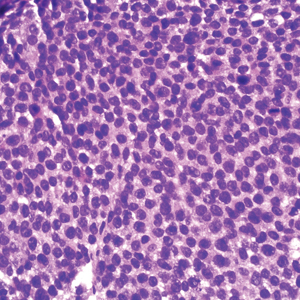
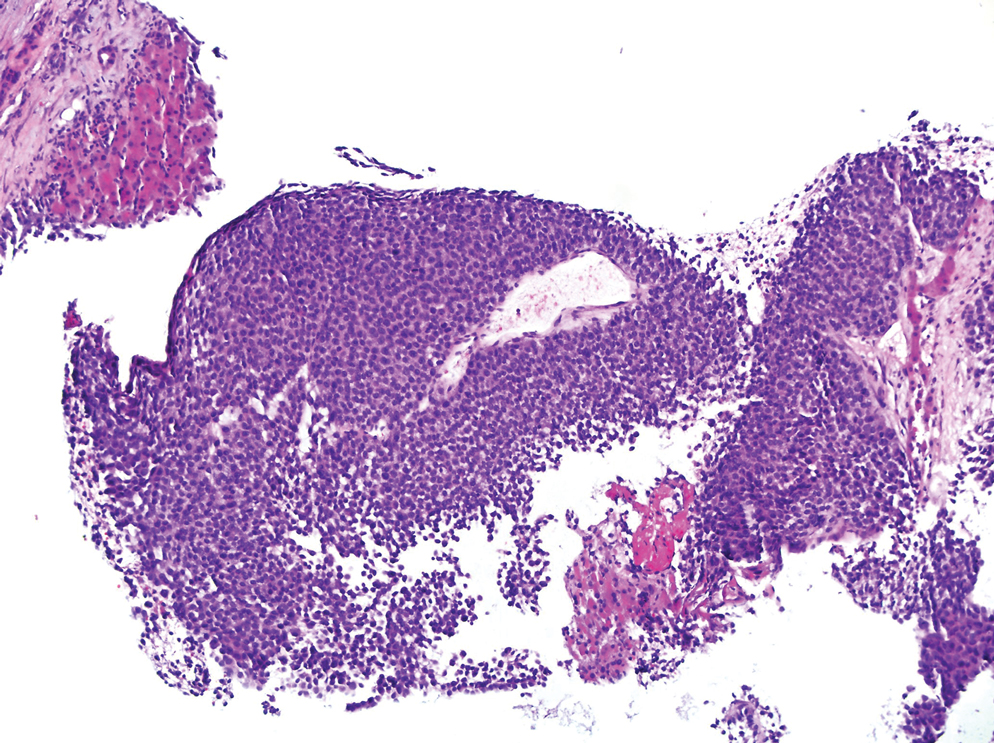
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
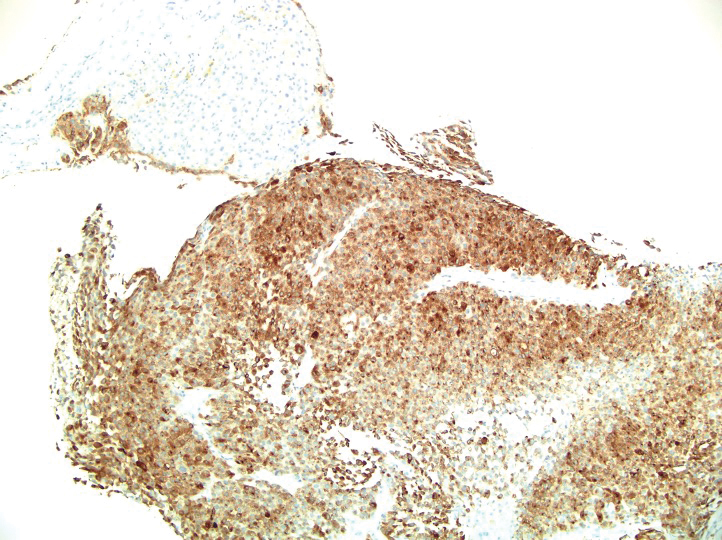
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
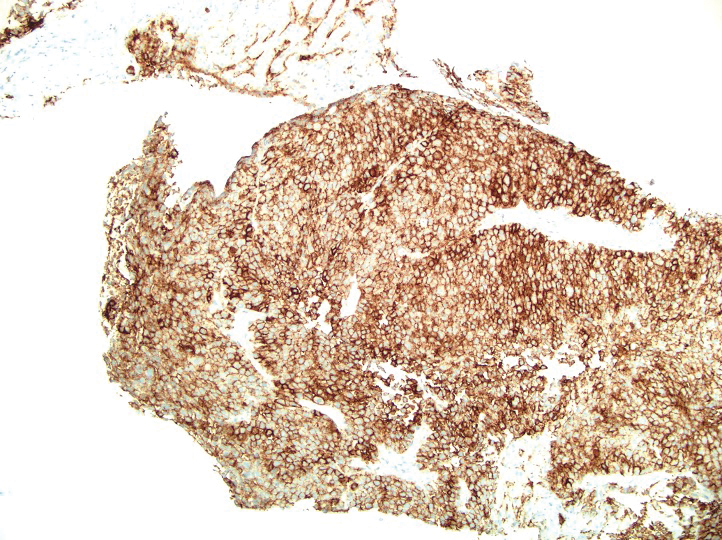
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.
