User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Hydroxychloroquine blood level ‘sweet spot’ may maximize efficacy in lupus
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood-level reference range of 750-1,200 ng/mL of hydroxychloroquine (HCQ) has been linked with 71% lower odds of active lupus, new research suggests.
Researchers, led by Shivani Garg, MD, assistant professor of rheumatology at the University of Wisconsin–Madison, also found that maintaining levels within that range lowered the odds for flares by 26% over 9 months of follow-up.
The findings, published in Arthritis Care & Research, could help clinicians personalize HCQ doses to maximize efficacy for each patient.
HCQ levels in whole blood and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) were measured during a baseline visit and again during a routine follow-up visit.
Among 158 baseline patient visits, 19% of the patients had active lupus. Researchers longitudinally followed 42 patients using convenience sampling, and among those patients, 7 (17%) had flares at the follow-up visit.
Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center in Baltimore, called the findings that suggest upper and lower efficacy and safety boundaries “very important.”
The findings highlight that guidelines for dosing don’t match efficacy needs, said Dr. Petri, who was not involved with the study.
“HCQ dosing has been under threat by guidelines insisting that the dose should be < 5 mg/kg even though this does not correlate with efficacy,” she said. “Basically, if we dose too low, the patient loses efficacy. If we dose too high, the risk of retinopathy increases, so this paper hones down the sweet spot.”
A 2014 study identified a higher eye toxicity risk with HCQ doses > 5 mg/kg per day, and the American Academy of Ophthalmology followed with guidelines for HCQ retinopathy screening that recommended reducing HCQ to ≤ 5 mg/kg per day.
Dr. Petri said that the range Dr. Garg and colleagues identified corroborates findings in one of her team’s studies.
That paper showed that thrombotic events dropped by 69% in patients with average HCQ blood levels ≥ 1,068 ng/mL vs. those with levels < 648 ng/mL (relative risk, 0.31; 95% confidence interval, 0.11-0.86; P = .024).
Dr. Garg and colleagues write that current lupus treatment guidelines do not universally recommend blood level monitoring for HCQ “as different cut-points have been used to define therapeutic HCQ blood levels and an effective range of HCQ levels with upper and lower bounds for efficacy has not been extensively examined.”
When to start checking levels
Blood levels of HCQ can be checked for any patient, although 1-3 months after starting the medication may be best to get steady levels, Dr. Garg told this news organization.
Dr. Petri said that she recommends HCQ whole blood levels be checked routinely for maximum dosing efficacy “but also to identify patients who are missing so many doses that they are subtherapeutic.”
She noted that nonadherence is a major issue among patients with systemic lupus erythematosus, especially among those who are younger and newly diagnosed.
Dr. Garg and Dr. Petri both said that insurance does not automatically cover the costs of checking HCQ levels in the blood, which has been a consistent frustration in the field.
“Having more data validates the reason to do it,” Dr. Garg said.
She added that “HCQ blood levels are still not done routinely in all patients, and at times the test needs to be sent to outside laboratories.”
Importance for patients with CKD
Many patient factors can affect how the body absorbs HCQ, Dr. Garg said, so finding the right level that is safe and maximizes benefit individually is important.
The findings are particularly important for patients with chronic kidney disease (CKD) of stage 3 or higher, Dr. Garg said.
The authors write that because kidneys clear more than half of all HCQ, impaired kidney function could boost HCQ blood levels, risking toxicity.
“Our study found a sixfold higher odds of having supratherapeutic HCQ blood levels in patients with CKD stage ≥ 3,” they write.
Dr. Garg added that if blood levels cannot be analyzed in all patients, they could be prioritized in patients with CKD stage 3 or above because these patients are at “higher risk of being underdosed with arbitrary reductions in HCQ doses and carry higher risk of toxicity if HCQ doses are not adjusted.”
More research will uncover other high-risk groups who would benefit most from close monitoring of HCQ blood levels, she said.
The study was supported by an award from the University of Wisconsin–Madison, and by an award to the institution from the National Institutes of Health National Center for Advancing Translational Sciences. Dr. Garg and coauthors as well as Dr. Petri report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
Company submits supplemental NDA for topical atopic dermatitis treatment
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
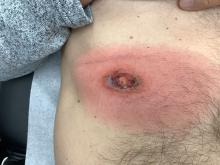
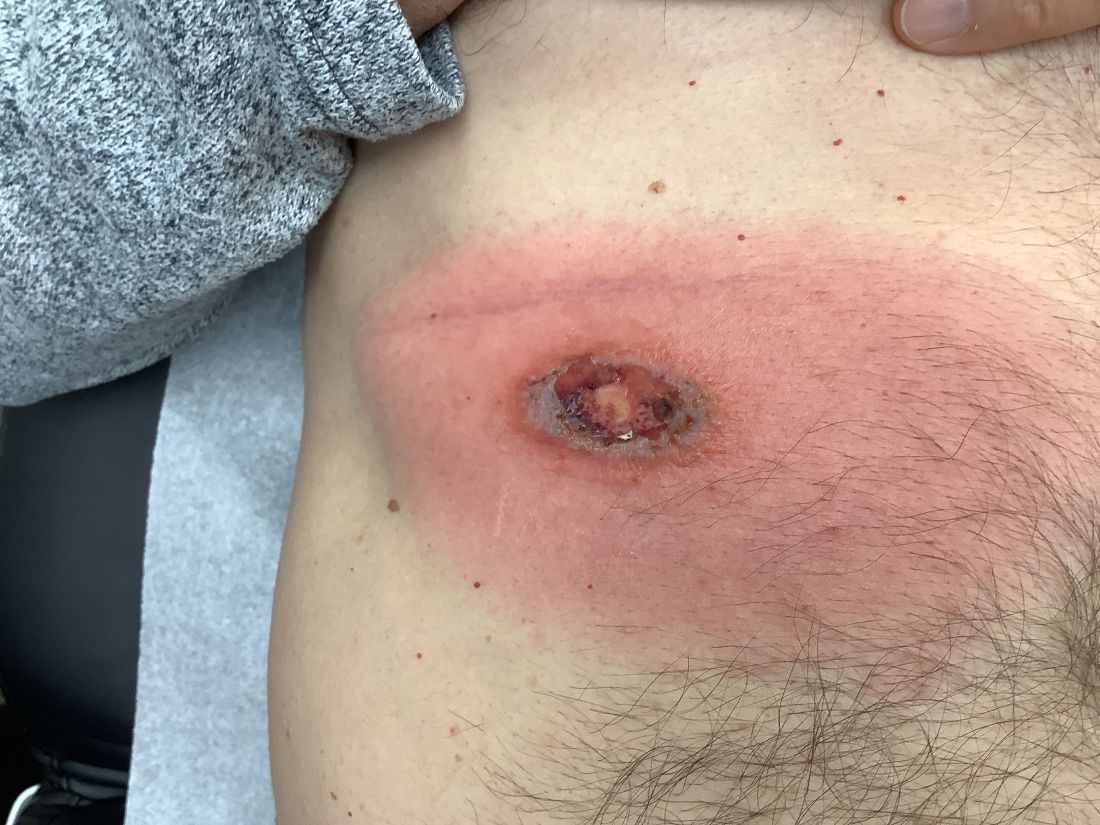
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Comorbidities, CV risk factors common in early PsA
TOPLINE:
Patients with early psoriatic arthritis (PsA) were significantly more likely to have multiple comorbidities and cardiovascular risk factors than controls.
METHODOLOGY:
- The study population included 67 adults with early PsA and 61 healthy matched controls with mean ages of 47.9 years and 45 years, respectively.
- Early PsA was defined as symptom duration of less than 2 years; patients with conditions including active infection, malignancy, or other rheumatic or systemic disease were excluded.
- The researchers examined the prevalence of comorbidities and cardiovascular risk factors in treatment-naive, newly diagnosed patients with PsA at baseline and after 1 year.
TAKEAWAY:
- , compared with healthy controls (odds ratios, 1.9 and 2.1, respectively).
- Dyslipidemia was the most prevalent comorbidity among patients with PsA and was more prevalent than in controls (64.2% vs. 39.3%; OR, 1.7).
- Obesity was more common in patients with PsA, compared with controls (40.3% vs. 18.3%, respectively), and more patients with PsA had cardiovascular disease at baseline than did controls (20.9% vs. 6.6%; OR, 3.2).
- Disease activity scores improved after 1 year, but the proportion of patients with comorbidities and CV risk factors remained stable.
IN PRACTICE:
The results support the early assessment of patients with PsA for comorbidities to inform treatment and suggest that comorbidities and CV risk factors are more than a consequence of long-term PsA and chronic systemic inflammation.
SOURCE:
The study was conducted by Alla Ishchenko, MD, and colleagues in the division of rheumatology at University Hospitals Leuven, Belgium. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The study was exploratory in nature, with a short follow-up period and a relatively small sample size.
DISCLOSURES:
Dr. Ishchenko disclosed support from PARTNER, an international fellowship program to study disease mechanisms in psoriatic arthritis, as well as grants from Lilly and from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with early psoriatic arthritis (PsA) were significantly more likely to have multiple comorbidities and cardiovascular risk factors than controls.
METHODOLOGY:
- The study population included 67 adults with early PsA and 61 healthy matched controls with mean ages of 47.9 years and 45 years, respectively.
- Early PsA was defined as symptom duration of less than 2 years; patients with conditions including active infection, malignancy, or other rheumatic or systemic disease were excluded.
- The researchers examined the prevalence of comorbidities and cardiovascular risk factors in treatment-naive, newly diagnosed patients with PsA at baseline and after 1 year.
TAKEAWAY:
- , compared with healthy controls (odds ratios, 1.9 and 2.1, respectively).
