User login
Studies add clarity to link between rosacea and Demodex, coffee
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
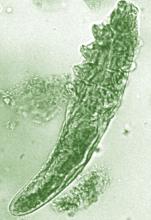
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
After PCI, stopping antiplatelet therapy for surgery appears safe
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
REPORTING FROM CRT 2020
OCT may help predict disease activity in CIS
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
WEST PALM BEACH, FLA. – according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that optical coherence tomography (OCT) could support patient monitoring and the initiation of disease-modifying therapy.
“Treatment of early MS [multiple sclerosis] is crucial to prevent neuroaxonal damage and, thus, sustained disability,” said Hanna G. Zimmermann, PhD, a research associate at NeuroCure Clinical Research Center at Charité Universitätsmedizin in Berlin. The ability to identify patients at high risk of future disease activity shortly after disease onset could help optimize patient management and guide the initiation of disease-modifying therapy. Dr. Zimmermann and colleagues investigated whether retinal OCT could predict disease activity in patients with CIS.
The investigators included 97 patients (mean age, 33.6 years; 62.9% female) with CIS in a prospective, longitudinal cohort study. Diagnoses of CIS were based on the 2010 revisions to the McDonald criteria. Patients were enrolled from two German centers within 12 months after a first clinical event. The researchers performed a neurologic examination, cerebral MRI, and retinal OCT for each participant and followed the population for 729 days (median, 664 days).
The primary OCT predictor was ganglion cell and inner plexiform (GCIP) layer thickness, because this parameter is stable and reliable for quantifying neuronal visual system damage in MS, said Dr. Zimmermann. Secondary OCT predictors were peripapillary retinal nerve fiber layer (pRNFL) thickness and inner nuclear layer (INL) thickness. The investigators only included eyes without a history of optic neuritis in the analysis.
The study’s primary outcome was failing the no evidence of disease activity (NEDA-3) criteria (no relapses, no disability progression, and no MRI activity). The secondary outcomes were MS diagnosis (according to the 2010 McDonald criteria) and worsening of disability.
At baseline, Dr. Zimmerman and colleagues found no differences in thickness of GCIP and pRNFL between patients and matched healthy controls. In all, 58 patients (59%) failed NEDA-3 criteria during follow-up. When Dr. Zimmermann and colleagues conducted Kaplan-Meier analysis, they found that patients with thinner GCIP thickness had a significantly higher risk of failing NEDA-3 criteria (thinnest vs. thickest tertile: hazard ratio, 3.33). A follow-up diagnosis of MS also was significantly more likely among patients with low GCIP thickness (thinnest vs. thickest tertile: HR, 4.05).
In addition, low pRNFL thickness indicated an increased risk of not meeting NEDA-3 criteria (thinnest vs. thickest tertile: HR, 2.46). However, neither INL thickness nor T2-weighted lesion count were associated with failing NEDA-3 criteria. Also, none of the OCT parameters were associated with future disability worsening.
Among the study’s limitations are its small sample size, the relatively short observation time, and the heterogeneity of patients between the two centers, which used different study protocols, said Dr. Zimmermann.
“OCT-assessed GCIP is promising for the early appraisal of future disease activity and might thus be helpful for risk-adjusted patient participation in clinical research,” she said. “It might also be helpful for clinicians for identifying CIS patients with worse prognosis and planning the care.” Dr. Zimmermann and colleagues plan to use advanced imaging techniques in future studies to understand the mechanisms behind the associations they identified. They hope to confirm their findings in a larger cohort and examine whether OCT can predict clinical outcomes such as relapses, disability worsening, and the extent of disease activity.
Dr. Zimmermann had no relevant disclosures and did not report a source of funding for the study.
SOURCE: Zimmermann HG et al. ACTRIMS Forum 2020, Abstract.
REPORTING FROM ACTRIMS FORUM 2020
Data overwhelmingly support use of dermoscopy in practice
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
LAHAINA, HAWAII – Multiple but there are still some “nonbelievers,” Ashfaq A. Marghoob, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In fact, not one study has shown that dermoscopy is less sensitive than the naked eye alone, and at clinics where dermoscopy is used, “two-thirds of the melanomas being detected now lack the classic ABCD features of melanoma,” added Dr. Marghoob, director of clinical dermatology at Memorial Sloan Kettering in Hauppauge, N.Y.
Dr. Marghoob cited a meta-analysis of 9 prospective trials, which concluded that dermoscopy was more accurate in diagnosing melanoma than the naked eye alone and did not lower specificity, with a sensitivity and specificity of 90%, compared with 71% and 81%, respectively for the naked eye (Br J Dermatol. 2008 Sep;159[3]:669-76).
“And at least from an evidence-based standpoint ... the Cochrane library has basically endorsed that, yes, dermoscopy does indeed improve your diagnostic accuracy,” Dr. Marghoob said.
He referred to a 2018 Cochrane review on 104 dermoscopy studies, with and without visual inspection, for diagnosing melanoma in adults (Cochrane Database Syst Rev. 2018 Dec 4;12:CD011902). The review authors concluded that “the evidence suggests that melanomas will be missed if visual inspection is used on its own,” and despite limitations in the evidence, “dermoscopy is a valuable tool to support the visual inspection of a suspicious skin lesion for the detection of melanoma and atypical intraepidermal melanocytic variants.”
As for the question of specificity, Dr. Marghoob said studies have found that dermoscopy results in the detection of more melanomas and reduces the number of biopsies of benign lesions and “every study looking into this has shown that, yes, it does improve” specificity.
A 10-year multicenter survey of about 300,000 cases, which included 17,172 melanomas and 283,043 melanocytic nevi, found that the number-needed-to-excise (NNE) values improved over time in specialized clinics where newer diagnostic techniques like dermoscopy were used (from 12.8 to 6.8), but the NNE did not appear to change in the nonspecialized settings (J Am Acad Dermatol. 2012 Jul;67[1]:54-9).
Looking at the benign-to-malignant ratio, there was no change among those not using dermoscopy, where the ratio remained at about 30 to 1. When dermoscopy was used, this ratio started to improve over the 10-year period, to about 5 to 1. In addition, “the dermoscopy users were finding many more melanomas than the non–dermoscopy users,” Dr. Marghoob added. And over the 10 years, the number of nevi being removed did not change among those not using dermoscopy but dropped among those using dermoscopy.
Adding photography with the ability to digitally monitor patients helps bring this ratio down further, Dr. Marghoob noted. He referred to a study that instead evaluated the ratio of melanoma to nonmelanomas diagnosed among dermatologists in three groups: those with no digital dermoscopy with little dermoscopy training (group A, the reference group), no digital dermoscopy but more dermoscopy training (group B), and those using digital dermoscopy (group C). In the group that used digital dermoscopy, that ratio was about 1 to 2.4, compared with about 1 to 8 in group B, and about 1 to 10.7 in group A (Br J Dermatol. 2012;167[4]778-86).