Asymptomatic Hair Loss in a Patient With Systemic Lupus Erythematosus
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4
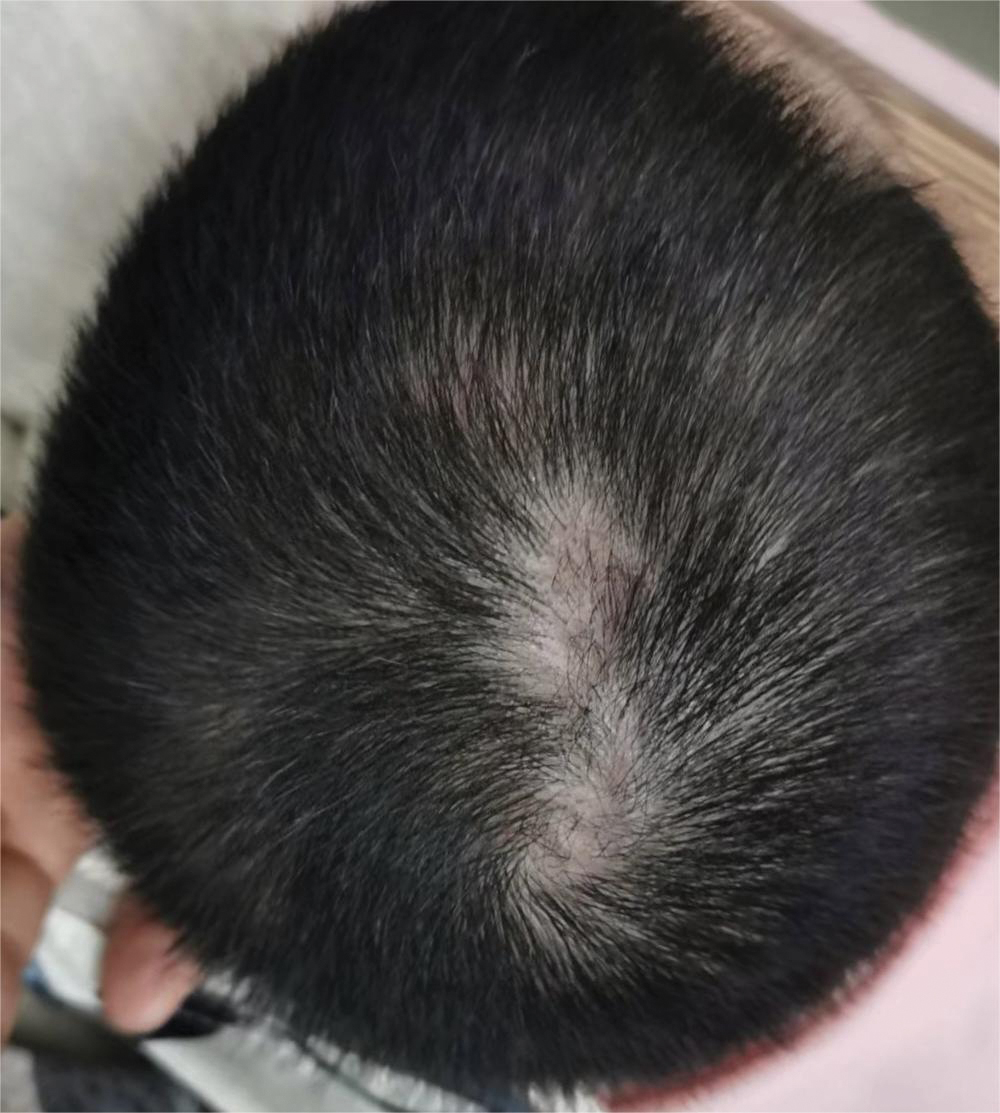
Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
A 51-year-old woman residing in the Hainan Province, China, was referred to our hospital for treatment of recurrent joint pain that could not be controlled at the local hospital. She had a history of systemic lupus erythematosus with a Systemic Lupus Erythematosus Disease Activity Index score of 8 (mild activity). Physical examination revealed irregular patches of hair loss on the head. There also were remnants of hair in some areas with black dots at the follicular opening and perifollicular keratotic papules interspersed as well as a few pale erythematous spots and white adherent scales.
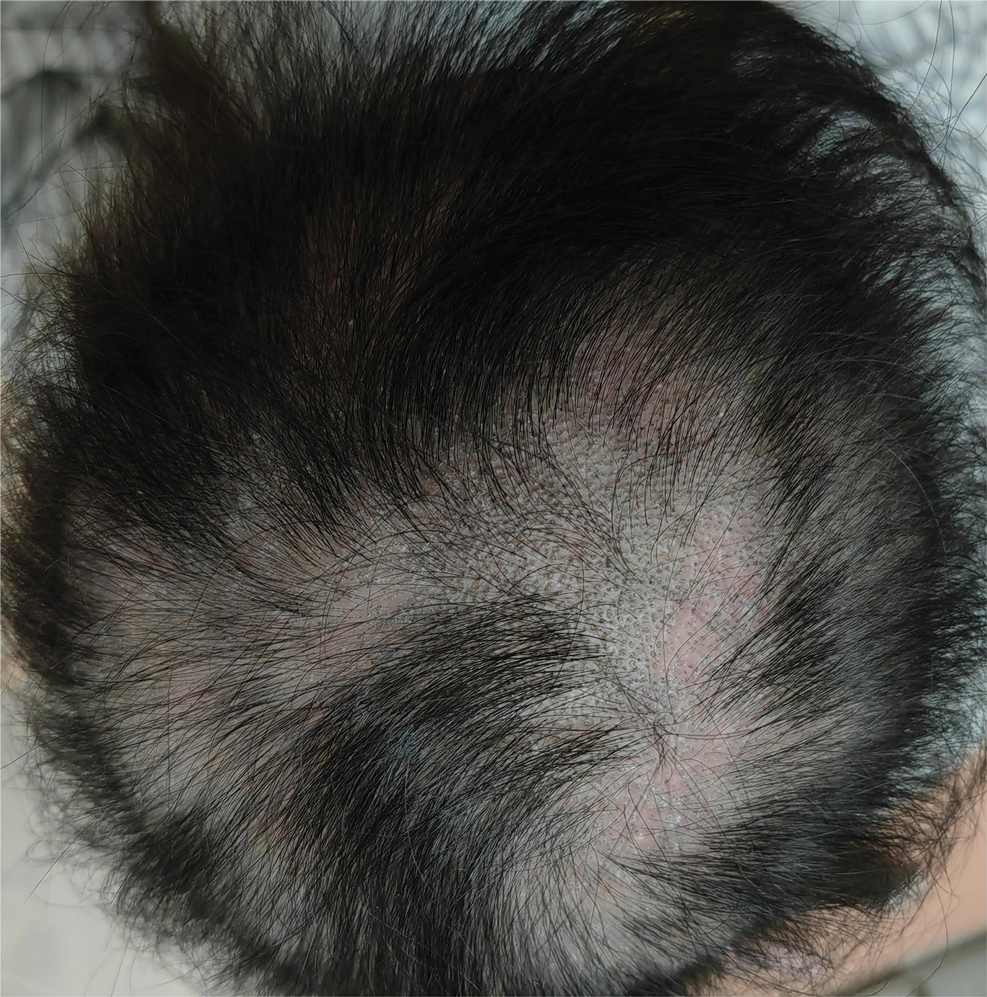
Alopecia Universalis Treated With Tofacitinib: The Role of JAK/STAT Inhibitors in Hair Regrowth
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).