- Dyslipidemia was the most prevalent comorbidity among patients with PsA and was more prevalent than in controls (64.2% vs. 39.3%; OR, 1.7).
- Obesity was more common in patients with PsA, compared with controls (40.3% vs. 18.3%, respectively), and more patients with PsA had cardiovascular disease at baseline than did controls (20.9% vs. 6.6%; OR, 3.2).
- Disease activity scores improved after 1 year, but the proportion of patients with comorbidities and CV risk factors remained stable.
IN PRACTICE:
The results support the early assessment of patients with PsA for comorbidities to inform treatment and suggest that comorbidities and CV risk factors are more than a consequence of long-term PsA and chronic systemic inflammation.
SOURCE:
The study was conducted by Alla Ishchenko, MD, and colleagues in the division of rheumatology at University Hospitals Leuven, Belgium. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The study was exploratory in nature, with a short follow-up period and a relatively small sample size.
DISCLOSURES:
Dr. Ishchenko disclosed support from PARTNER, an international fellowship program to study disease mechanisms in psoriatic arthritis, as well as grants from Lilly and from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with early psoriatic arthritis (PsA) were significantly more likely to have multiple comorbidities and cardiovascular risk factors than controls.
METHODOLOGY:
- The study population included 67 adults with early PsA and 61 healthy matched controls with mean ages of 47.9 years and 45 years, respectively.
- Early PsA was defined as symptom duration of less than 2 years; patients with conditions including active infection, malignancy, or other rheumatic or systemic disease were excluded.
- The researchers examined the prevalence of comorbidities and cardiovascular risk factors in treatment-naive, newly diagnosed patients with PsA at baseline and after 1 year.
TAKEAWAY:
- , compared with healthy controls (odds ratios, 1.9 and 2.1, respectively).
- Dyslipidemia was the most prevalent comorbidity among patients with PsA and was more prevalent than in controls (64.2% vs. 39.3%; OR, 1.7).
- Obesity was more common in patients with PsA, compared with controls (40.3% vs. 18.3%, respectively), and more patients with PsA had cardiovascular disease at baseline than did controls (20.9% vs. 6.6%; OR, 3.2).
- Disease activity scores improved after 1 year, but the proportion of patients with comorbidities and CV risk factors remained stable.
IN PRACTICE:
The results support the early assessment of patients with PsA for comorbidities to inform treatment and suggest that comorbidities and CV risk factors are more than a consequence of long-term PsA and chronic systemic inflammation.
SOURCE:
The study was conducted by Alla Ishchenko, MD, and colleagues in the division of rheumatology at University Hospitals Leuven, Belgium. The study was published online in Arthritis Care & Research.
LIMITATIONS:
The study was exploratory in nature, with a short follow-up period and a relatively small sample size.
DISCLOSURES:
Dr. Ishchenko disclosed support from PARTNER, an international fellowship program to study disease mechanisms in psoriatic arthritis, as well as grants from Lilly and from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
A version of this article first appeared on Medscape.com.
Seeking help for burnout may be a gamble for doctors
By the end of 2021, Anuj Peddada, MD, had hit a wall. He couldn’t sleep, couldn’t concentrate, erupted in anger, and felt isolated personally and professionally. To temper pandemic-driven pressures, the Colorado radiation oncologist took an 8-week stress management and resiliency course, but the feelings kept creeping back.
Still, Dr. Peddada, in his own private practice, pushed through, working 60-hour weeks and carrying the workload of two physicians. It wasn’t until he caught himself making uncharacteristic medical errors, including radiation planning for the wrong site, that he knew he needed help – and possibly a temporary break from medicine.
There was just one hitch: He was closing his private practice to start a new in-house job with Centura Health, the Colorado Springs hospital he’d contracted with for over 20 years.
Given the long-standing relationship – Dr. Peddada’s image graced some of the company’s marketing billboards – he expected Centura would understand when, on his doctor’s recommendation, he requested a short-term medical leave that would delay his start date by 1 month.
Instead, Centura abruptly rescinded the employment offer, leaving Dr. Peddada jobless and with no recourse but to sue.
“I was blindsided. The hospital had a physician resiliency program that claimed to encourage physicians to seek help, [so] I thought they would be completely supportive and understanding,” Dr. Peddada said.
He told this news organization that he was naive to have been so honest with the hospital he’d long served as a contractor, including the decade-plus he›d spent directing its radiation oncology department.
“It is exceedingly painful to see hospital leadership use me in their advertisement[s] ... trying to profit off my reputation and work after devastating my career.”
The lawsuit Dr. Peddada filed in July in Colorado federal district court may offer a rare glimpse of the potential career ramifications of seeking help for physician burnout. Despite employers’ oft-stated support for physician wellness, Dr. Peddada’s experience may serve as a cautionary tale for doctors who are open about their struggles.
Centura Health did not respond to requests for comment. In court documents, the health system’s attorneys asked for more time to respond to Dr. Peddada’s complaint.
A plea for help
In the complaint, Dr. Peddada and his attorneys claim that Centura violated the state’s Anti-Discrimination Act and the Americans with Disabilities Act (ADA) when it failed to offer reasonable accommodations after he began experiencing “physiological and psychological symptoms corresponding to burnout.”
Since 1999, Dr. Peddada had contracted exclusively with Centura to provide oncology services at its hospital, Penrose Cancer Center, and began covering a second Centura location in 2021. As medical director of Penrose’s radiation oncology department, he helped establish a community nurse navigator program and accounted for 75% of Centura’s radiation oncology referrals, according to the complaint.
But when his symptoms and fear for the safety of his patients became unbearable, Dr. Peddada requested an urgent evaluation from his primary care physician, who diagnosed him with “physician burnout” and recommended medical leave.
Shortly after presenting the leave request to Centura, rumors began circulating that he was having a “nervous breakdown,” the complaint noted. Dr. Peddada worried that perhaps his private health information was being shared with hospital employees.
After meeting with the hospital’s head of physician resiliency and agreeing to undergo a peer review evaluation by the Colorado Physician Health Program, which would decide the reinstatement timeline and if further therapy was necessary, Dr. Peddada was assured his leave would be approved.
Five days later, his job offer was revoked.
In an email from hospital leadership, the oncologist was informed that he had “declined employment” by failing to sign a revised employment contract sent to him 2 weeks prior when he was out of state on a preapproved vacation, according to the lawsuit.
The lawsuit alleges that Dr. Peddada was wrongfully discharged due to his disability after Centura “exploited [his] extensive patient base, referral network, and reputation to generate growth and profit.”
Colorado employment law attorney Deborah Yim, Esq., who is not involved in Peddada’s case, told this news organization that the ADA requires employers to provide reasonable accommodations for physical or mental impairments that substantially limit at least one major life activity, except when the request imposes an undue hardship on the employer.
“Depression and related mental health conditions would qualify, depending on the circumstances, and courts have certainly found them to be qualifying disabilities entitled to ADA protection in the past,” she said.
Not all employers are receptive to doctors’ needs, says the leadership team at Physicians Just Equity, an organization providing peer support to doctors experiencing workplace conflicts like discrimination and retaliation. They say that Dr. Peddada’s experience, where disclosing burnout results in being “ostracized, penalized, and ultimately ousted,” is the rule rather than the exception.
“Dr. Peddada’s case represents the unfortunate reality faced by many physicians in today’s clinical landscape,” the organization’s board of directors said in a written statement. “The imbalance of unreasonable professional demands, the lack of autonomy, moral injury, and disintegrating practice rewards is unsustainable for the medical professional.”
“Retaliation by employers after speaking up against this imbalance [and] requesting support and time to rejuvenate is a grave failure of health care systems that prioritize the business of delivering health care over the health, well-being, and satisfaction of their most valuable resource – the physician,” the board added in their statement.
Dr. Peddada has since closed his private practice and works as an independent contractor and consultant, his attorney, Iris Halpern, JD, said in an interview. She says Centura could have honored the accommodation request or suggested another option that met his needs, but “not only were they unsupportive, they terminated him.”
Ms. Yim says the parties will have opportunities to reach a settlement and resolve the dispute as the case works through the court system. Otherwise, Dr. Peddada and Centura may eventually head to trial.
Current state of physician burnout
The state of physician burnout is certainly a concerning one. More than half (53%) of physicians responding to this year’s Medscape Physician Burnout & Depression Report said they are burned out. Nearly one-quarter reported feeling depressed. Some of the top reasons they cited were too many bureaucratic tasks (61%), too many work hours (37%), and lack of autonomy (31%).
A 2022 study by the Mayo Clinic found a substantial increase in physician burnout in the first 2 years of the pandemic, with doctors reporting rising emotional exhaustion and depersonalization.
Although burnout affects many physicians and is a priority focus of the National Academy of Medicine’s plan to restore workforce well-being, admitting it is often seen as taboo and can imperil a doctor’s career. In the Medscape report, for example, 39% of physicians said they would not even consider professional treatment for burnout, with many commenting that they would just deal with it themselves.
“Many physicians are frightened to take time out for self-care because [they] fear losing their job, being stigmatized, and potentially ending their careers,” said Dr. Peddada, adding that physicians are commonly asked questions about their mental health when applying for hospital privileges. He says this dynamic forces them to choose between getting help or ignoring their true feelings, leading to poor quality of care and patient safety risks.
Medical licensing boards probe physicians’ mental health, too. As part of its #FightingForDocs campaign, the American Medical Association hopes to remove the stigma around burnout and depression and advocates for licensing boards to revise questions that may discourage physicians from seeking assistance. The AMA recommends that physicians only disclose current physical or mental conditions affecting their ability to practice.
Pringl Miller, MD, founder and executive director of Physician Just Equity, told Medscape that improving physician wellness requires structural change.