The use of dermoscopy is also associated with thinner tumors. Among dermoscopy users, the thickness of the tumors detected drops, and the proportion of thin to thick lesions detected increases, Dr. Marghoob said.
For example, in one study, the mean thickness in melanomas detected with dermoscopy was 1.4 mm versus 2.59 mm when dermoscopy was not used (J Eur Acad Dermatol Venereol. 2015 Jan; 29[1]:102-8). About 55% of the tumors detected with dermoscopic examination were 1 mm or less in thickness versus 23.4% of those detected without dermoscopy, “so the dermoscopy users from a proportion standpoint were also finding thinner tumors,” Dr. Marghoob said. Dermoscopy was also identified as an independent predictor of finding thinner tumors.
Even without a study, it could be assumed that the use of dermoscopy would reduce costs, since dermoscopy increases sensitivity and the total number of melanomas detected, decreases the number of benign nevi removed, and helps detect disease earlier. But there are data showing that dermoscopy reduces health care costs, Dr. Marghoob said.* For example, a Belgian study that looked at dermoscopy in two cohorts of melanoma patients concluded that adequate dermoscopy training was cost effective (Eur J Cancer. 2016 Nov;67:38-45).
It has also been shown that adding dermoscopy to a primary care setting has cost benefits, Dr. Marghoob said. He cited a study of Dutch general practices that found that the probability of a correct diagnosis was 1.25 times higher when dermoscopy was used to evaluate suspicious skin lesions and concluded that the use of dermoscopy appeared to be cost effective (J Eur Acad Dermatol Venereol. 2014 Nov;28[11]:1442-9).
Dr. Marghoob had no disclosures relevant to this presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
*Correction, 2/28/20: An earlier version of this article mischaracterized the cost implications of dermoscopy use.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Obesity expert: Time to embrace growing array of options
MAUI, HAWAII – Specialists who study obesity and embrace the increasing number of treatment options are poised to lead the way in stemming the disease, which Andres Acosta, MD, PhD, calls the “epidemic of the century.”
“Gastroenterologists are in the first line of treatment for obesity management,” said Acosta, who runs the precision medicine for obesity lab at the Mayo Clinic in Rochester, Minn.
“Patients with obesity are already in our clinics,” he said in an interview. And too many physicians “are ignoring the problem.”
The vast majority of people with acid reflux have obesity, as do those with nonalcoholic fatty liver disease, he explained. “By targeting those two areas, we’ll be targeting more than 50% of our patients.” Recurring polyps and colon cancer are also often associated with obesity, he said.
Because of their skill as endoscopists, internists, and nutrition experts, gastroenterologists are uniquely positioned to care for obesity, said Acosta, who is first author of a white paper – Practice Guide on Obesity and Weight Management, Education and Resources – developed by the American Gastroenterological Association with input from nine medical societies.
More treatment choices
Physicians heard an update on options available in the continuum of obesity care from Christopher Thompson, MD, director of endoscopy at Brigham and Women’s Hospital in Boston, at the Gastroenterology Updates IBD Liver Disease Conference 2020. He discussed the potential weight-loss range and safety profile of each.
Some medications result in a body-weight loss of 5%, whereas gastric bypass surgeries can result in a loss of up to 40%, he said in an interview. And weight loss is typically 10% with intragastric balloon, 15%-20% with aspiration therapies and with endoscopic suturing techniques, and 25%-30% with sleeve gastrectomy.
“It’s nice to be able to offer all of those to patients,” he said, adding that he wants to get the message across to hesitant physicians that obesity management “is not as difficult as they think.”
Physicians can be reluctant to address obesity because of the social stigma associated with excess weight and a discomfort in talking about it.
But “there are ways to open that conversation, and it needs to start happening more,” said Thompson, who pointed out that obesity is the underlying cause of many other illnesses, including diabetes and heart diseases.
And new strategies are in the offing, he explained. His team at Brigham is currently involved in clinical trials to test whether the diversion of food and bile to the lower part of the bowel will generate a metabolic signal that affects insulin resistance and weight, he reported.
They are also testing whether gastric procedures can be combined with small bowel procedures to achieve the weight loss seen with bariatric surgery.
As treatment options for obesity increase, precision medicine will help maximize their potential, said Acosta.
Precision medicine will amp up treatments
Acosta outlined the four categories that patients who are obese generally fall into: those with a “hungry brain,” who think they need to eat more than they do; those with a “hungry gut,” whose gut is not sending the proper signal to the brain that it is full; those with “emotional hunger”; and those with abnormal metabolism.
“For each of those, there are genetic circumstances, metabolism, a hormonal profile, as well as pathophysiologic aspects of obesity, that make these groups unique,” he said.
Deciding which patients should get which treatment is the next frontier, he explained. “For example, if you give an intragastric balloon to all comers, patients will lose about 12% of their body weight. But if you separate responders from nonresponders and you select the right intervention, you can achieve an 18% loss of body weight in the right responders.”
At Mayo, they are working on a blood test to break down phenotypes and identify who will respond best to which treatment, he reported. That could lead to a much more efficient use of scarce resources.
“At the same time, I hope that more insurance companies will cover more obesity treatments,” said Acosta.
This article first appeared on Medscape.com.
MAUI, HAWAII – Specialists who study obesity and embrace the increasing number of treatment options are poised to lead the way in stemming the disease, which Andres Acosta, MD, PhD, calls the “epidemic of the century.”
“Gastroenterologists are in the first line of treatment for obesity management,” said Acosta, who runs the precision medicine for obesity lab at the Mayo Clinic in Rochester, Minn.
“Patients with obesity are already in our clinics,” he said in an interview. And too many physicians “are ignoring the problem.”
The vast majority of people with acid reflux have obesity, as do those with nonalcoholic fatty liver disease, he explained. “By targeting those two areas, we’ll be targeting more than 50% of our patients.” Recurring polyps and colon cancer are also often associated with obesity, he said.
Because of their skill as endoscopists, internists, and nutrition experts, gastroenterologists are uniquely positioned to care for obesity, said Acosta, who is first author of a white paper – Practice Guide on Obesity and Weight Management, Education and Resources – developed by the American Gastroenterological Association with input from nine medical societies.
More treatment choices
Physicians heard an update on options available in the continuum of obesity care from Christopher Thompson, MD, director of endoscopy at Brigham and Women’s Hospital in Boston, at the Gastroenterology Updates IBD Liver Disease Conference 2020. He discussed the potential weight-loss range and safety profile of each.