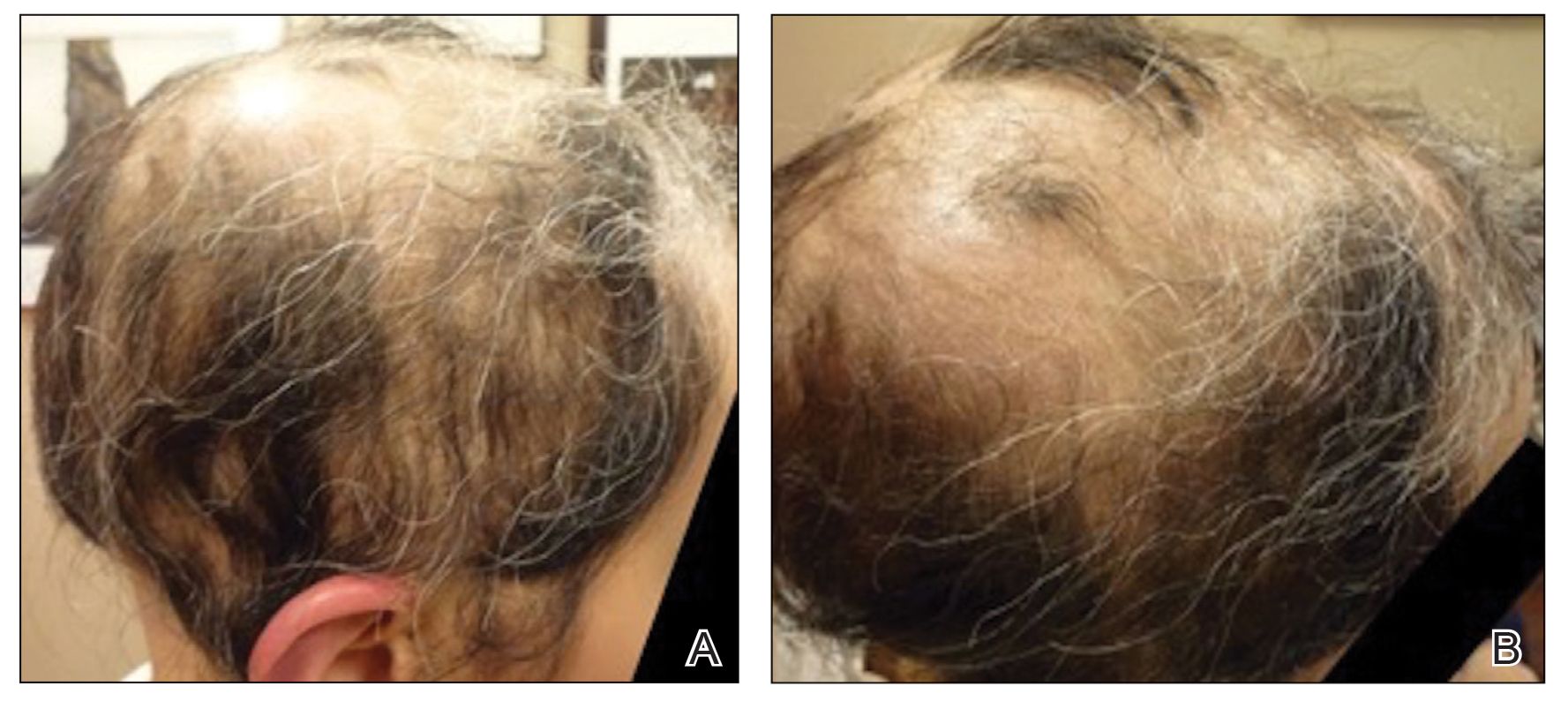
The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.
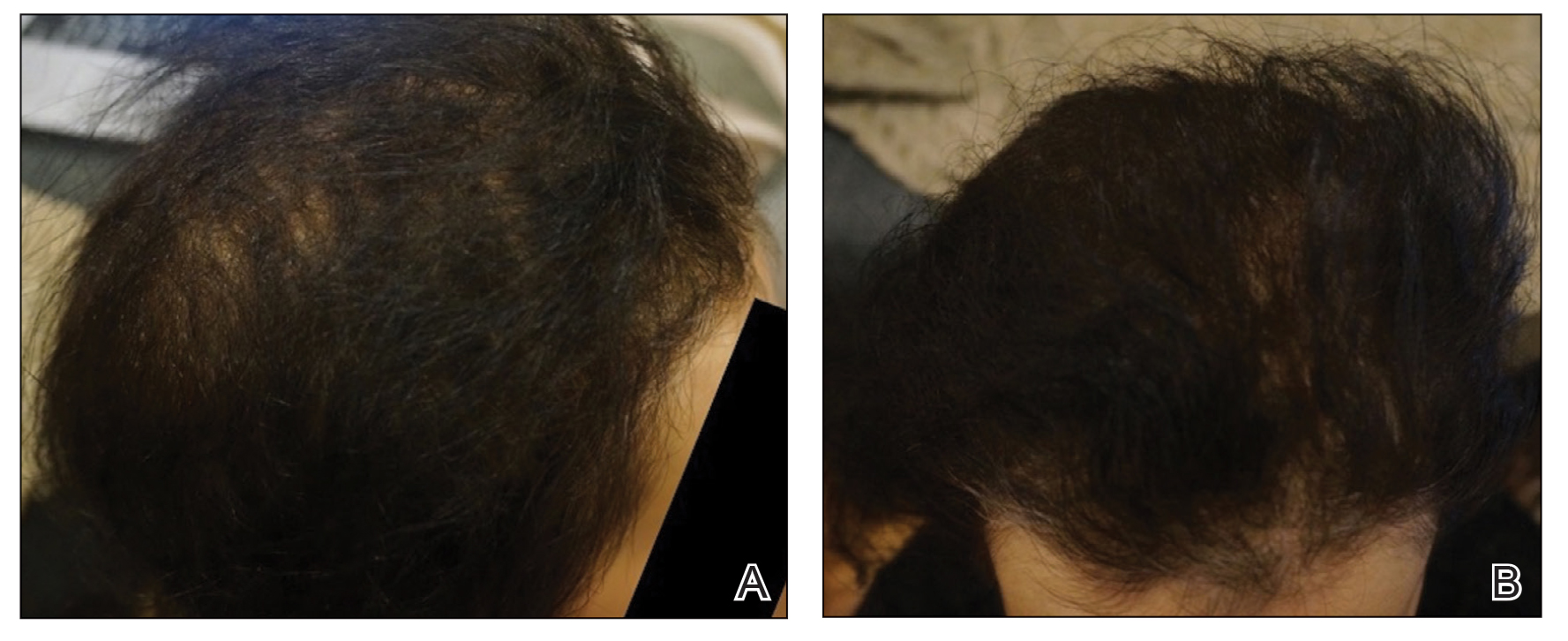
Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.
Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4
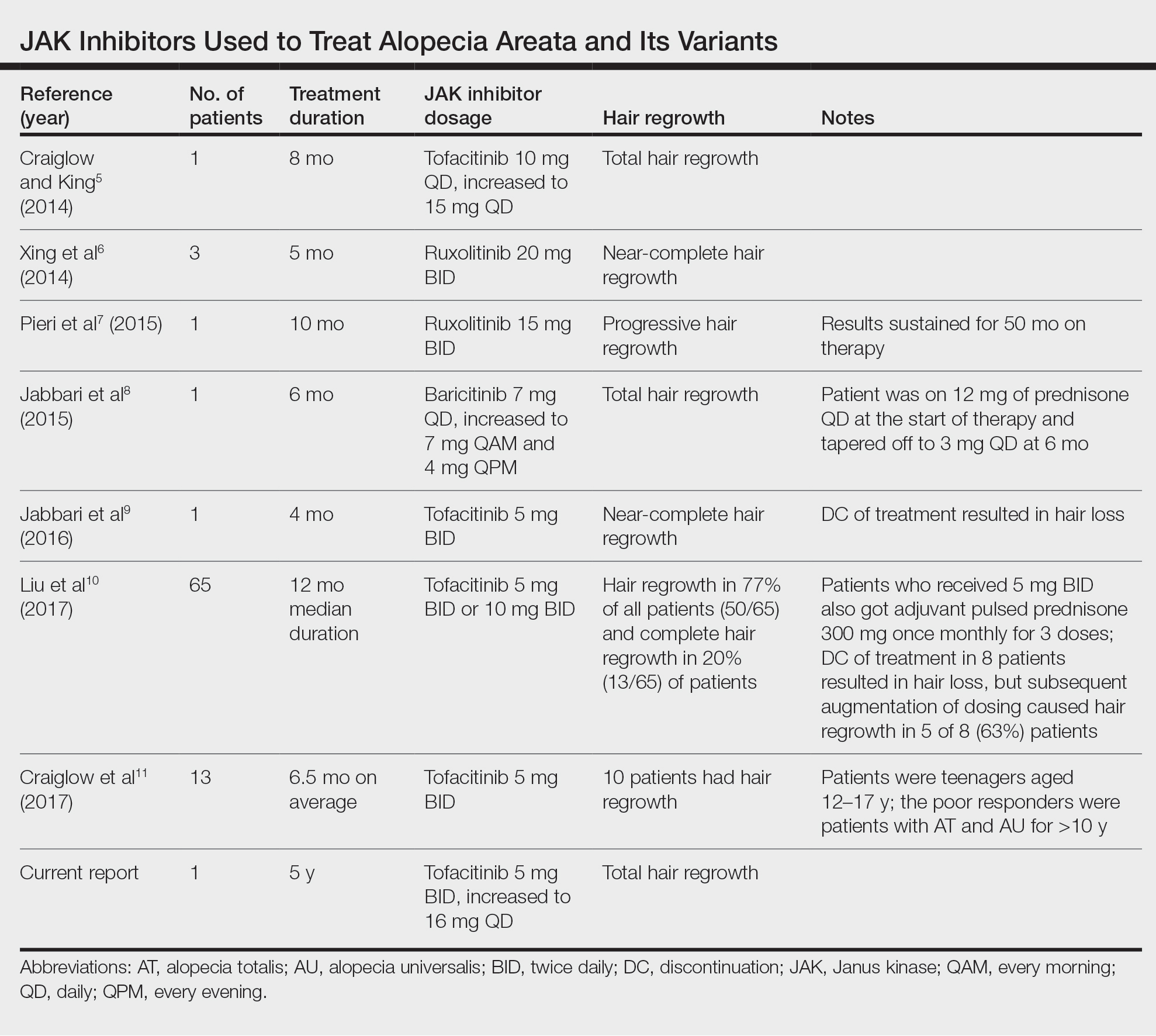
In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Practice Points
- Janus kinase inhibitors target one of the cellular pathogeneses of alopecia areata.
- Janus kinase inhibitors may be an option for patients who have exhausted other treatment modalities for alopecia.
Pediatric Primary Cutaneous Marginal Zone Lymphoma Treated With Doxycycline
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.
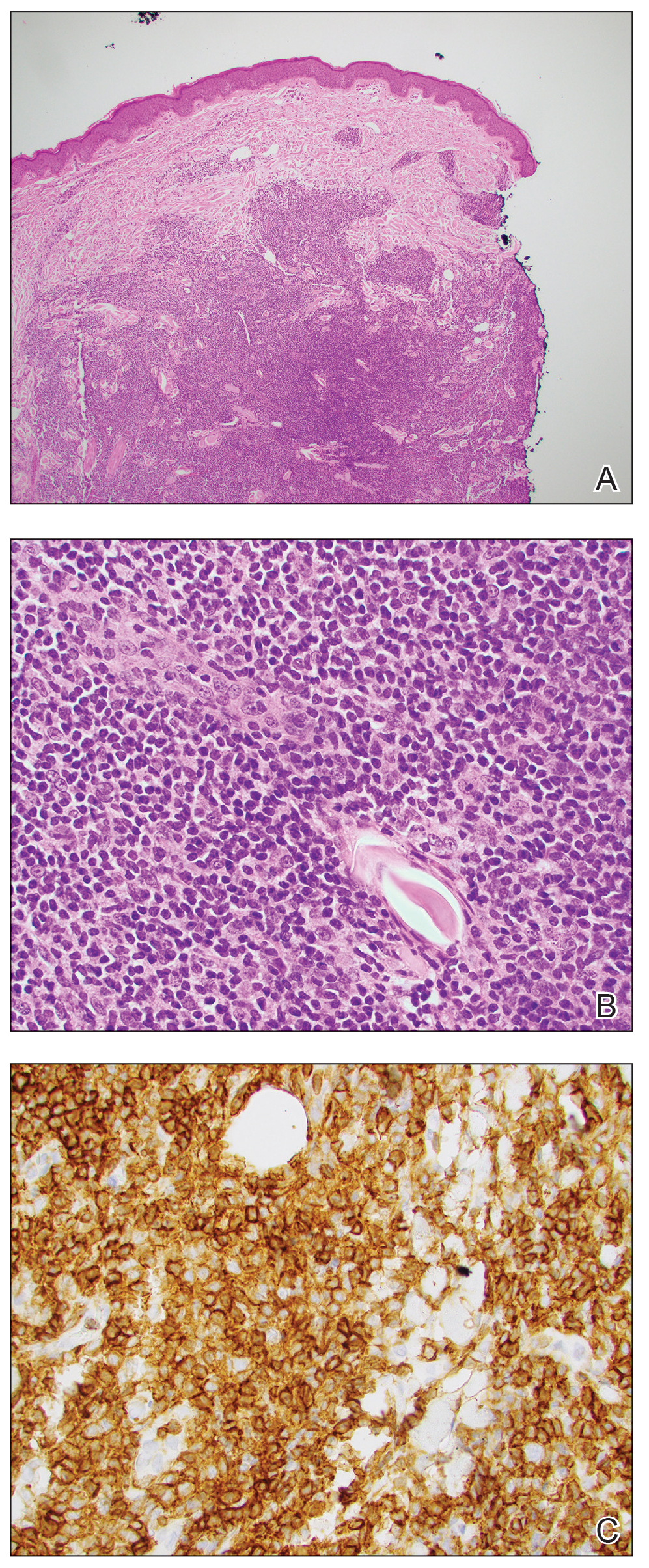
The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.