“Physicians (who) experience burnout without the proper accommodations run the risk of personal harm, because most physicians will prioritize the health and well-being of their patients over themselves ... [resulting in] suboptimal and unsafe patient care,” she said.
Helping doctors regain a sense of purpose
One change involves reframing how the health care industry thinks about and approaches burnout, says Steven Siegel, MD, chief mental health and wellness officer with Keck Medicine of USC. He told this news organization that these discussions should enhance the physician’s sense of purpose.
“Some people treat burnout as a concrete disorder like cancer, instead of saying, ‘I’m feeling exhausted, demoralized, and don’t enjoy my job anymore. What can we do to restore my enthusiasm for work?’ ”
Dr. Siegel recognizes that these issues existed before the pandemic and have only worsened as physicians feel less connected to and satisfied with their profession – a byproduct, he says, of the commercialization of medicine.
“We’ve moved from practices to systems, then from small to large systems, where it seems the path to survival is cutting costs and increasing margins, even among nonprofits.”
The road ahead
Making headway on these problems will take time. Last year, Keck Medicine received a $2 million grant to launch a 3-year randomized clinical trial to help reconnect physicians and other clinicians with their work. Dr. Siegel says the trial may serve as a national pilot program and will eventually grow to include 400 volunteers.
The trial will investigate the effectiveness of three possible interventions: (1) teaching people how to regulate their internal narratives and emotions through techniques like cognitive behavioral therapy and acceptance and commitment therapy; (2) providing customized EHR training to reduce the burden of navigating the system; and (3) allowing physicians to weigh in on workflow changes.
“We put physicians on teams that make the decisions about workflows,” said Dr. Siegel. The arrangement can give people the agency they desire and help them understand why an idea might not be plausible, which enriches future suggestions and discussions, he says.
A version of this article first appeared on Medscape.com.
By the end of 2021, Anuj Peddada, MD, had hit a wall. He couldn’t sleep, couldn’t concentrate, erupted in anger, and felt isolated personally and professionally. To temper pandemic-driven pressures, the Colorado radiation oncologist took an 8-week stress management and resiliency course, but the feelings kept creeping back.
Still, Dr. Peddada, in his own private practice, pushed through, working 60-hour weeks and carrying the workload of two physicians. It wasn’t until he caught himself making uncharacteristic medical errors, including radiation planning for the wrong site, that he knew he needed help – and possibly a temporary break from medicine.
There was just one hitch: He was closing his private practice to start a new in-house job with Centura Health, the Colorado Springs hospital he’d contracted with for over 20 years.
Given the long-standing relationship – Dr. Peddada’s image graced some of the company’s marketing billboards – he expected Centura would understand when, on his doctor’s recommendation, he requested a short-term medical leave that would delay his start date by 1 month.
Instead, Centura abruptly rescinded the employment offer, leaving Dr. Peddada jobless and with no recourse but to sue.
“I was blindsided. The hospital had a physician resiliency program that claimed to encourage physicians to seek help, [so] I thought they would be completely supportive and understanding,” Dr. Peddada said.
He told this news organization that he was naive to have been so honest with the hospital he’d long served as a contractor, including the decade-plus he›d spent directing its radiation oncology department.
“It is exceedingly painful to see hospital leadership use me in their advertisement[s] ... trying to profit off my reputation and work after devastating my career.”
The lawsuit Dr. Peddada filed in July in Colorado federal district court may offer a rare glimpse of the potential career ramifications of seeking help for physician burnout. Despite employers’ oft-stated support for physician wellness, Dr. Peddada’s experience may serve as a cautionary tale for doctors who are open about their struggles.
Centura Health did not respond to requests for comment. In court documents, the health system’s attorneys asked for more time to respond to Dr. Peddada’s complaint.
A plea for help
In the complaint, Dr. Peddada and his attorneys claim that Centura violated the state’s Anti-Discrimination Act and the Americans with Disabilities Act (ADA) when it failed to offer reasonable accommodations after he began experiencing “physiological and psychological symptoms corresponding to burnout.”
Since 1999, Dr. Peddada had contracted exclusively with Centura to provide oncology services at its hospital, Penrose Cancer Center, and began covering a second Centura location in 2021. As medical director of Penrose’s radiation oncology department, he helped establish a community nurse navigator program and accounted for 75% of Centura’s radiation oncology referrals, according to the complaint.
But when his symptoms and fear for the safety of his patients became unbearable, Dr. Peddada requested an urgent evaluation from his primary care physician, who diagnosed him with “physician burnout” and recommended medical leave.
Shortly after presenting the leave request to Centura, rumors began circulating that he was having a “nervous breakdown,” the complaint noted. Dr. Peddada worried that perhaps his private health information was being shared with hospital employees.
After meeting with the hospital’s head of physician resiliency and agreeing to undergo a peer review evaluation by the Colorado Physician Health Program, which would decide the reinstatement timeline and if further therapy was necessary, Dr. Peddada was assured his leave would be approved.
Five days later, his job offer was revoked.
In an email from hospital leadership, the oncologist was informed that he had “declined employment” by failing to sign a revised employment contract sent to him 2 weeks prior when he was out of state on a preapproved vacation, according to the lawsuit.
The lawsuit alleges that Dr. Peddada was wrongfully discharged due to his disability after Centura “exploited [his] extensive patient base, referral network, and reputation to generate growth and profit.”
Colorado employment law attorney Deborah Yim, Esq., who is not involved in Peddada’s case, told this news organization that the ADA requires employers to provide reasonable accommodations for physical or mental impairments that substantially limit at least one major life activity, except when the request imposes an undue hardship on the employer.
“Depression and related mental health conditions would qualify, depending on the circumstances, and courts have certainly found them to be qualifying disabilities entitled to ADA protection in the past,” she said.
Not all employers are receptive to doctors’ needs, says the leadership team at Physicians Just Equity, an organization providing peer support to doctors experiencing workplace conflicts like discrimination and retaliation. They say that Dr. Peddada’s experience, where disclosing burnout results in being “ostracized, penalized, and ultimately ousted,” is the rule rather than the exception.
“Dr. Peddada’s case represents the unfortunate reality faced by many physicians in today’s clinical landscape,” the organization’s board of directors said in a written statement. “The imbalance of unreasonable professional demands, the lack of autonomy, moral injury, and disintegrating practice rewards is unsustainable for the medical professional.”
“Retaliation by employers after speaking up against this imbalance [and] requesting support and time to rejuvenate is a grave failure of health care systems that prioritize the business of delivering health care over the health, well-being, and satisfaction of their most valuable resource – the physician,” the board added in their statement.
Dr. Peddada has since closed his private practice and works as an independent contractor and consultant, his attorney, Iris Halpern, JD, said in an interview. She says Centura could have honored the accommodation request or suggested another option that met his needs, but “not only were they unsupportive, they terminated him.”
Ms. Yim says the parties will have opportunities to reach a settlement and resolve the dispute as the case works through the court system. Otherwise, Dr. Peddada and Centura may eventually head to trial.
Current state of physician burnout
The state of physician burnout is certainly a concerning one. More than half (53%) of physicians responding to this year’s Medscape Physician Burnout & Depression Report said they are burned out. Nearly one-quarter reported feeling depressed. Some of the top reasons they cited were too many bureaucratic tasks (61%), too many work hours (37%), and lack of autonomy (31%).
A 2022 study by the Mayo Clinic found a substantial increase in physician burnout in the first 2 years of the pandemic, with doctors reporting rising emotional exhaustion and depersonalization.
Although burnout affects many physicians and is a priority focus of the National Academy of Medicine’s plan to restore workforce well-being, admitting it is often seen as taboo and can imperil a doctor’s career. In the Medscape report, for example, 39% of physicians said they would not even consider professional treatment for burnout, with many commenting that they would just deal with it themselves.
“Many physicians are frightened to take time out for self-care because [they] fear losing their job, being stigmatized, and potentially ending their careers,” said Dr. Peddada, adding that physicians are commonly asked questions about their mental health when applying for hospital privileges. He says this dynamic forces them to choose between getting help or ignoring their true feelings, leading to poor quality of care and patient safety risks.
Medical licensing boards probe physicians’ mental health, too. As part of its #FightingForDocs campaign, the American Medical Association hopes to remove the stigma around burnout and depression and advocates for licensing boards to revise questions that may discourage physicians from seeking assistance. The AMA recommends that physicians only disclose current physical or mental conditions affecting their ability to practice.
Pringl Miller, MD, founder and executive director of Physician Just Equity, told Medscape that improving physician wellness requires structural change.
“Physicians (who) experience burnout without the proper accommodations run the risk of personal harm, because most physicians will prioritize the health and well-being of their patients over themselves ... [resulting in] suboptimal and unsafe patient care,” she said.
Helping doctors regain a sense of purpose
One change involves reframing how the health care industry thinks about and approaches burnout, says Steven Siegel, MD, chief mental health and wellness officer with Keck Medicine of USC. He told this news organization that these discussions should enhance the physician’s sense of purpose.
“Some people treat burnout as a concrete disorder like cancer, instead of saying, ‘I’m feeling exhausted, demoralized, and don’t enjoy my job anymore. What can we do to restore my enthusiasm for work?’ ”
Dr. Siegel recognizes that these issues existed before the pandemic and have only worsened as physicians feel less connected to and satisfied with their profession – a byproduct, he says, of the commercialization of medicine.
“We’ve moved from practices to systems, then from small to large systems, where it seems the path to survival is cutting costs and increasing margins, even among nonprofits.”
The road ahead
Making headway on these problems will take time. Last year, Keck Medicine received a $2 million grant to launch a 3-year randomized clinical trial to help reconnect physicians and other clinicians with their work. Dr. Siegel says the trial may serve as a national pilot program and will eventually grow to include 400 volunteers.