Some medications result in a body-weight loss of 5%, whereas gastric bypass surgeries can result in a loss of up to 40%, he said in an interview. And weight loss is typically 10% with intragastric balloon, 15%-20% with aspiration therapies and with endoscopic suturing techniques, and 25%-30% with sleeve gastrectomy.
“It’s nice to be able to offer all of those to patients,” he said, adding that he wants to get the message across to hesitant physicians that obesity management “is not as difficult as they think.”
Physicians can be reluctant to address obesity because of the social stigma associated with excess weight and a discomfort in talking about it.
But “there are ways to open that conversation, and it needs to start happening more,” said Thompson, who pointed out that obesity is the underlying cause of many other illnesses, including diabetes and heart diseases.
And new strategies are in the offing, he explained. His team at Brigham is currently involved in clinical trials to test whether the diversion of food and bile to the lower part of the bowel will generate a metabolic signal that affects insulin resistance and weight, he reported.
They are also testing whether gastric procedures can be combined with small bowel procedures to achieve the weight loss seen with bariatric surgery.
As treatment options for obesity increase, precision medicine will help maximize their potential, said Acosta.
Precision medicine will amp up treatments
Acosta outlined the four categories that patients who are obese generally fall into: those with a “hungry brain,” who think they need to eat more than they do; those with a “hungry gut,” whose gut is not sending the proper signal to the brain that it is full; those with “emotional hunger”; and those with abnormal metabolism.
“For each of those, there are genetic circumstances, metabolism, a hormonal profile, as well as pathophysiologic aspects of obesity, that make these groups unique,” he said.
Deciding which patients should get which treatment is the next frontier, he explained. “For example, if you give an intragastric balloon to all comers, patients will lose about 12% of their body weight. But if you separate responders from nonresponders and you select the right intervention, you can achieve an 18% loss of body weight in the right responders.”
At Mayo, they are working on a blood test to break down phenotypes and identify who will respond best to which treatment, he reported. That could lead to a much more efficient use of scarce resources.
“At the same time, I hope that more insurance companies will cover more obesity treatments,” said Acosta.
This article first appeared on Medscape.com.
MAUI, HAWAII – Specialists who study obesity and embrace the increasing number of treatment options are poised to lead the way in stemming the disease, which Andres Acosta, MD, PhD, calls the “epidemic of the century.”
“Gastroenterologists are in the first line of treatment for obesity management,” said Acosta, who runs the precision medicine for obesity lab at the Mayo Clinic in Rochester, Minn.
“Patients with obesity are already in our clinics,” he said in an interview. And too many physicians “are ignoring the problem.”
The vast majority of people with acid reflux have obesity, as do those with nonalcoholic fatty liver disease, he explained. “By targeting those two areas, we’ll be targeting more than 50% of our patients.” Recurring polyps and colon cancer are also often associated with obesity, he said.
Because of their skill as endoscopists, internists, and nutrition experts, gastroenterologists are uniquely positioned to care for obesity, said Acosta, who is first author of a white paper – Practice Guide on Obesity and Weight Management, Education and Resources – developed by the American Gastroenterological Association with input from nine medical societies.
More treatment choices
Physicians heard an update on options available in the continuum of obesity care from Christopher Thompson, MD, director of endoscopy at Brigham and Women’s Hospital in Boston, at the Gastroenterology Updates IBD Liver Disease Conference 2020. He discussed the potential weight-loss range and safety profile of each.
Some medications result in a body-weight loss of 5%, whereas gastric bypass surgeries can result in a loss of up to 40%, he said in an interview. And weight loss is typically 10% with intragastric balloon, 15%-20% with aspiration therapies and with endoscopic suturing techniques, and 25%-30% with sleeve gastrectomy.
“It’s nice to be able to offer all of those to patients,” he said, adding that he wants to get the message across to hesitant physicians that obesity management “is not as difficult as they think.”
Physicians can be reluctant to address obesity because of the social stigma associated with excess weight and a discomfort in talking about it.
But “there are ways to open that conversation, and it needs to start happening more,” said Thompson, who pointed out that obesity is the underlying cause of many other illnesses, including diabetes and heart diseases.
And new strategies are in the offing, he explained. His team at Brigham is currently involved in clinical trials to test whether the diversion of food and bile to the lower part of the bowel will generate a metabolic signal that affects insulin resistance and weight, he reported.
They are also testing whether gastric procedures can be combined with small bowel procedures to achieve the weight loss seen with bariatric surgery.
As treatment options for obesity increase, precision medicine will help maximize their potential, said Acosta.
Precision medicine will amp up treatments
Acosta outlined the four categories that patients who are obese generally fall into: those with a “hungry brain,” who think they need to eat more than they do; those with a “hungry gut,” whose gut is not sending the proper signal to the brain that it is full; those with “emotional hunger”; and those with abnormal metabolism.
“For each of those, there are genetic circumstances, metabolism, a hormonal profile, as well as pathophysiologic aspects of obesity, that make these groups unique,” he said.
Deciding which patients should get which treatment is the next frontier, he explained. “For example, if you give an intragastric balloon to all comers, patients will lose about 12% of their body weight. But if you separate responders from nonresponders and you select the right intervention, you can achieve an 18% loss of body weight in the right responders.”
At Mayo, they are working on a blood test to break down phenotypes and identify who will respond best to which treatment, he reported. That could lead to a much more efficient use of scarce resources.
“At the same time, I hope that more insurance companies will cover more obesity treatments,” said Acosta.
This article first appeared on Medscape.com.
EXPERT ANALYSIS FROM GUILD 2020
Older adults with IBD often undertreated
MAUI, HAWAII – said Christina Ha, MD, from the Inflammatory Bowel Disease Center at Cedars-Sinai in Los Angeles.
Clinicians sometimes fall back on steroids because they are typically inexpensive and because there are fears that the new anti-TNF biologics can cause adverse events in older patients, Ha said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“There are not a lot of safety data for the age group, which is not well represented in clinical trials,” she explained. “We can’t necessarily extrapolate data from a study of people with an average age in the 40s to someone in their 70s.”
But, she emphasized, steroid use for more than 3 months is potentially inappropriate.
“If we have a patient on steroids, we should be saying which steroid-sparing strategy will be incorporated into their regimen when we start them on their course of steroids,” she explained.
Ha said she gets asked frequently whether the man-made steroid budesonide, which is readily available, should be considered an acceptable alternative to prednisone.
“Steroids are not maintenance therapies,” she pointed out. “One could argue that maybe someone who has symptomatic mild Crohn’s disease could be kept on low doses of budesonide. But I would argue whether it is really the budesonide that’s helping them or some other disease process related to polypharmacy.”
There are no long-term safety or efficacy data for budesonide in patients with ulcerative colitis or Crohn’s disease, she added.