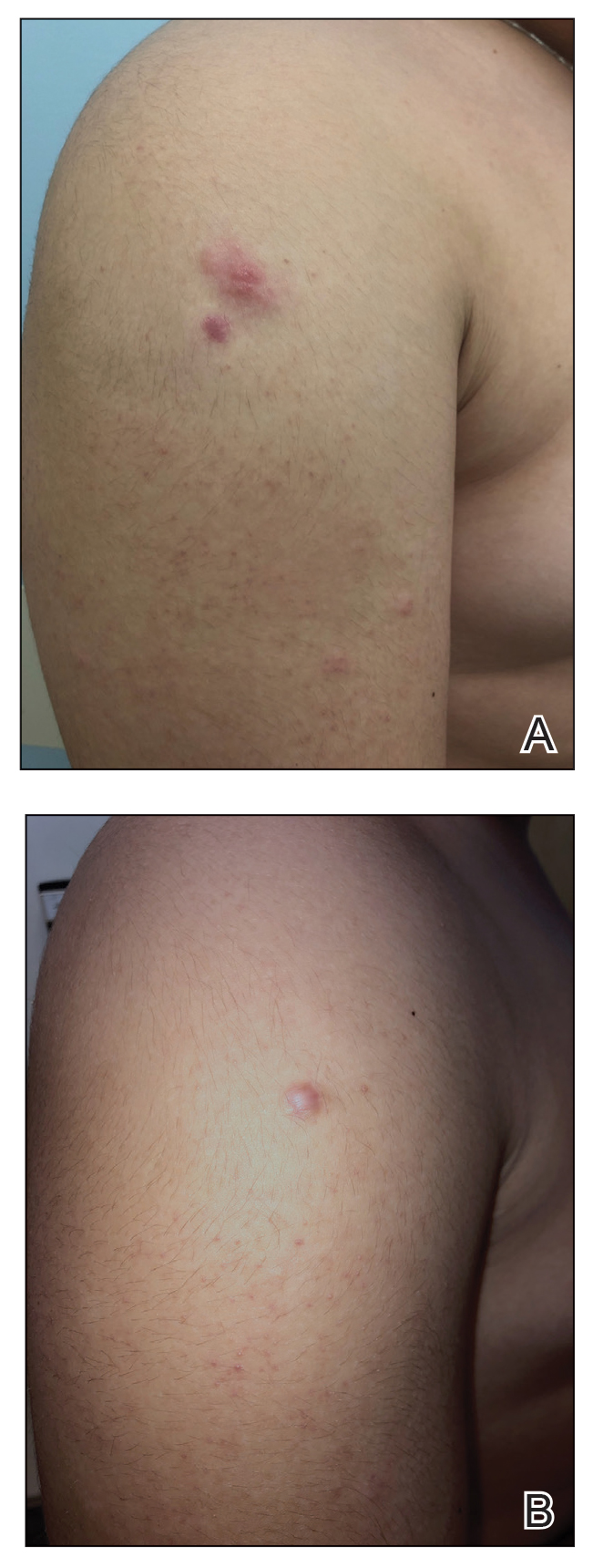
Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Practice Points
- When skin biopsy reveals marginal zone lymphoma, laboratory workup should include a complete blood cell count, chemistry, and serum lactate dehydrogenase levels. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed.
- For patients with multifocal skin lesions, positive emission tomography with computed tomography is utilized to exclude systemic disease and assess lymph node involvement.
- Treatments for primary cutaneous marginal zone lymphoma include excision, topical steroids, intralesional steroids, intralesional rituximab, radiation therapy, and antibiotics.
- Doxycycline can be considered as a treatment option for pediatric patients with widespread cutaneous involvement.
Pustular Eruption on the Face
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
A 52-year-old man developed a sudden eruption of small pustules on background erythema and edema covering the forehead, nasal bridge, periorbital region, cheeks, and perioral region on day 3 of hospitalization in the intensive care unit for management of septic shock secondary to a complicated urinary tract infection. He had a medical history of benign prostatic hyperplasia, sarcoidosis, and atopic dermatitis. He initially presented to the emergency department with fever, chills, and dysuria of 2 days’ duration. Because he received ceftriaxone, vancomycin, ciprofloxacin, and tamsulosin while hospitalized for the infection, the primary medical team suspected a drug reaction and empirically started applying hydrocortisone cream 2.5%. The rash continued to spread over the ensuing day, prompting a dermatology consultation to rule out a drug eruption and to help guide further management. The patient was in substantial distress and pain. Physical examination revealed numerous discrete and confluent monomorphic pustules on background erythema with faint collarettes of scale covering most of the face. Substantial periorbital and facial edema forced the eyes closed. There was no mucous membrane involvement. A review of systems was negative for dyspnea and dysphagia, and the rash was not present elsewhere on the body. Ophthalmologic evaluation revealed no ocular involvement or vision changes. Laboratory studies demonstrated neutrophilia (17.27×109 cells/L [reference range, 2.0–6.9×109 cells/L]). The eosinophil count, blood urea nitrogen/creatinine, and liver function tests were within reference range.
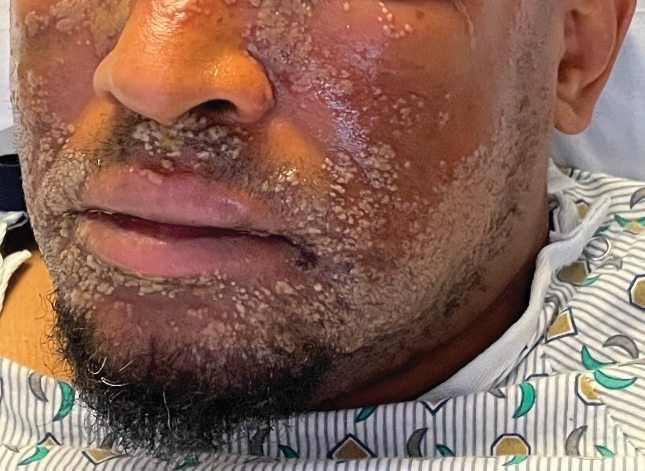
Keratotic Nodules in a Patient With End-Stage Renal Disease
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.
Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.