The trial will investigate the effectiveness of three possible interventions: (1) teaching people how to regulate their internal narratives and emotions through techniques like cognitive behavioral therapy and acceptance and commitment therapy; (2) providing customized EHR training to reduce the burden of navigating the system; and (3) allowing physicians to weigh in on workflow changes.
“We put physicians on teams that make the decisions about workflows,” said Dr. Siegel. The arrangement can give people the agency they desire and help them understand why an idea might not be plausible, which enriches future suggestions and discussions, he says.
A version of this article first appeared on Medscape.com.
By the end of 2021, Anuj Peddada, MD, had hit a wall. He couldn’t sleep, couldn’t concentrate, erupted in anger, and felt isolated personally and professionally. To temper pandemic-driven pressures, the Colorado radiation oncologist took an 8-week stress management and resiliency course, but the feelings kept creeping back.
Still, Dr. Peddada, in his own private practice, pushed through, working 60-hour weeks and carrying the workload of two physicians. It wasn’t until he caught himself making uncharacteristic medical errors, including radiation planning for the wrong site, that he knew he needed help – and possibly a temporary break from medicine.
There was just one hitch: He was closing his private practice to start a new in-house job with Centura Health, the Colorado Springs hospital he’d contracted with for over 20 years.
Given the long-standing relationship – Dr. Peddada’s image graced some of the company’s marketing billboards – he expected Centura would understand when, on his doctor’s recommendation, he requested a short-term medical leave that would delay his start date by 1 month.
Instead, Centura abruptly rescinded the employment offer, leaving Dr. Peddada jobless and with no recourse but to sue.
“I was blindsided. The hospital had a physician resiliency program that claimed to encourage physicians to seek help, [so] I thought they would be completely supportive and understanding,” Dr. Peddada said.
He told this news organization that he was naive to have been so honest with the hospital he’d long served as a contractor, including the decade-plus he›d spent directing its radiation oncology department.
“It is exceedingly painful to see hospital leadership use me in their advertisement[s] ... trying to profit off my reputation and work after devastating my career.”
The lawsuit Dr. Peddada filed in July in Colorado federal district court may offer a rare glimpse of the potential career ramifications of seeking help for physician burnout. Despite employers’ oft-stated support for physician wellness, Dr. Peddada’s experience may serve as a cautionary tale for doctors who are open about their struggles.
Centura Health did not respond to requests for comment. In court documents, the health system’s attorneys asked for more time to respond to Dr. Peddada’s complaint.
A plea for help
In the complaint, Dr. Peddada and his attorneys claim that Centura violated the state’s Anti-Discrimination Act and the Americans with Disabilities Act (ADA) when it failed to offer reasonable accommodations after he began experiencing “physiological and psychological symptoms corresponding to burnout.”
Since 1999, Dr. Peddada had contracted exclusively with Centura to provide oncology services at its hospital, Penrose Cancer Center, and began covering a second Centura location in 2021. As medical director of Penrose’s radiation oncology department, he helped establish a community nurse navigator program and accounted for 75% of Centura’s radiation oncology referrals, according to the complaint.
But when his symptoms and fear for the safety of his patients became unbearable, Dr. Peddada requested an urgent evaluation from his primary care physician, who diagnosed him with “physician burnout” and recommended medical leave.
Shortly after presenting the leave request to Centura, rumors began circulating that he was having a “nervous breakdown,” the complaint noted. Dr. Peddada worried that perhaps his private health information was being shared with hospital employees.
After meeting with the hospital’s head of physician resiliency and agreeing to undergo a peer review evaluation by the Colorado Physician Health Program, which would decide the reinstatement timeline and if further therapy was necessary, Dr. Peddada was assured his leave would be approved.
Five days later, his job offer was revoked.
In an email from hospital leadership, the oncologist was informed that he had “declined employment” by failing to sign a revised employment contract sent to him 2 weeks prior when he was out of state on a preapproved vacation, according to the lawsuit.
The lawsuit alleges that Dr. Peddada was wrongfully discharged due to his disability after Centura “exploited [his] extensive patient base, referral network, and reputation to generate growth and profit.”
Colorado employment law attorney Deborah Yim, Esq., who is not involved in Peddada’s case, told this news organization that the ADA requires employers to provide reasonable accommodations for physical or mental impairments that substantially limit at least one major life activity, except when the request imposes an undue hardship on the employer.
“Depression and related mental health conditions would qualify, depending on the circumstances, and courts have certainly found them to be qualifying disabilities entitled to ADA protection in the past,” she said.
Not all employers are receptive to doctors’ needs, says the leadership team at Physicians Just Equity, an organization providing peer support to doctors experiencing workplace conflicts like discrimination and retaliation. They say that Dr. Peddada’s experience, where disclosing burnout results in being “ostracized, penalized, and ultimately ousted,” is the rule rather than the exception.
“Dr. Peddada’s case represents the unfortunate reality faced by many physicians in today’s clinical landscape,” the organization’s board of directors said in a written statement. “The imbalance of unreasonable professional demands, the lack of autonomy, moral injury, and disintegrating practice rewards is unsustainable for the medical professional.”
“Retaliation by employers after speaking up against this imbalance [and] requesting support and time to rejuvenate is a grave failure of health care systems that prioritize the business of delivering health care over the health, well-being, and satisfaction of their most valuable resource – the physician,” the board added in their statement.
Dr. Peddada has since closed his private practice and works as an independent contractor and consultant, his attorney, Iris Halpern, JD, said in an interview. She says Centura could have honored the accommodation request or suggested another option that met his needs, but “not only were they unsupportive, they terminated him.”
Ms. Yim says the parties will have opportunities to reach a settlement and resolve the dispute as the case works through the court system. Otherwise, Dr. Peddada and Centura may eventually head to trial.
Current state of physician burnout
The state of physician burnout is certainly a concerning one. More than half (53%) of physicians responding to this year’s Medscape Physician Burnout & Depression Report said they are burned out. Nearly one-quarter reported feeling depressed. Some of the top reasons they cited were too many bureaucratic tasks (61%), too many work hours (37%), and lack of autonomy (31%).
A 2022 study by the Mayo Clinic found a substantial increase in physician burnout in the first 2 years of the pandemic, with doctors reporting rising emotional exhaustion and depersonalization.
Although burnout affects many physicians and is a priority focus of the National Academy of Medicine’s plan to restore workforce well-being, admitting it is often seen as taboo and can imperil a doctor’s career. In the Medscape report, for example, 39% of physicians said they would not even consider professional treatment for burnout, with many commenting that they would just deal with it themselves.
“Many physicians are frightened to take time out for self-care because [they] fear losing their job, being stigmatized, and potentially ending their careers,” said Dr. Peddada, adding that physicians are commonly asked questions about their mental health when applying for hospital privileges. He says this dynamic forces them to choose between getting help or ignoring their true feelings, leading to poor quality of care and patient safety risks.
Medical licensing boards probe physicians’ mental health, too. As part of its #FightingForDocs campaign, the American Medical Association hopes to remove the stigma around burnout and depression and advocates for licensing boards to revise questions that may discourage physicians from seeking assistance. The AMA recommends that physicians only disclose current physical or mental conditions affecting their ability to practice.
Pringl Miller, MD, founder and executive director of Physician Just Equity, told Medscape that improving physician wellness requires structural change.
“Physicians (who) experience burnout without the proper accommodations run the risk of personal harm, because most physicians will prioritize the health and well-being of their patients over themselves ... [resulting in] suboptimal and unsafe patient care,” she said.
Helping doctors regain a sense of purpose
One change involves reframing how the health care industry thinks about and approaches burnout, says Steven Siegel, MD, chief mental health and wellness officer with Keck Medicine of USC. He told this news organization that these discussions should enhance the physician’s sense of purpose.
“Some people treat burnout as a concrete disorder like cancer, instead of saying, ‘I’m feeling exhausted, demoralized, and don’t enjoy my job anymore. What can we do to restore my enthusiasm for work?’ ”
Dr. Siegel recognizes that these issues existed before the pandemic and have only worsened as physicians feel less connected to and satisfied with their profession – a byproduct, he says, of the commercialization of medicine.
“We’ve moved from practices to systems, then from small to large systems, where it seems the path to survival is cutting costs and increasing margins, even among nonprofits.”
The road ahead
Making headway on these problems will take time. Last year, Keck Medicine received a $2 million grant to launch a 3-year randomized clinical trial to help reconnect physicians and other clinicians with their work. Dr. Siegel says the trial may serve as a national pilot program and will eventually grow to include 400 volunteers.
The trial will investigate the effectiveness of three possible interventions: (1) teaching people how to regulate their internal narratives and emotions through techniques like cognitive behavioral therapy and acceptance and commitment therapy; (2) providing customized EHR training to reduce the burden of navigating the system; and (3) allowing physicians to weigh in on workflow changes.
“We put physicians on teams that make the decisions about workflows,” said Dr. Siegel. The arrangement can give people the agency they desire and help them understand why an idea might not be plausible, which enriches future suggestions and discussions, he says.
A version of this article first appeared on Medscape.com.
New Moderna vaccine to work against recent COVID variant
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
New AI-enhanced bandages poised to transform wound treatment
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
Almonds and almond oil
Almonds and almond oil are known to exhibit anti-inflammatory, antihepatotoxicity, and immunity-boosting activity.1 The seed from the deciduous almond tree (Oleum amygdalae), which is native to Iran and parts of the Levant, almonds contain copious amounts of phenols and polyphenols, fatty acids, and vitamin E, all of which are known to exert antioxidant activity.2-5 These seeds have been found to have a substantial impact on serum lipids.4 Emollient and sclerosant characteristics have also been linked to almond oil, which has been found to ameliorate complexion and skin tone.5 Significantly, in vitro and in vivo studies have shown that UVB-induced photoaging can be attenuated through the use of almond oil and almond skin extract.2 Further, in traditional Chinese Medicine, Ayurveda, and ancient Greco-Persian medicine, almond oil was used to treat cutaneous conditions, including eczema and psoriasis.1.