Special considerations
Older patients with IBD have a decreased ability to handle disease activity; they have more comorbidities and a susceptibility to falls, said Ha. Early control of the disease is therefore essential.
Sarcopenia, an inherent part of aging when muscle mass decreases over time, is central to physiologic changes, which have implications for older adults with IBD, she said.
“We’re learning that sarcopenia is also prevalent in our patients with moderate to severe inflammatory bowel disease,” she explained. “Sarcopenia is associated with increased risk of infections, hospitalizations, and postoperative complications.”
Other changes occur in the intestines as patients age, Ha reported. “Recent studies have shown that there are changes in the intestinal barrier in terms of the junctions within the mucosal lining that increase intestinal permeability, which may help explain why some patients respond to treatments and others don’t.”
Physical therapy underused
Other treatment options, such as physical therapy, have also been underused in older patients with IBD. For example, there’s often considerable pushback against doing a physical therapy assessment on a hospitalized older patient, said Ha.
Medicare covers up to 80% of those services, but referral wording is key. “They’re not going to cover it for a primary diagnosis of ulcerative colitis or Crohn’s,” she explained. However, “they will cover it for a primary diagnosis of deconditioning with a secondary diagnoses of steroid exposure, anemia, Crohn’s disease, or ulcerative colitis.”
Physical therapy can improve muscle function, decrease muscle pain, potentially decrease analgesics, improve bone mass, and decrease joint pain, stress, fatigue, and debility. Fatigue is prevalent in patients with IBD, Ha explained.
Another underused resource is psychosocial assessment, she added. Although depression is not part of the aging process, it is common in those with chronic conditions.
Visits with licensed psychiatrists and clinical psychologists are covered under Medicare Part B, Ha pointed out, as are psychiatric evaluation and testing and individual and group therapy.
Older patients with IBD are often not receiving the care they need, said Uma Mahadevan, MD, a gastroenterologist at UCSF Health in San Francisco.
The need for awareness of polypharmacy, which Ha also discussed, is a concern in all older patients, but especially those with IBD, Mahadevan said in an interview. Clinicians need to be aware of the cascading effect of pharmacy, in which one drug’s adverse effect leads to the prescription of another drug, with different adverse effects.
Ha gave the example of a patient with IBD who started to have diarrhea as an adverse effect of a medication. A clinician might then prescribe a medication for Clostridium difficile, but that might lead to nausea, leading to the prescription of an antinausea medicine.
A multidisciplinary team is needed to perform medication reconciliation, Ha noted.
Correcting anemia important for IBD
Anemia is also underidentified and undertreated in older patients with IBD, Ha said.
“Across the board with inflammatory bowel disease, we don’t do a great job of being aggressive and correcting anemia. That has implications for fatigue and implications with functional status and circulating volume,” she said.
In older patients, it might be that the decline in hemoglobin over time is more important to outcomes than the number itself, she said. “A hemoglobin of 8 g/dL is one thing, but if it was at 12 g/dL 6 months ago, that’s a different story.”
“For older patients, anemia is associated with a high incidence of cardiovascular disease, cognitive impairment, increased risks of falls and fractures, longer hospitalizations (and thus increased costs of care), increased frailty and dementia, and increased risk of mortality,” Ha said. But, she pointed out, Medicare benefits do cover intravenous iron formulations.
This article originally appeared on Medscape.com.
MAUI, HAWAII – said Christina Ha, MD, from the Inflammatory Bowel Disease Center at Cedars-Sinai in Los Angeles.
Clinicians sometimes fall back on steroids because they are typically inexpensive and because there are fears that the new anti-TNF biologics can cause adverse events in older patients, Ha said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“There are not a lot of safety data for the age group, which is not well represented in clinical trials,” she explained. “We can’t necessarily extrapolate data from a study of people with an average age in the 40s to someone in their 70s.”
But, she emphasized, steroid use for more than 3 months is potentially inappropriate.
“If we have a patient on steroids, we should be saying which steroid-sparing strategy will be incorporated into their regimen when we start them on their course of steroids,” she explained.
Ha said she gets asked frequently whether the man-made steroid budesonide, which is readily available, should be considered an acceptable alternative to prednisone.
“Steroids are not maintenance therapies,” she pointed out. “One could argue that maybe someone who has symptomatic mild Crohn’s disease could be kept on low doses of budesonide. But I would argue whether it is really the budesonide that’s helping them or some other disease process related to polypharmacy.”
There are no long-term safety or efficacy data for budesonide in patients with ulcerative colitis or Crohn’s disease, she added.
Special considerations
Older patients with IBD have a decreased ability to handle disease activity; they have more comorbidities and a susceptibility to falls, said Ha. Early control of the disease is therefore essential.
Sarcopenia, an inherent part of aging when muscle mass decreases over time, is central to physiologic changes, which have implications for older adults with IBD, she said.
“We’re learning that sarcopenia is also prevalent in our patients with moderate to severe inflammatory bowel disease,” she explained. “Sarcopenia is associated with increased risk of infections, hospitalizations, and postoperative complications.”
Other changes occur in the intestines as patients age, Ha reported. “Recent studies have shown that there are changes in the intestinal barrier in terms of the junctions within the mucosal lining that increase intestinal permeability, which may help explain why some patients respond to treatments and others don’t.”
Physical therapy underused
Other treatment options, such as physical therapy, have also been underused in older patients with IBD. For example, there’s often considerable pushback against doing a physical therapy assessment on a hospitalized older patient, said Ha.
Medicare covers up to 80% of those services, but referral wording is key. “They’re not going to cover it for a primary diagnosis of ulcerative colitis or Crohn’s,” she explained. However, “they will cover it for a primary diagnosis of deconditioning with a secondary diagnoses of steroid exposure, anemia, Crohn’s disease, or ulcerative colitis.”
Physical therapy can improve muscle function, decrease muscle pain, potentially decrease analgesics, improve bone mass, and decrease joint pain, stress, fatigue, and debility. Fatigue is prevalent in patients with IBD, Ha explained.
Another underused resource is psychosocial assessment, she added. Although depression is not part of the aging process, it is common in those with chronic conditions.
Visits with licensed psychiatrists and clinical psychologists are covered under Medicare Part B, Ha pointed out, as are psychiatric evaluation and testing and individual and group therapy.
Older patients with IBD are often not receiving the care they need, said Uma Mahadevan, MD, a gastroenterologist at UCSF Health in San Francisco.
The need for awareness of polypharmacy, which Ha also discussed, is a concern in all older patients, but especially those with IBD, Mahadevan said in an interview. Clinicians need to be aware of the cascading effect of pharmacy, in which one drug’s adverse effect leads to the prescription of another drug, with different adverse effects.