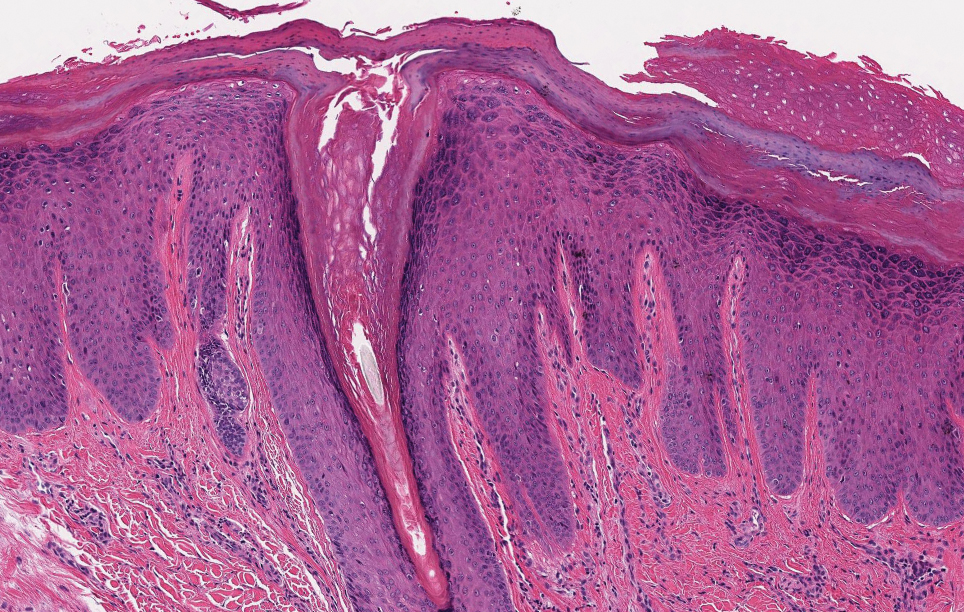
Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15
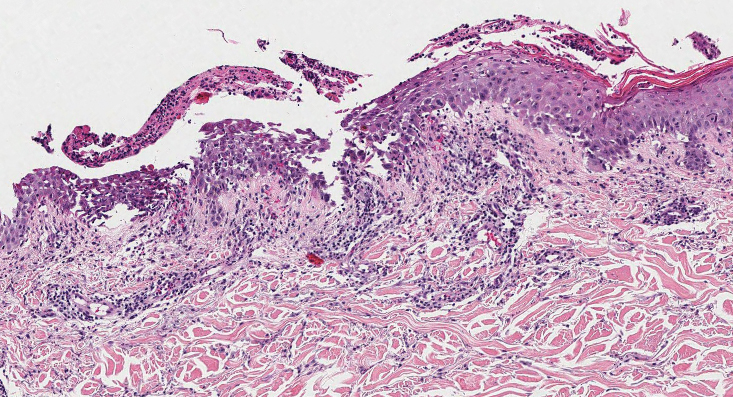
Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16
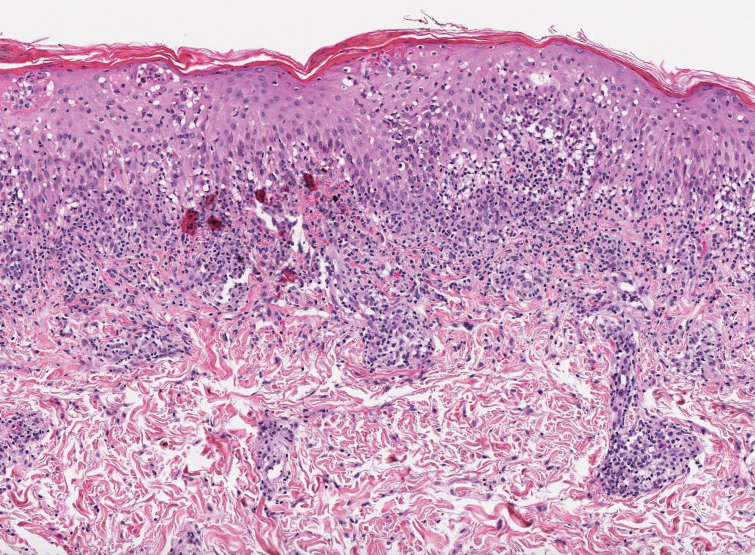
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
A 42-year-old man with end-stage renal disease on hemodialysis presented with generalized body itching and nodules on the scalp and back of 1 year’s duration. Physical examination revealed diffuse, hyperpigmented, pruritic, keratotic nodules and macules on the scalp and back (top). A punch biopsy was performed (bottom).
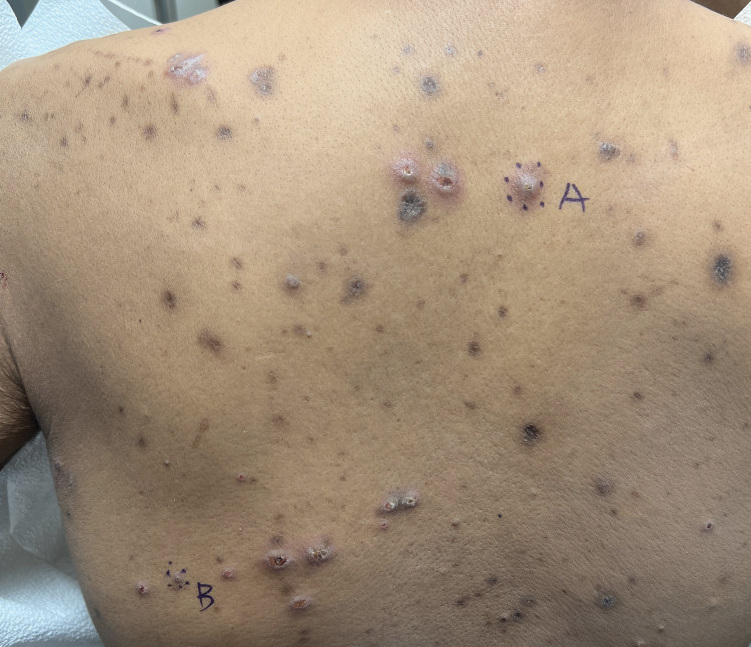
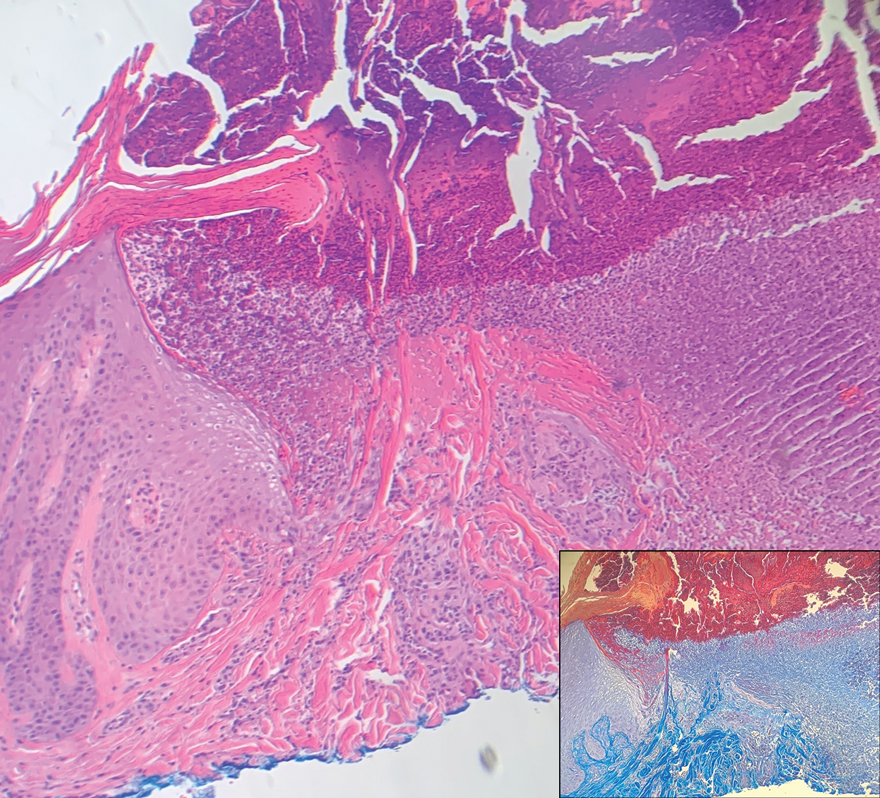
Attitudes Toward Utilization of Minimally Invasive Cosmetic Procedures in Black Women: Results of a Cross-sectional Survey
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.