Antiphotoaging activity
In 2019, Foolad and Vaughn conducted a prospective, investigator-blind, randomized controlled trial to determine the effects of almond consumption on facial sebum production and wrinkles. Participants (28 postmenopausal women with Fitzpatrick skin types I and II completed the study) consumed 20% of their daily energy intake in almonds or a calorie-matched snack over 16 weeks through the UC Davis Dermatology Clinic. Photographic analysis revealed that the almond group experienced significantly diminished wrinkle severity, compared with the control group. The investigators concluded that daily almond consumption has the potential to decrease wrinkle severity in postmenopausal women and that almonds may confer natural antiaging effects.4
In a similar investigation 2 years later, Rybak et al. reported on a prospective, randomized controlled study to ascertain the effects of almond consumption on photoaging in postmenopausal women with Fitzpatrick skin types I or II who obtained 20% of their daily energy consumption via almonds or a calorie-matched snack for 24 weeks. Results demonstrated significant effects conferred by almond consumption, with average wrinkle severity substantially diminished in the almond group at weeks 16 (by 15%) and 24 (by 16%), compared with baseline. In addition, facial pigment intensity was reduced by 20% in the almond group by week 16 and this was maintained through the end of the study. Further, sebum excretion was higher in the control group. The investigators concluded that the daily consumption of almonds may have the potential to enhance protection against photoaging, particularly in terms of facial wrinkles and pigment intensity, in postmenopausal women.3
Later in 2021, Li et al. conducted a study in 39 healthy Asian women (18-45 years old) with Fitzpatrick skin types II to IV to investigate the effects of almond consumption on UVB resistance. The researchers randomized participants to eat either 1.5 oz of almonds or 1.8 oz of pretzels daily for 12 weeks. Results showed that the minimal erythema dose was higher in the almond group as compared with the control group. No differences were observed in hydration, melanin, roughness, or sebum on facial skin. The authors concluded that daily oral almond intake may improve photoprotection by raising the minimal erythema dose.2
In a 2022 review on the cutaneous benefits of sweet almond, evening primrose, and jojoba oils, Blaak and Staib noted that all three have been used for hundreds if not thousands of years in traditional medicine to treat various conditions, including skin disorders. Further, they concluded that the longstanding uses of these oils has been borne out by contemporary data, which reveal cutaneous benefits for adult and young skin, particularly in bolstering stratum corneum integrity, recovery, and lipid ratio.6
Later that year, Sanju et al., reporting on the development and assessment of a broad-spectrum polyherbal sunscreen delivered through solid lipid nanoparticles, noted that almond oil was among the natural ingredients used because of its photoprotective characteristics. Overall, the sunscreen formulation, Safranal, was found to impart robust protection against UV radiation.7
Wound healing
In 2020, Borzou et al. conducted a single-blind randomized clinical trial to ascertain the impact of topical almond oil in preventing pressure injuries. Data collection occurred over 8 months in a hospital setting, with 108 patients randomly assigned to receive almond oil, placebo (liquid paraffin), or the control (standard of care). The researchers found that topically applied almond oil was linked to a lower incidence of pressure injuries, and they arose later in the study as compared with those injuries in the groups receiving paraffin or standard of care. Pressure injury incidence was 5.6% in the almond oil group, 13.9% in the placebo group, and 25.1% in the control group.8
That same year, Caglar et al. completed a randomized controlled trial in 90 preterm infants to assess the effects of sunflower seed oil and almond oil on the stratum corneum. Infants were randomly selected for treatment with either oil or control. A nurse researcher applied oils to the whole body except for the head and face four times daily for 5 days. Investigators determined that stratum corneum hydration was better in the oil groups as compared with control, with no difference found between sunflower seed and almond oils.9
Eczema, hand dermatitis, and striae
In 2018, Simon et al. performed a randomized, double-blind study to determine the short- and long-term effects of two emollients on pruritus and skin restoration in xerotic eczema. The emollients contained lactic acid and refined almond oil, with one also including polidocanol. Both emollients were effective in reducing the severity of itching, with skin moisture and lipid content found to have risen after the initial administration and yielding steady improvement over 2 weeks.10
Earlier that year, Zeichner et al. found that the use of an OTC sweet almond oil, rich in fatty acids and a standard-bearing treatment for eczema and psoriasis for centuries, was effective in treating hand dermatitis. Specifically, the moisturizer, which contained 7% sweet almond oil and 2% colloidal oatmeal, was identified as safe and effective in resolving moderate to severe hand dermatitis.11
Some studies have also shown almond oil to be effective against striae gravidarum. Hajhashemi et al. conducted a double-blind clinical trial in 160 nulliparous women to compare the effects of aloe vera gel and sweet almond oil on striae gravidarum in 2018. Volunteers were randomly assigned to one of three case groups (Aloe vera, sweet almond oil, or base cream) who received topical treatment on the abdomen, or the fourth group, which received no treatment. Results showed that both treatment creams were effective in decreasing erythema and the pruritus associated with striae as well as in preventing their expansion.12 Previously, Tashan and Kafkasli showed in a nonrandomized study that massage with bitter almond oil may diminish the visibility of present striae gravidarum and prevent the emergence of new striae.13
Conclusion
Almonds and almond oil have been used as food and in traditional medical practices dating back several centuries. In the last decade, intriguing results have emerged regarding the effects of almond consumption or topical almond oil administration on skin health. While much more research is necessary, the recent data seem to support the traditional uses of this tree seed for dermatologic purposes.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology” (New York: McGraw Hill), was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Ahmad Z. Complement Ther Clin Pract. 2010 Feb;16(1):10-2.
2. Li JN et al. J Cosmet Dermatol. 2021 Sep;20(9):2975-80.
3. Rybak I et al. Nutrients. 2021 Feb 27;13(3):785.
4. Foolad N et al. Phytother Res. 2019 Dec;33(12):3212-7.
5. Lin TK et al. Int J Mol Sci. 2017 Dec 27;19(1):70.
6. Blaak J, Staib P. Int J Cosmet Sci. 2022 Feb;44(1):1-9.
7. Sanju N et al. J Cosmet Dermatol. 2022 Oct;21(10):4433-46.
8. Borzou SR et al. J Wound Ostomy Continence Nurs. 2020 Jul/Aug;47(4):336-42.
9. Caglar S et al. Adv Skin Wound Care. 2020 Aug;33(8):1-6.
10. Simon D et al. Dermatol Ther. 2018 Nov;31(6):e12692.
11. Zeichner JA at al. J Drugs Dermatol. 2018 Jan 1;17(1):78-82.
12. Hajhashemi M et al. J Matern Fetal Neonatal Med. 2018 Jul;31(13):1703-8.
13. Timur Tashan S and Kafkasli A. J Clin Nurs. 2012 Jun;21(11-12):1570-6.
Almonds and almond oil are known to exhibit anti-inflammatory, antihepatotoxicity, and immunity-boosting activity.1 The seed from the deciduous almond tree (Oleum amygdalae), which is native to Iran and parts of the Levant, almonds contain copious amounts of phenols and polyphenols, fatty acids, and vitamin E, all of which are known to exert antioxidant activity.2-5 These seeds have been found to have a substantial impact on serum lipids.4 Emollient and sclerosant characteristics have also been linked to almond oil, which has been found to ameliorate complexion and skin tone.5 Significantly, in vitro and in vivo studies have shown that UVB-induced photoaging can be attenuated through the use of almond oil and almond skin extract.2 Further, in traditional Chinese Medicine, Ayurveda, and ancient Greco-Persian medicine, almond oil was used to treat cutaneous conditions, including eczema and psoriasis.1.