Ha gave the example of a patient with IBD who started to have diarrhea as an adverse effect of a medication. A clinician might then prescribe a medication for Clostridium difficile, but that might lead to nausea, leading to the prescription of an antinausea medicine.
A multidisciplinary team is needed to perform medication reconciliation, Ha noted.
Correcting anemia important for IBD
Anemia is also underidentified and undertreated in older patients with IBD, Ha said.
“Across the board with inflammatory bowel disease, we don’t do a great job of being aggressive and correcting anemia. That has implications for fatigue and implications with functional status and circulating volume,” she said.
In older patients, it might be that the decline in hemoglobin over time is more important to outcomes than the number itself, she said. “A hemoglobin of 8 g/dL is one thing, but if it was at 12 g/dL 6 months ago, that’s a different story.”
“For older patients, anemia is associated with a high incidence of cardiovascular disease, cognitive impairment, increased risks of falls and fractures, longer hospitalizations (and thus increased costs of care), increased frailty and dementia, and increased risk of mortality,” Ha said. But, she pointed out, Medicare benefits do cover intravenous iron formulations.
This article originally appeared on Medscape.com.
MAUI, HAWAII – said Christina Ha, MD, from the Inflammatory Bowel Disease Center at Cedars-Sinai in Los Angeles.
Clinicians sometimes fall back on steroids because they are typically inexpensive and because there are fears that the new anti-TNF biologics can cause adverse events in older patients, Ha said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“There are not a lot of safety data for the age group, which is not well represented in clinical trials,” she explained. “We can’t necessarily extrapolate data from a study of people with an average age in the 40s to someone in their 70s.”
But, she emphasized, steroid use for more than 3 months is potentially inappropriate.
“If we have a patient on steroids, we should be saying which steroid-sparing strategy will be incorporated into their regimen when we start them on their course of steroids,” she explained.
Ha said she gets asked frequently whether the man-made steroid budesonide, which is readily available, should be considered an acceptable alternative to prednisone.
“Steroids are not maintenance therapies,” she pointed out. “One could argue that maybe someone who has symptomatic mild Crohn’s disease could be kept on low doses of budesonide. But I would argue whether it is really the budesonide that’s helping them or some other disease process related to polypharmacy.”
There are no long-term safety or efficacy data for budesonide in patients with ulcerative colitis or Crohn’s disease, she added.
Special considerations
Older patients with IBD have a decreased ability to handle disease activity; they have more comorbidities and a susceptibility to falls, said Ha. Early control of the disease is therefore essential.
Sarcopenia, an inherent part of aging when muscle mass decreases over time, is central to physiologic changes, which have implications for older adults with IBD, she said.
“We’re learning that sarcopenia is also prevalent in our patients with moderate to severe inflammatory bowel disease,” she explained. “Sarcopenia is associated with increased risk of infections, hospitalizations, and postoperative complications.”
Other changes occur in the intestines as patients age, Ha reported. “Recent studies have shown that there are changes in the intestinal barrier in terms of the junctions within the mucosal lining that increase intestinal permeability, which may help explain why some patients respond to treatments and others don’t.”
Physical therapy underused
Other treatment options, such as physical therapy, have also been underused in older patients with IBD. For example, there’s often considerable pushback against doing a physical therapy assessment on a hospitalized older patient, said Ha.
Medicare covers up to 80% of those services, but referral wording is key. “They’re not going to cover it for a primary diagnosis of ulcerative colitis or Crohn’s,” she explained. However, “they will cover it for a primary diagnosis of deconditioning with a secondary diagnoses of steroid exposure, anemia, Crohn’s disease, or ulcerative colitis.”
Physical therapy can improve muscle function, decrease muscle pain, potentially decrease analgesics, improve bone mass, and decrease joint pain, stress, fatigue, and debility. Fatigue is prevalent in patients with IBD, Ha explained.
Another underused resource is psychosocial assessment, she added. Although depression is not part of the aging process, it is common in those with chronic conditions.
Visits with licensed psychiatrists and clinical psychologists are covered under Medicare Part B, Ha pointed out, as are psychiatric evaluation and testing and individual and group therapy.
Older patients with IBD are often not receiving the care they need, said Uma Mahadevan, MD, a gastroenterologist at UCSF Health in San Francisco.
The need for awareness of polypharmacy, which Ha also discussed, is a concern in all older patients, but especially those with IBD, Mahadevan said in an interview. Clinicians need to be aware of the cascading effect of pharmacy, in which one drug’s adverse effect leads to the prescription of another drug, with different adverse effects.
Ha gave the example of a patient with IBD who started to have diarrhea as an adverse effect of a medication. A clinician might then prescribe a medication for Clostridium difficile, but that might lead to nausea, leading to the prescription of an antinausea medicine.
A multidisciplinary team is needed to perform medication reconciliation, Ha noted.
Correcting anemia important for IBD
Anemia is also underidentified and undertreated in older patients with IBD, Ha said.
“Across the board with inflammatory bowel disease, we don’t do a great job of being aggressive and correcting anemia. That has implications for fatigue and implications with functional status and circulating volume,” she said.
In older patients, it might be that the decline in hemoglobin over time is more important to outcomes than the number itself, she said. “A hemoglobin of 8 g/dL is one thing, but if it was at 12 g/dL 6 months ago, that’s a different story.”
“For older patients, anemia is associated with a high incidence of cardiovascular disease, cognitive impairment, increased risks of falls and fractures, longer hospitalizations (and thus increased costs of care), increased frailty and dementia, and increased risk of mortality,” Ha said. But, she pointed out, Medicare benefits do cover intravenous iron formulations.
This article originally appeared on Medscape.com.
EXPERT ANALYSIS FROM GUILD 2020
A case is building for proactive anti-TNF monitoring in IBD
MAUI, HAWAII – Gastroenterologists are starting to embrace proactive therapeutic drug monitoring for inflammatory bowel disease patients on tumor necrosis factor inhibitors, according to reports at the Gastroenterology Updates, IBD, and Liver Disease conference.
Proactive therapeutic drug monitoring (TDM) is an alternative to standard combination therapy for inflammatory bowel disease, which involves giving a tumor necrosis factor inhibitor (TNFi) with an immunomodulator, usually azathioprine.
The immunomodulator is meant to prevent antibodies from forming against the TNFi and short-circuiting its effect. Growing evidence suggests that proactive TDM can do the same thing without the second drug and its sometimes fatal adverse events.
Serum TNFi levels are checked during induction, when the risk of antibody formation is highest if levels are too low, and increased upward if they are. Levels are also checked to make sure they aren’t too high, and sometimes followed during maintenance, speakers said at the meeting.
Commercially available serum assays make it easy; the turnaround time is a few days.