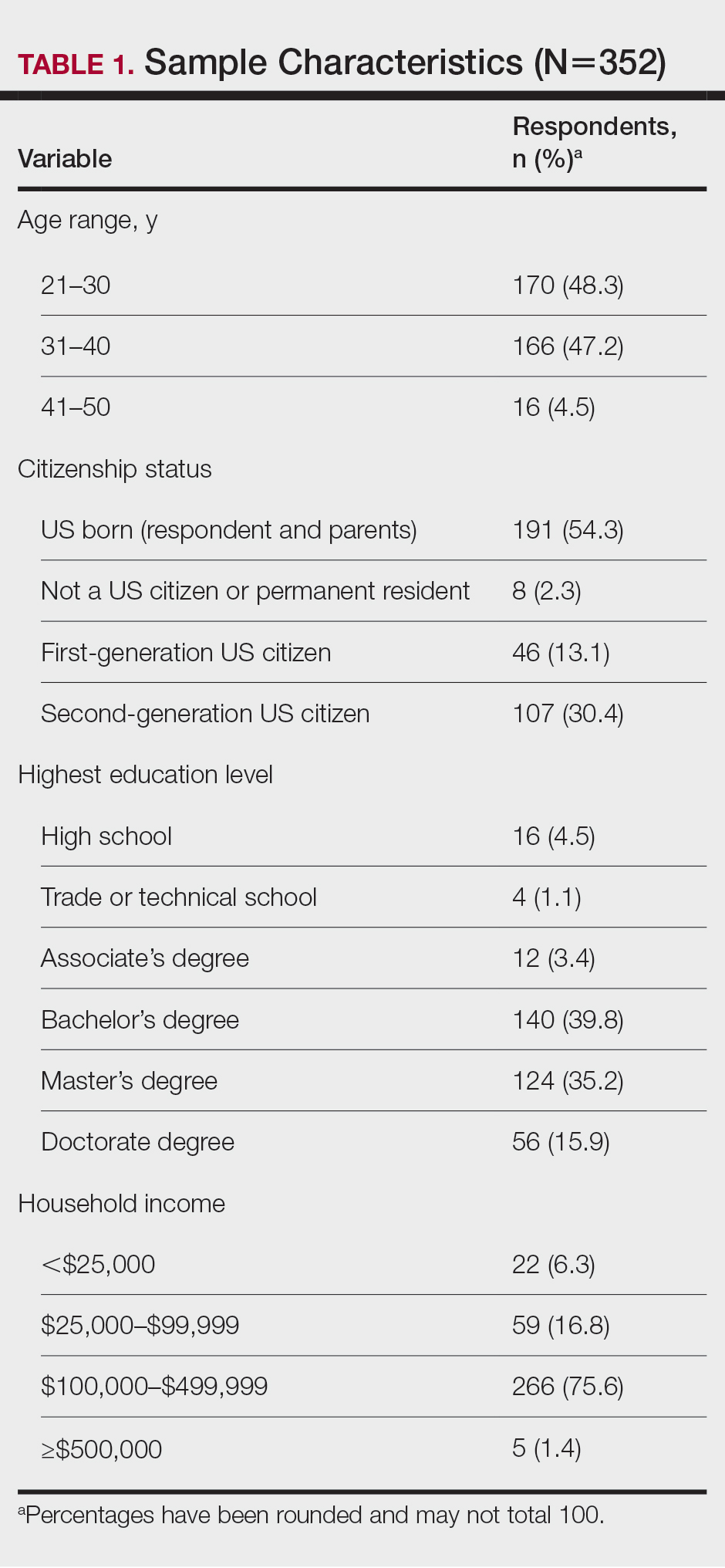
Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.