Antiphotoaging activity
In 2019, Foolad and Vaughn conducted a prospective, investigator-blind, randomized controlled trial to determine the effects of almond consumption on facial sebum production and wrinkles. Participants (28 postmenopausal women with Fitzpatrick skin types I and II completed the study) consumed 20% of their daily energy intake in almonds or a calorie-matched snack over 16 weeks through the UC Davis Dermatology Clinic. Photographic analysis revealed that the almond group experienced significantly diminished wrinkle severity, compared with the control group. The investigators concluded that daily almond consumption has the potential to decrease wrinkle severity in postmenopausal women and that almonds may confer natural antiaging effects.4
In a similar investigation 2 years later, Rybak et al. reported on a prospective, randomized controlled study to ascertain the effects of almond consumption on photoaging in postmenopausal women with Fitzpatrick skin types I or II who obtained 20% of their daily energy consumption via almonds or a calorie-matched snack for 24 weeks. Results demonstrated significant effects conferred by almond consumption, with average wrinkle severity substantially diminished in the almond group at weeks 16 (by 15%) and 24 (by 16%), compared with baseline. In addition, facial pigment intensity was reduced by 20% in the almond group by week 16 and this was maintained through the end of the study. Further, sebum excretion was higher in the control group. The investigators concluded that the daily consumption of almonds may have the potential to enhance protection against photoaging, particularly in terms of facial wrinkles and pigment intensity, in postmenopausal women.3
Later in 2021, Li et al. conducted a study in 39 healthy Asian women (18-45 years old) with Fitzpatrick skin types II to IV to investigate the effects of almond consumption on UVB resistance. The researchers randomized participants to eat either 1.5 oz of almonds or 1.8 oz of pretzels daily for 12 weeks. Results showed that the minimal erythema dose was higher in the almond group as compared with the control group. No differences were observed in hydration, melanin, roughness, or sebum on facial skin. The authors concluded that daily oral almond intake may improve photoprotection by raising the minimal erythema dose.2
In a 2022 review on the cutaneous benefits of sweet almond, evening primrose, and jojoba oils, Blaak and Staib noted that all three have been used for hundreds if not thousands of years in traditional medicine to treat various conditions, including skin disorders. Further, they concluded that the longstanding uses of these oils has been borne out by contemporary data, which reveal cutaneous benefits for adult and young skin, particularly in bolstering stratum corneum integrity, recovery, and lipid ratio.6
Later that year, Sanju et al., reporting on the development and assessment of a broad-spectrum polyherbal sunscreen delivered through solid lipid nanoparticles, noted that almond oil was among the natural ingredients used because of its photoprotective characteristics. Overall, the sunscreen formulation, Safranal, was found to impart robust protection against UV radiation.7
Wound healing
In 2020, Borzou et al. conducted a single-blind randomized clinical trial to ascertain the impact of topical almond oil in preventing pressure injuries. Data collection occurred over 8 months in a hospital setting, with 108 patients randomly assigned to receive almond oil, placebo (liquid paraffin), or the control (standard of care). The researchers found that topically applied almond oil was linked to a lower incidence of pressure injuries, and they arose later in the study as compared with those injuries in the groups receiving paraffin or standard of care. Pressure injury incidence was 5.6% in the almond oil group, 13.9% in the placebo group, and 25.1% in the control group.8
That same year, Caglar et al. completed a randomized controlled trial in 90 preterm infants to assess the effects of sunflower seed oil and almond oil on the stratum corneum. Infants were randomly selected for treatment with either oil or control. A nurse researcher applied oils to the whole body except for the head and face four times daily for 5 days. Investigators determined that stratum corneum hydration was better in the oil groups as compared with control, with no difference found between sunflower seed and almond oils.9
Eczema, hand dermatitis, and striae
In 2018, Simon et al. performed a randomized, double-blind study to determine the short- and long-term effects of two emollients on pruritus and skin restoration in xerotic eczema. The emollients contained lactic acid and refined almond oil, with one also including polidocanol. Both emollients were effective in reducing the severity of itching, with skin moisture and lipid content found to have risen after the initial administration and yielding steady improvement over 2 weeks.10
Earlier that year, Zeichner et al. found that the use of an OTC sweet almond oil, rich in fatty acids and a standard-bearing treatment for eczema and psoriasis for centuries, was effective in treating hand dermatitis. Specifically, the moisturizer, which contained 7% sweet almond oil and 2% colloidal oatmeal, was identified as safe and effective in resolving moderate to severe hand dermatitis.11
Some studies have also shown almond oil to be effective against striae gravidarum. Hajhashemi et al. conducted a double-blind clinical trial in 160 nulliparous women to compare the effects of aloe vera gel and sweet almond oil on striae gravidarum in 2018. Volunteers were randomly assigned to one of three case groups (Aloe vera, sweet almond oil, or base cream) who received topical treatment on the abdomen, or the fourth group, which received no treatment. Results showed that both treatment creams were effective in decreasing erythema and the pruritus associated with striae as well as in preventing their expansion.12 Previously, Tashan and Kafkasli showed in a nonrandomized study that massage with bitter almond oil may diminish the visibility of present striae gravidarum and prevent the emergence of new striae.13
Conclusion
Almonds and almond oil have been used as food and in traditional medical practices dating back several centuries. In the last decade, intriguing results have emerged regarding the effects of almond consumption or topical almond oil administration on skin health. While much more research is necessary, the recent data seem to support the traditional uses of this tree seed for dermatologic purposes.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology” (New York: McGraw Hill), was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Ahmad Z. Complement Ther Clin Pract. 2010 Feb;16(1):10-2.
2. Li JN et al. J Cosmet Dermatol. 2021 Sep;20(9):2975-80.
3. Rybak I et al. Nutrients. 2021 Feb 27;13(3):785.
4. Foolad N et al. Phytother Res. 2019 Dec;33(12):3212-7.
5. Lin TK et al. Int J Mol Sci. 2017 Dec 27;19(1):70.
6. Blaak J, Staib P. Int J Cosmet Sci. 2022 Feb;44(1):1-9.
7. Sanju N et al. J Cosmet Dermatol. 2022 Oct;21(10):4433-46.
8. Borzou SR et al. J Wound Ostomy Continence Nurs. 2020 Jul/Aug;47(4):336-42.
9. Caglar S et al. Adv Skin Wound Care. 2020 Aug;33(8):1-6.
10. Simon D et al. Dermatol Ther. 2018 Nov;31(6):e12692.
11. Zeichner JA at al. J Drugs Dermatol. 2018 Jan 1;17(1):78-82.
12. Hajhashemi M et al. J Matern Fetal Neonatal Med. 2018 Jul;31(13):1703-8.
13. Timur Tashan S and Kafkasli A. J Clin Nurs. 2012 Jun;21(11-12):1570-6.
Almonds and almond oil are known to exhibit anti-inflammatory, antihepatotoxicity, and immunity-boosting activity.1 The seed from the deciduous almond tree (Oleum amygdalae), which is native to Iran and parts of the Levant, almonds contain copious amounts of phenols and polyphenols, fatty acids, and vitamin E, all of which are known to exert antioxidant activity.2-5 These seeds have been found to have a substantial impact on serum lipids.4 Emollient and sclerosant characteristics have also been linked to almond oil, which has been found to ameliorate complexion and skin tone.5 Significantly, in vitro and in vivo studies have shown that UVB-induced photoaging can be attenuated through the use of almond oil and almond skin extract.2 Further, in traditional Chinese Medicine, Ayurveda, and ancient Greco-Persian medicine, almond oil was used to treat cutaneous conditions, including eczema and psoriasis.1.
Antiphotoaging activity
In 2019, Foolad and Vaughn conducted a prospective, investigator-blind, randomized controlled trial to determine the effects of almond consumption on facial sebum production and wrinkles. Participants (28 postmenopausal women with Fitzpatrick skin types I and II completed the study) consumed 20% of their daily energy intake in almonds or a calorie-matched snack over 16 weeks through the UC Davis Dermatology Clinic. Photographic analysis revealed that the almond group experienced significantly diminished wrinkle severity, compared with the control group. The investigators concluded that daily almond consumption has the potential to decrease wrinkle severity in postmenopausal women and that almonds may confer natural antiaging effects.4
In a similar investigation 2 years later, Rybak et al. reported on a prospective, randomized controlled study to ascertain the effects of almond consumption on photoaging in postmenopausal women with Fitzpatrick skin types I or II who obtained 20% of their daily energy consumption via almonds or a calorie-matched snack for 24 weeks. Results demonstrated significant effects conferred by almond consumption, with average wrinkle severity substantially diminished in the almond group at weeks 16 (by 15%) and 24 (by 16%), compared with baseline. In addition, facial pigment intensity was reduced by 20% in the almond group by week 16 and this was maintained through the end of the study. Further, sebum excretion was higher in the control group. The investigators concluded that the daily consumption of almonds may have the potential to enhance protection against photoaging, particularly in terms of facial wrinkles and pigment intensity, in postmenopausal women.3
Later in 2021, Li et al. conducted a study in 39 healthy Asian women (18-45 years old) with Fitzpatrick skin types II to IV to investigate the effects of almond consumption on UVB resistance. The researchers randomized participants to eat either 1.5 oz of almonds or 1.8 oz of pretzels daily for 12 weeks. Results showed that the minimal erythema dose was higher in the almond group as compared with the control group. No differences were observed in hydration, melanin, roughness, or sebum on facial skin. The authors concluded that daily oral almond intake may improve photoprotection by raising the minimal erythema dose.2
In a 2022 review on the cutaneous benefits of sweet almond, evening primrose, and jojoba oils, Blaak and Staib noted that all three have been used for hundreds if not thousands of years in traditional medicine to treat various conditions, including skin disorders. Further, they concluded that the longstanding uses of these oils has been borne out by contemporary data, which reveal cutaneous benefits for adult and young skin, particularly in bolstering stratum corneum integrity, recovery, and lipid ratio.6
Later that year, Sanju et al., reporting on the development and assessment of a broad-spectrum polyherbal sunscreen delivered through solid lipid nanoparticles, noted that almond oil was among the natural ingredients used because of its photoprotective characteristics. Overall, the sunscreen formulation, Safranal, was found to impart robust protection against UV radiation.7
Wound healing
In 2020, Borzou et al. conducted a single-blind randomized clinical trial to ascertain the impact of topical almond oil in preventing pressure injuries. Data collection occurred over 8 months in a hospital setting, with 108 patients randomly assigned to receive almond oil, placebo (liquid paraffin), or the control (standard of care). The researchers found that topically applied almond oil was linked to a lower incidence of pressure injuries, and they arose later in the study as compared with those injuries in the groups receiving paraffin or standard of care. Pressure injury incidence was 5.6% in the almond oil group, 13.9% in the placebo group, and 25.1% in the control group.8
That same year, Caglar et al. completed a randomized controlled trial in 90 preterm infants to assess the effects of sunflower seed oil and almond oil on the stratum corneum. Infants were randomly selected for treatment with either oil or control. A nurse researcher applied oils to the whole body except for the head and face four times daily for 5 days. Investigators determined that stratum corneum hydration was better in the oil groups as compared with control, with no difference found between sunflower seed and almond oils.9
Eczema, hand dermatitis, and striae
In 2018, Simon et al. performed a randomized, double-blind study to determine the short- and long-term effects of two emollients on pruritus and skin restoration in xerotic eczema. The emollients contained lactic acid and refined almond oil, with one also including polidocanol. Both emollients were effective in reducing the severity of itching, with skin moisture and lipid content found to have risen after the initial administration and yielding steady improvement over 2 weeks.10
Earlier that year, Zeichner et al. found that the use of an OTC sweet almond oil, rich in fatty acids and a standard-bearing treatment for eczema and psoriasis for centuries, was effective in treating hand dermatitis. Specifically, the moisturizer, which contained 7% sweet almond oil and 2% colloidal oatmeal, was identified as safe and effective in resolving moderate to severe hand dermatitis.11
Some studies have also shown almond oil to be effective against striae gravidarum. Hajhashemi et al. conducted a double-blind clinical trial in 160 nulliparous women to compare the effects of aloe vera gel and sweet almond oil on striae gravidarum in 2018. Volunteers were randomly assigned to one of three case groups (Aloe vera, sweet almond oil, or base cream) who received topical treatment on the abdomen, or the fourth group, which received no treatment. Results showed that both treatment creams were effective in decreasing erythema and the pruritus associated with striae as well as in preventing their expansion.12 Previously, Tashan and Kafkasli showed in a nonrandomized study that massage with bitter almond oil may diminish the visibility of present striae gravidarum and prevent the emergence of new striae.13
Conclusion
Almonds and almond oil have been used as food and in traditional medical practices dating back several centuries. In the last decade, intriguing results have emerged regarding the effects of almond consumption or topical almond oil administration on skin health. While much more research is necessary, the recent data seem to support the traditional uses of this tree seed for dermatologic purposes.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology” (New York: McGraw Hill), was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Ahmad Z. Complement Ther Clin Pract. 2010 Feb;16(1):10-2.