Reactive testing is too late
Reactive, instead of proactive, TNFi level testing is standard at the moment, which means levels are checked when people stop responding, but by then it’s often too late. “Why wait until a patient isn’t doing well and potentially has antibodies you can’t overcome before optimizing them? Why not deal with dose optimizing [tumor necrosis factor inhibitors] as opposed to using another drug like a thiopurine? This is common sense,” said gastroenterologist Adam Cheifetz, MD, director of the Center for Inflammatory Bowel Disease at Beth Israel Deaconess Medical Center and professor of medicine at Harvard Medical School, Boston, and a long-time advocate for proactive TDM.
Not too long ago, Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., doubted the approach, but he’s since come around “now that we are starting to see higher level data.” In his own practice, he said he now checks infliximab levels at week 14, before the fourth infusion. Other clinicians check at week 6 before the third infusion.
Whatever the timing, “If we are going to put somebody into remission, it’s going to be in that first 3-month window, so I think early optimization is important. Once you’ve adjusted the dose and optimized it, it might be something you want to check once a year. In my own practice, I’ve been inclined to do more combination therapy, but we’ll see how this goes,” Dr. Loftus said.
The evidence
Both presenters reviewed a recent randomized trial from Israel in pediatric Crohn’s patients induced with adalimumab. Trough levels were checked and adjusted as necessary to concentrations of 5 mcg/mL at weeks 4 and 8, and every 8 weeks thereafter, in 38 children; 40 others were randomized to reactive monitoring, with adjustments to the same trough level. Most of the children in the proactive group had dose intensifications, versus fewer than two-thirds in the reactive group.
At week 72, 31 children (82%) in the proactive group, but only 19 children (48%) in the reactive group, met the study’s primary endpoint, sustained corticosteroid-free clinical remission at all visits (Gastroenterology. 2019 Oct;157[4]:985-96.e2).
In short, “proactive TDM was far superior to reactive testing,” Dr. Cheifetz said.
There’s a question if the results would translate to adults, but, Dr. Loftus said, they beg “the issue of if I should be checking adalimumab trough levels at weeks 4 and 8. It certainly gives you pause to think about doing that.”
Among other reports, Dr. Cheifetz also reviewed a retrospective study of 264 patients – almost two-thirds with Crohn’s disease, the rest with ulcerative colitis - on infliximab maintenance therapy; half had proactive TDM, and the rest reactive monitoring. The target trough concentration was 5-10 mcg/mL; median follow-up was 2.4 years. He was the senior investigator.
On multiple Cox regression analysis, the proactive group had an 84% lower risk of treatment failure, an 84% lower risk of IBD hospitalization, a 75% lower risk of antibody formation, an 83% lower risk of serious infusion reactions, and a 70% lower risk of IBD surgery (Clin Gastroenterol Hepatol. 2017 Oct;15[10]:1580-8.e3).
Dr. Cheifetz said he uses assays from Prometheus Laboratories for proactive TDM, but there are other options. Prometheus turnaround times are a few days. He and his colleagues also have a website – www.bridgeibd.com – where doctors can plug in patient characteristics and get proactive TDM advice.
Dr. Cheifetz is a consultant for Prometheus, as well as Janssen, Abbvie and other companies. He disclosed research funding form Inform Diagnostics. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies.
MAUI, HAWAII – Gastroenterologists are starting to embrace proactive therapeutic drug monitoring for inflammatory bowel disease patients on tumor necrosis factor inhibitors, according to reports at the Gastroenterology Updates, IBD, and Liver Disease conference.
Proactive therapeutic drug monitoring (TDM) is an alternative to standard combination therapy for inflammatory bowel disease, which involves giving a tumor necrosis factor inhibitor (TNFi) with an immunomodulator, usually azathioprine.
The immunomodulator is meant to prevent antibodies from forming against the TNFi and short-circuiting its effect. Growing evidence suggests that proactive TDM can do the same thing without the second drug and its sometimes fatal adverse events.
Serum TNFi levels are checked during induction, when the risk of antibody formation is highest if levels are too low, and increased upward if they are. Levels are also checked to make sure they aren’t too high, and sometimes followed during maintenance, speakers said at the meeting.
Commercially available serum assays make it easy; the turnaround time is a few days.
Reactive testing is too late
Reactive, instead of proactive, TNFi level testing is standard at the moment, which means levels are checked when people stop responding, but by then it’s often too late. “Why wait until a patient isn’t doing well and potentially has antibodies you can’t overcome before optimizing them? Why not deal with dose optimizing [tumor necrosis factor inhibitors] as opposed to using another drug like a thiopurine? This is common sense,” said gastroenterologist Adam Cheifetz, MD, director of the Center for Inflammatory Bowel Disease at Beth Israel Deaconess Medical Center and professor of medicine at Harvard Medical School, Boston, and a long-time advocate for proactive TDM.
Not too long ago, Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., doubted the approach, but he’s since come around “now that we are starting to see higher level data.” In his own practice, he said he now checks infliximab levels at week 14, before the fourth infusion. Other clinicians check at week 6 before the third infusion.
Whatever the timing, “If we are going to put somebody into remission, it’s going to be in that first 3-month window, so I think early optimization is important. Once you’ve adjusted the dose and optimized it, it might be something you want to check once a year. In my own practice, I’ve been inclined to do more combination therapy, but we’ll see how this goes,” Dr. Loftus said.
The evidence
Both presenters reviewed a recent randomized trial from Israel in pediatric Crohn’s patients induced with adalimumab. Trough levels were checked and adjusted as necessary to concentrations of 5 mcg/mL at weeks 4 and 8, and every 8 weeks thereafter, in 38 children; 40 others were randomized to reactive monitoring, with adjustments to the same trough level. Most of the children in the proactive group had dose intensifications, versus fewer than two-thirds in the reactive group.
At week 72, 31 children (82%) in the proactive group, but only 19 children (48%) in the reactive group, met the study’s primary endpoint, sustained corticosteroid-free clinical remission at all visits (Gastroenterology. 2019 Oct;157[4]:985-96.e2).
In short, “proactive TDM was far superior to reactive testing,” Dr. Cheifetz said.
There’s a question if the results would translate to adults, but, Dr. Loftus said, they beg “the issue of if I should be checking adalimumab trough levels at weeks 4 and 8. It certainly gives you pause to think about doing that.”
Among other reports, Dr. Cheifetz also reviewed a retrospective study of 264 patients – almost two-thirds with Crohn’s disease, the rest with ulcerative colitis - on infliximab maintenance therapy; half had proactive TDM, and the rest reactive monitoring. The target trough concentration was 5-10 mcg/mL; median follow-up was 2.4 years. He was the senior investigator.