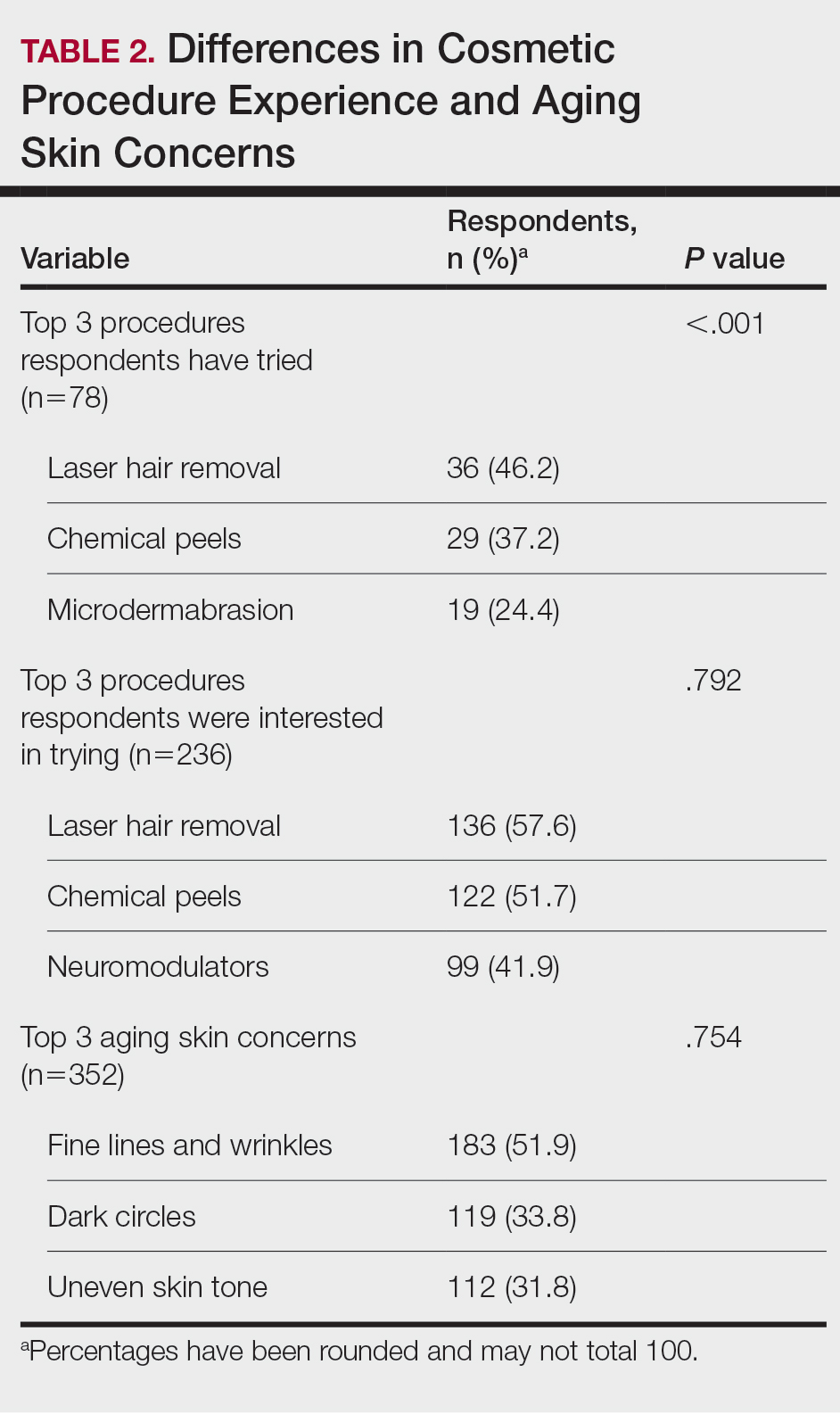
Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).
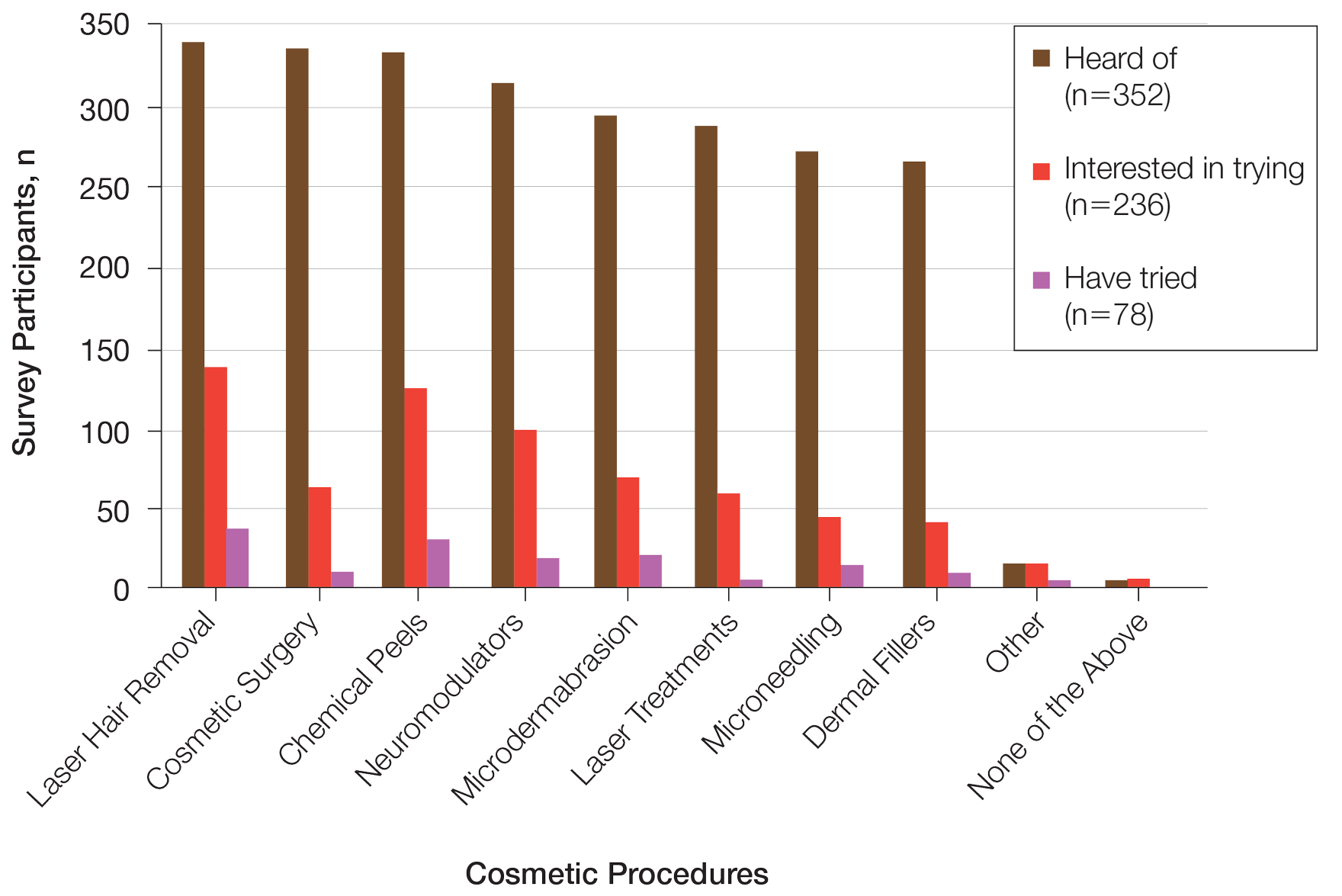
Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Practice Points
- Cosmetic procedures may be more widely accepted among younger Black women than older Black women.
- Age has a considerable influence on the types of cosmetic procedures that Black women are interested in trying.
- Microdermabrasion, chemical peels, and laser hair removal were the most frequently utilized procedures in this study population.
- As attitudes and perceptions of young Black women are changing and favoring more frequent sunscreen use, dermatologists should remain on top of current trends to provide culturally sensitive and relevant recommendations to patients with darker skin tones.