2. Li JN et al. J Cosmet Dermatol. 2021 Sep;20(9):2975-80.
3. Rybak I et al. Nutrients. 2021 Feb 27;13(3):785.
4. Foolad N et al. Phytother Res. 2019 Dec;33(12):3212-7.
5. Lin TK et al. Int J Mol Sci. 2017 Dec 27;19(1):70.
6. Blaak J, Staib P. Int J Cosmet Sci. 2022 Feb;44(1):1-9.
7. Sanju N et al. J Cosmet Dermatol. 2022 Oct;21(10):4433-46.
8. Borzou SR et al. J Wound Ostomy Continence Nurs. 2020 Jul/Aug;47(4):336-42.
9. Caglar S et al. Adv Skin Wound Care. 2020 Aug;33(8):1-6.
10. Simon D et al. Dermatol Ther. 2018 Nov;31(6):e12692.
11. Zeichner JA at al. J Drugs Dermatol. 2018 Jan 1;17(1):78-82.
12. Hajhashemi M et al. J Matern Fetal Neonatal Med. 2018 Jul;31(13):1703-8.
13. Timur Tashan S and Kafkasli A. J Clin Nurs. 2012 Jun;21(11-12):1570-6.
Skin has different daytime and nighttime needs, emerging circadian research suggests
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2023
CoolSculpting remains most popular procedure for noninvasive fat removal, expert says
SAN DIEGO –, some aesthetic experts wondered how consumers would embrace the fat reduction procedure going forward.
The negative publicity surrounding this case “is thought to have detracted from some of the volume of it [in terms of demand], but it looks like it’s coming back again,” Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, said during a presentation on noninvasive fat removal treatment options at the annual Masters of Aesthetics Symposium.
In fact, he said, CoolSculpting accounts for an estimated 72% of noninvasive fat removal treatments performed in the United States. “By and large, there is high satisfaction with this procedure,” said Dr. Ibrahimi. “There have been about 17 million procedures done worldwide. Paradoxical adipose hyperplasia is a very rare side effect. As newer iterations of this technology have come out, I think there is an even lower incidence.”
CoolSculpting, or cryolipolysis, freezes excess fat to remove it from stubborn areas via panniculitis. The technology was developed by Dieter Manstein MD, PhD, and R. Rox Anderson, MD, at Massachusetts General Hospital and Harvard Medical School, both in Boston, and cleared by the U.S. Food and Drug Administration for noninvasive fat removal in 2010.
“If you kill a fat cell in an adult, it can’t come back,” Dr. Ibrahimi said. “When this technology first came out it was very simple. We treated an area once and were done. Now we know to treat the area multiple times, and you can treat a much larger volume in a patient during one session safely. You can bring about dramatic results, but it often takes a series of 35-minute treatment cycles and about 3 months to see clinical results. There are published studies showing that results are persisting even 10 years after treatment. This is nice, because I tell my patients, ‘if you keep up with your diet and exercise, we don’t expect the fat to come back.’ ”
Other noninvasive options for fat removal include the following:
- Ultrasound. Options include high-intensity focused ultrasound (Liposonix) and pulsed focused ultrasound (UltraShape). Dr. Ibrahimi described these devices as “very painful, and the results were very difficult to reproduce from the initial clinical studies.”
- Low-level light therapy. Early devices on the market include Zerona and UltraSlim. “Oftentimes these lacked any sort of histological analysis,” he said. “There was no obvious mechanism of action, and questionable efficacy.”
- Laser. Powered by a 1060-nm laser, SculpSure can reduce fat cells safely in 25-minute treatment sessions, Dr. Ibrahimi said. Each session is delivered with one of four available applicators and involves 4 minutes of heating and the next 21 minutes alternating between heating and cooling. “You’re trying to reach a target temperature that kills fat cells,” he explained. “The beauty of having these applicators is that you can kind of customize to the individual patient; it uses contact cooling, and it’s safe for all Fitzpatrick skin types. This device results in a 10%-12% reduction in fat, so it’s clinically significant but very modest.”
A robotic version of the technology, known as the Robotic Fat Killer, is also available. So is the EON, a touchless 1064-nm laser FDA cleared for abdominal, flank, thigh, and back fat reduction. “It adapts to the body shape of the area and individual to deliver a customized treatment,” Dr. Ibrahimi said.
- Radiofrequency. Most devices on the market, such as truSculpt and Vanquish, are powered by monopolar radiofrequency (RF) energy. “Similar to the 1060-nm laser, you can customize these treatments,” he said. “You’re treating to a target temperature. It involves 15-minute cycles, and there are clinical, histology, and ultrasound data supporting this technology.”
Dr. Ibrahimi uses truSculpt and CoolSculpting in his practice, “but sometimes you have patients who are ‘too fit’ for CoolSculpting; they don’t fit the handpiece perfectly,” he said. “That’s where having a monopolar RF or a 1060-nm laser is useful, to help you hone in on those stubborn pockets of fat.”
- Deoxycholic acid. While not a device, deoxycholic acid (Kybella), administered subcutaneously, is approved by the FDA for improving “the appearance of moderate to severe convexity or fullness associated with submental fat” in adults. “A lot of people use it off-label on the abdomen and other stubborn areas,” Dr. Ibrahimi said. “It often requires a series of treatments. That’s the biggest limiting issue with using this technology. It works well, but compared to CoolSculpting, there is a lot of swelling and bruising, which you would expect with an injectable. Managing that down time and hand holding is difficult. But if you can get patients to buy into the downtime, [it yields] pretty impressive results.”
Dr. Ibrahimi also discussed the promise of electrical muscle stimulation for strengthening, firming, and toning muscles. The technology applies an electrical current through electrodes placed on the skin, which stimulates muscles, or through an electromagnetic field.
In a published study of 45 men and women, Dr. Ibrahimi, Anne Chapas, MD, medical director of UnionDerm in New York, and colleagues evaluated the safety and efficacy of an electrical muscle stimulation system for improving muscle strength and toning of the upper extremities.
For the treatments, they used disposable contact pads to place pairs of electrodes on the biceps and on the triceps. All patients (median age 42) received 30-minute treatments twice weekly for 2 or 3 weeks, corresponding to four or six total sessions respectively, depending on the study site. Follow-ups were conducted 30 and 90 days after treatment. They used a validated dynamometer device to measure strength at baseline, at the final treatment session, and at the post-treatment 30- and 90-day visits.
“We saw about a 40% increase in strength in the biceps and about a 30% increase in strength in the triceps,” Dr. Ibrahimi said. “Interestingly, the effect got greater at 30 and 90 days, so this is something that lingers on for quite a while.” In addition to the increase in strength, the researchers and patients noted an improvement in the appearance of the arms. He predicted that this technology “is going to play a role in functional medicine and getting injured athletes back to their sports faster.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera (manufacturer of truSculpt), Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies (none are relevant to the treatments mentioned in this story).
SAN DIEGO –, some aesthetic experts wondered how consumers would embrace the fat reduction procedure going forward.
The negative publicity surrounding this case “is thought to have detracted from some of the volume of it [in terms of demand], but it looks like it’s coming back again,” Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, said during a presentation on noninvasive fat removal treatment options at the annual Masters of Aesthetics Symposium.
In fact, he said, CoolSculpting accounts for an estimated 72% of noninvasive fat removal treatments performed in the United States. “By and large, there is high satisfaction with this procedure,” said Dr. Ibrahimi. “There have been about 17 million procedures done worldwide. Paradoxical adipose hyperplasia is a very rare side effect. As newer iterations of this technology have come out, I think there is an even lower incidence.”
CoolSculpting, or cryolipolysis, freezes excess fat to remove it from stubborn areas via panniculitis. The technology was developed by Dieter Manstein MD, PhD, and R. Rox Anderson, MD, at Massachusetts General Hospital and Harvard Medical School, both in Boston, and cleared by the U.S. Food and Drug Administration for noninvasive fat removal in 2010.