On multiple Cox regression analysis, the proactive group had an 84% lower risk of treatment failure, an 84% lower risk of IBD hospitalization, a 75% lower risk of antibody formation, an 83% lower risk of serious infusion reactions, and a 70% lower risk of IBD surgery (Clin Gastroenterol Hepatol. 2017 Oct;15[10]:1580-8.e3).
Dr. Cheifetz said he uses assays from Prometheus Laboratories for proactive TDM, but there are other options. Prometheus turnaround times are a few days. He and his colleagues also have a website – www.bridgeibd.com – where doctors can plug in patient characteristics and get proactive TDM advice.
Dr. Cheifetz is a consultant for Prometheus, as well as Janssen, Abbvie and other companies. He disclosed research funding form Inform Diagnostics. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies.
MAUI, HAWAII – Gastroenterologists are starting to embrace proactive therapeutic drug monitoring for inflammatory bowel disease patients on tumor necrosis factor inhibitors, according to reports at the Gastroenterology Updates, IBD, and Liver Disease conference.
Proactive therapeutic drug monitoring (TDM) is an alternative to standard combination therapy for inflammatory bowel disease, which involves giving a tumor necrosis factor inhibitor (TNFi) with an immunomodulator, usually azathioprine.
The immunomodulator is meant to prevent antibodies from forming against the TNFi and short-circuiting its effect. Growing evidence suggests that proactive TDM can do the same thing without the second drug and its sometimes fatal adverse events.
Serum TNFi levels are checked during induction, when the risk of antibody formation is highest if levels are too low, and increased upward if they are. Levels are also checked to make sure they aren’t too high, and sometimes followed during maintenance, speakers said at the meeting.
Commercially available serum assays make it easy; the turnaround time is a few days.
Reactive testing is too late
Reactive, instead of proactive, TNFi level testing is standard at the moment, which means levels are checked when people stop responding, but by then it’s often too late. “Why wait until a patient isn’t doing well and potentially has antibodies you can’t overcome before optimizing them? Why not deal with dose optimizing [tumor necrosis factor inhibitors] as opposed to using another drug like a thiopurine? This is common sense,” said gastroenterologist Adam Cheifetz, MD, director of the Center for Inflammatory Bowel Disease at Beth Israel Deaconess Medical Center and professor of medicine at Harvard Medical School, Boston, and a long-time advocate for proactive TDM.
Not too long ago, Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., doubted the approach, but he’s since come around “now that we are starting to see higher level data.” In his own practice, he said he now checks infliximab levels at week 14, before the fourth infusion. Other clinicians check at week 6 before the third infusion.
Whatever the timing, “If we are going to put somebody into remission, it’s going to be in that first 3-month window, so I think early optimization is important. Once you’ve adjusted the dose and optimized it, it might be something you want to check once a year. In my own practice, I’ve been inclined to do more combination therapy, but we’ll see how this goes,” Dr. Loftus said.
The evidence
Both presenters reviewed a recent randomized trial from Israel in pediatric Crohn’s patients induced with adalimumab. Trough levels were checked and adjusted as necessary to concentrations of 5 mcg/mL at weeks 4 and 8, and every 8 weeks thereafter, in 38 children; 40 others were randomized to reactive monitoring, with adjustments to the same trough level. Most of the children in the proactive group had dose intensifications, versus fewer than two-thirds in the reactive group.
At week 72, 31 children (82%) in the proactive group, but only 19 children (48%) in the reactive group, met the study’s primary endpoint, sustained corticosteroid-free clinical remission at all visits (Gastroenterology. 2019 Oct;157[4]:985-96.e2).
In short, “proactive TDM was far superior to reactive testing,” Dr. Cheifetz said.
There’s a question if the results would translate to adults, but, Dr. Loftus said, they beg “the issue of if I should be checking adalimumab trough levels at weeks 4 and 8. It certainly gives you pause to think about doing that.”
Among other reports, Dr. Cheifetz also reviewed a retrospective study of 264 patients – almost two-thirds with Crohn’s disease, the rest with ulcerative colitis - on infliximab maintenance therapy; half had proactive TDM, and the rest reactive monitoring. The target trough concentration was 5-10 mcg/mL; median follow-up was 2.4 years. He was the senior investigator.
On multiple Cox regression analysis, the proactive group had an 84% lower risk of treatment failure, an 84% lower risk of IBD hospitalization, a 75% lower risk of antibody formation, an 83% lower risk of serious infusion reactions, and a 70% lower risk of IBD surgery (Clin Gastroenterol Hepatol. 2017 Oct;15[10]:1580-8.e3).
Dr. Cheifetz said he uses assays from Prometheus Laboratories for proactive TDM, but there are other options. Prometheus turnaround times are a few days. He and his colleagues also have a website – www.bridgeibd.com – where doctors can plug in patient characteristics and get proactive TDM advice.
Dr. Cheifetz is a consultant for Prometheus, as well as Janssen, Abbvie and other companies. He disclosed research funding form Inform Diagnostics. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies.
REPORTING FROM GUILD 2020
Pembrolizumab plus chemoradiotherapy shows early promise in NSCLC
Combination pembrolizumab and chemoradiotherapy appears safe and active for patients with locally advanced non–small cell lung cancer (NSCLC), results from a phase 1 trial suggest.
Nearly 90% of evaluable patients responded to the combination, and the 12-month progression-free survival rate was 69.7%. Pneumonitis was common, but most patients responded to high-dose corticosteroids.
Salma K. Jabbour, MD, of Rutgers Cancer Institute of New Jersey, in New Brunswick, and colleagues reported these results in JAMA Oncology.
The phase 1 study included 21 patients with locally advanced, unresectable, stage III NSCLC. Planned treatment consisted of pembrolizumab (Keytruda) at various dosing schedules, chemotherapy (weekly carboplatin and paclitaxel), and radiation (2 Gy/day, 60 Gy total).
The researchers used a standard 3 + 3 design to evaluate the safety and tolerability of pembrolizumab in five dosing cohorts.
In cohort 1, patients received pembrolizumab at 200 mg every 21 days within 2-6 weeks of completing chemoradiotherapy. In cohort 2, patients received pembrolizumab at 100 mg every 21 days, starting on day 29 of chemoradiotherapy. Cohort 3 received the 200 mg dose of pembrolizumab, starting on day 29.
Pembrolizumab was started on day 1 of chemoradiotherapy at the 100-mg dose in cohort 4 and at the 200-mg dose in cohort 5. An additional six-patient safety expansion cohort received the same treatment as cohort 5.
The median follow-up was 16 months. There was one dose-limiting toxicity – grade 5 pneumonitis, which occurred in the safety expansion cohort.
Grade 2 or higher immune-related toxicities were observed in 14 patients (67%), and grade 2 or higher pneumonitis occurred in 7 patients (33%).