“If you kill a fat cell in an adult, it can’t come back,” Dr. Ibrahimi said. “When this technology first came out it was very simple. We treated an area once and were done. Now we know to treat the area multiple times, and you can treat a much larger volume in a patient during one session safely. You can bring about dramatic results, but it often takes a series of 35-minute treatment cycles and about 3 months to see clinical results. There are published studies showing that results are persisting even 10 years after treatment. This is nice, because I tell my patients, ‘if you keep up with your diet and exercise, we don’t expect the fat to come back.’ ”
Other noninvasive options for fat removal include the following:
- Ultrasound. Options include high-intensity focused ultrasound (Liposonix) and pulsed focused ultrasound (UltraShape). Dr. Ibrahimi described these devices as “very painful, and the results were very difficult to reproduce from the initial clinical studies.”
- Low-level light therapy. Early devices on the market include Zerona and UltraSlim. “Oftentimes these lacked any sort of histological analysis,” he said. “There was no obvious mechanism of action, and questionable efficacy.”
- Laser. Powered by a 1060-nm laser, SculpSure can reduce fat cells safely in 25-minute treatment sessions, Dr. Ibrahimi said. Each session is delivered with one of four available applicators and involves 4 minutes of heating and the next 21 minutes alternating between heating and cooling. “You’re trying to reach a target temperature that kills fat cells,” he explained. “The beauty of having these applicators is that you can kind of customize to the individual patient; it uses contact cooling, and it’s safe for all Fitzpatrick skin types. This device results in a 10%-12% reduction in fat, so it’s clinically significant but very modest.”
A robotic version of the technology, known as the Robotic Fat Killer, is also available. So is the EON, a touchless 1064-nm laser FDA cleared for abdominal, flank, thigh, and back fat reduction. “It adapts to the body shape of the area and individual to deliver a customized treatment,” Dr. Ibrahimi said.
- Radiofrequency. Most devices on the market, such as truSculpt and Vanquish, are powered by monopolar radiofrequency (RF) energy. “Similar to the 1060-nm laser, you can customize these treatments,” he said. “You’re treating to a target temperature. It involves 15-minute cycles, and there are clinical, histology, and ultrasound data supporting this technology.”
Dr. Ibrahimi uses truSculpt and CoolSculpting in his practice, “but sometimes you have patients who are ‘too fit’ for CoolSculpting; they don’t fit the handpiece perfectly,” he said. “That’s where having a monopolar RF or a 1060-nm laser is useful, to help you hone in on those stubborn pockets of fat.”
- Deoxycholic acid. While not a device, deoxycholic acid (Kybella), administered subcutaneously, is approved by the FDA for improving “the appearance of moderate to severe convexity or fullness associated with submental fat” in adults. “A lot of people use it off-label on the abdomen and other stubborn areas,” Dr. Ibrahimi said. “It often requires a series of treatments. That’s the biggest limiting issue with using this technology. It works well, but compared to CoolSculpting, there is a lot of swelling and bruising, which you would expect with an injectable. Managing that down time and hand holding is difficult. But if you can get patients to buy into the downtime, [it yields] pretty impressive results.”
Dr. Ibrahimi also discussed the promise of electrical muscle stimulation for strengthening, firming, and toning muscles. The technology applies an electrical current through electrodes placed on the skin, which stimulates muscles, or through an electromagnetic field.
In a published study of 45 men and women, Dr. Ibrahimi, Anne Chapas, MD, medical director of UnionDerm in New York, and colleagues evaluated the safety and efficacy of an electrical muscle stimulation system for improving muscle strength and toning of the upper extremities.
For the treatments, they used disposable contact pads to place pairs of electrodes on the biceps and on the triceps. All patients (median age 42) received 30-minute treatments twice weekly for 2 or 3 weeks, corresponding to four or six total sessions respectively, depending on the study site. Follow-ups were conducted 30 and 90 days after treatment. They used a validated dynamometer device to measure strength at baseline, at the final treatment session, and at the post-treatment 30- and 90-day visits.
“We saw about a 40% increase in strength in the biceps and about a 30% increase in strength in the triceps,” Dr. Ibrahimi said. “Interestingly, the effect got greater at 30 and 90 days, so this is something that lingers on for quite a while.” In addition to the increase in strength, the researchers and patients noted an improvement in the appearance of the arms. He predicted that this technology “is going to play a role in functional medicine and getting injured athletes back to their sports faster.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera (manufacturer of truSculpt), Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies (none are relevant to the treatments mentioned in this story).
SAN DIEGO –, some aesthetic experts wondered how consumers would embrace the fat reduction procedure going forward.
The negative publicity surrounding this case “is thought to have detracted from some of the volume of it [in terms of demand], but it looks like it’s coming back again,” Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, said during a presentation on noninvasive fat removal treatment options at the annual Masters of Aesthetics Symposium.
In fact, he said, CoolSculpting accounts for an estimated 72% of noninvasive fat removal treatments performed in the United States. “By and large, there is high satisfaction with this procedure,” said Dr. Ibrahimi. “There have been about 17 million procedures done worldwide. Paradoxical adipose hyperplasia is a very rare side effect. As newer iterations of this technology have come out, I think there is an even lower incidence.”
CoolSculpting, or cryolipolysis, freezes excess fat to remove it from stubborn areas via panniculitis. The technology was developed by Dieter Manstein MD, PhD, and R. Rox Anderson, MD, at Massachusetts General Hospital and Harvard Medical School, both in Boston, and cleared by the U.S. Food and Drug Administration for noninvasive fat removal in 2010.
“If you kill a fat cell in an adult, it can’t come back,” Dr. Ibrahimi said. “When this technology first came out it was very simple. We treated an area once and were done. Now we know to treat the area multiple times, and you can treat a much larger volume in a patient during one session safely. You can bring about dramatic results, but it often takes a series of 35-minute treatment cycles and about 3 months to see clinical results. There are published studies showing that results are persisting even 10 years after treatment. This is nice, because I tell my patients, ‘if you keep up with your diet and exercise, we don’t expect the fat to come back.’ ”
Other noninvasive options for fat removal include the following:
- Ultrasound. Options include high-intensity focused ultrasound (Liposonix) and pulsed focused ultrasound (UltraShape). Dr. Ibrahimi described these devices as “very painful, and the results were very difficult to reproduce from the initial clinical studies.”
- Low-level light therapy. Early devices on the market include Zerona and UltraSlim. “Oftentimes these lacked any sort of histological analysis,” he said. “There was no obvious mechanism of action, and questionable efficacy.”
- Laser. Powered by a 1060-nm laser, SculpSure can reduce fat cells safely in 25-minute treatment sessions, Dr. Ibrahimi said. Each session is delivered with one of four available applicators and involves 4 minutes of heating and the next 21 minutes alternating between heating and cooling. “You’re trying to reach a target temperature that kills fat cells,” he explained. “The beauty of having these applicators is that you can kind of customize to the individual patient; it uses contact cooling, and it’s safe for all Fitzpatrick skin types. This device results in a 10%-12% reduction in fat, so it’s clinically significant but very modest.”
A robotic version of the technology, known as the Robotic Fat Killer, is also available. So is the EON, a touchless 1064-nm laser FDA cleared for abdominal, flank, thigh, and back fat reduction. “It adapts to the body shape of the area and individual to deliver a customized treatment,” Dr. Ibrahimi said.
- Radiofrequency. Most devices on the market, such as truSculpt and Vanquish, are powered by monopolar radiofrequency (RF) energy. “Similar to the 1060-nm laser, you can customize these treatments,” he said. “You’re treating to a target temperature. It involves 15-minute cycles, and there are clinical, histology, and ultrasound data supporting this technology.”
Dr. Ibrahimi uses truSculpt and CoolSculpting in his practice, “but sometimes you have patients who are ‘too fit’ for CoolSculpting; they don’t fit the handpiece perfectly,” he said. “That’s where having a monopolar RF or a 1060-nm laser is useful, to help you hone in on those stubborn pockets of fat.”
- Deoxycholic acid. While not a device, deoxycholic acid (Kybella), administered subcutaneously, is approved by the FDA for improving “the appearance of moderate to severe convexity or fullness associated with submental fat” in adults. “A lot of people use it off-label on the abdomen and other stubborn areas,” Dr. Ibrahimi said. “It often requires a series of treatments. That’s the biggest limiting issue with using this technology. It works well, but compared to CoolSculpting, there is a lot of swelling and bruising, which you would expect with an injectable. Managing that down time and hand holding is difficult. But if you can get patients to buy into the downtime, [it yields] pretty impressive results.”
Dr. Ibrahimi also discussed the promise of electrical muscle stimulation for strengthening, firming, and toning muscles. The technology applies an electrical current through electrodes placed on the skin, which stimulates muscles, or through an electromagnetic field.
In a published study of 45 men and women, Dr. Ibrahimi, Anne Chapas, MD, medical director of UnionDerm in New York, and colleagues evaluated the safety and efficacy of an electrical muscle stimulation system for improving muscle strength and toning of the upper extremities.
For the treatments, they used disposable contact pads to place pairs of electrodes on the biceps and on the triceps. All patients (median age 42) received 30-minute treatments twice weekly for 2 or 3 weeks, corresponding to four or six total sessions respectively, depending on the study site. Follow-ups were conducted 30 and 90 days after treatment. They used a validated dynamometer device to measure strength at baseline, at the final treatment session, and at the post-treatment 30- and 90-day visits.
“We saw about a 40% increase in strength in the biceps and about a 30% increase in strength in the triceps,” Dr. Ibrahimi said. “Interestingly, the effect got greater at 30 and 90 days, so this is something that lingers on for quite a while.” In addition to the increase in strength, the researchers and patients noted an improvement in the appearance of the arms. He predicted that this technology “is going to play a role in functional medicine and getting injured athletes back to their sports faster.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera (manufacturer of truSculpt), Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies (none are relevant to the treatments mentioned in this story).