“Although we observed an increased rate of pneumonitis, most patients had pneumonitis that responded to high-dose corticosteroid treatment,” the researchers noted.
There were 19 patients who received at least two cycles of pembrolizumab and were evaluable for response. The best response was complete response in 3 patients (16%), partial response in 14 patients (74%), and stable disease in 1 patient (5%).
The progression-free survival rate was 81.0% at 6 months and 69.7% at 12 months. The overall survival rate was 95.2% at 6 months and 85.2% at 12 months.
The researchers acknowledged that two key limitations of this study were the small sample size and short duration of follow-up.
“Pembrolizumab with concurrent chemoradiotherapy is tolerable for patients with locally advanced, unresectable NSCLC, although the risk of pneumonitis and long-term outcomes should be evaluated in additional studies,” commented Enriqueta Felip, MD, PhD, of Vall D’Hebron Institute of Oncology in Barcelona, who was not involved in this study.
The study was funded by Merck. The authors and Dr. Felip disclosed relationships with Merck and other companies.
SOURCE: Jabbour SK et al. JAMA Oncol. 2020 Feb 20. doi: 10.1001/jamaoncol.2019.6731.
Combination pembrolizumab and chemoradiotherapy appears safe and active for patients with locally advanced non–small cell lung cancer (NSCLC), results from a phase 1 trial suggest.
Nearly 90% of evaluable patients responded to the combination, and the 12-month progression-free survival rate was 69.7%. Pneumonitis was common, but most patients responded to high-dose corticosteroids.
Salma K. Jabbour, MD, of Rutgers Cancer Institute of New Jersey, in New Brunswick, and colleagues reported these results in JAMA Oncology.
The phase 1 study included 21 patients with locally advanced, unresectable, stage III NSCLC. Planned treatment consisted of pembrolizumab (Keytruda) at various dosing schedules, chemotherapy (weekly carboplatin and paclitaxel), and radiation (2 Gy/day, 60 Gy total).
The researchers used a standard 3 + 3 design to evaluate the safety and tolerability of pembrolizumab in five dosing cohorts.
In cohort 1, patients received pembrolizumab at 200 mg every 21 days within 2-6 weeks of completing chemoradiotherapy. In cohort 2, patients received pembrolizumab at 100 mg every 21 days, starting on day 29 of chemoradiotherapy. Cohort 3 received the 200 mg dose of pembrolizumab, starting on day 29.
Pembrolizumab was started on day 1 of chemoradiotherapy at the 100-mg dose in cohort 4 and at the 200-mg dose in cohort 5. An additional six-patient safety expansion cohort received the same treatment as cohort 5.
The median follow-up was 16 months. There was one dose-limiting toxicity – grade 5 pneumonitis, which occurred in the safety expansion cohort.
Grade 2 or higher immune-related toxicities were observed in 14 patients (67%), and grade 2 or higher pneumonitis occurred in 7 patients (33%).
“Although we observed an increased rate of pneumonitis, most patients had pneumonitis that responded to high-dose corticosteroid treatment,” the researchers noted.
There were 19 patients who received at least two cycles of pembrolizumab and were evaluable for response. The best response was complete response in 3 patients (16%), partial response in 14 patients (74%), and stable disease in 1 patient (5%).
The progression-free survival rate was 81.0% at 6 months and 69.7% at 12 months. The overall survival rate was 95.2% at 6 months and 85.2% at 12 months.
The researchers acknowledged that two key limitations of this study were the small sample size and short duration of follow-up.
“Pembrolizumab with concurrent chemoradiotherapy is tolerable for patients with locally advanced, unresectable NSCLC, although the risk of pneumonitis and long-term outcomes should be evaluated in additional studies,” commented Enriqueta Felip, MD, PhD, of Vall D’Hebron Institute of Oncology in Barcelona, who was not involved in this study.
The study was funded by Merck. The authors and Dr. Felip disclosed relationships with Merck and other companies.
SOURCE: Jabbour SK et al. JAMA Oncol. 2020 Feb 20. doi: 10.1001/jamaoncol.2019.6731.
Combination pembrolizumab and chemoradiotherapy appears safe and active for patients with locally advanced non–small cell lung cancer (NSCLC), results from a phase 1 trial suggest.
Nearly 90% of evaluable patients responded to the combination, and the 12-month progression-free survival rate was 69.7%. Pneumonitis was common, but most patients responded to high-dose corticosteroids.
Salma K. Jabbour, MD, of Rutgers Cancer Institute of New Jersey, in New Brunswick, and colleagues reported these results in JAMA Oncology.
The phase 1 study included 21 patients with locally advanced, unresectable, stage III NSCLC. Planned treatment consisted of pembrolizumab (Keytruda) at various dosing schedules, chemotherapy (weekly carboplatin and paclitaxel), and radiation (2 Gy/day, 60 Gy total).
The researchers used a standard 3 + 3 design to evaluate the safety and tolerability of pembrolizumab in five dosing cohorts.
In cohort 1, patients received pembrolizumab at 200 mg every 21 days within 2-6 weeks of completing chemoradiotherapy. In cohort 2, patients received pembrolizumab at 100 mg every 21 days, starting on day 29 of chemoradiotherapy. Cohort 3 received the 200 mg dose of pembrolizumab, starting on day 29.
Pembrolizumab was started on day 1 of chemoradiotherapy at the 100-mg dose in cohort 4 and at the 200-mg dose in cohort 5. An additional six-patient safety expansion cohort received the same treatment as cohort 5.
The median follow-up was 16 months. There was one dose-limiting toxicity – grade 5 pneumonitis, which occurred in the safety expansion cohort.
Grade 2 or higher immune-related toxicities were observed in 14 patients (67%), and grade 2 or higher pneumonitis occurred in 7 patients (33%).
“Although we observed an increased rate of pneumonitis, most patients had pneumonitis that responded to high-dose corticosteroid treatment,” the researchers noted.
There were 19 patients who received at least two cycles of pembrolizumab and were evaluable for response. The best response was complete response in 3 patients (16%), partial response in 14 patients (74%), and stable disease in 1 patient (5%).
The progression-free survival rate was 81.0% at 6 months and 69.7% at 12 months. The overall survival rate was 95.2% at 6 months and 85.2% at 12 months.
The researchers acknowledged that two key limitations of this study were the small sample size and short duration of follow-up.
“Pembrolizumab with concurrent chemoradiotherapy is tolerable for patients with locally advanced, unresectable NSCLC, although the risk of pneumonitis and long-term outcomes should be evaluated in additional studies,” commented Enriqueta Felip, MD, PhD, of Vall D’Hebron Institute of Oncology in Barcelona, who was not involved in this study.
The study was funded by Merck. The authors and Dr. Felip disclosed relationships with Merck and other companies.
SOURCE: Jabbour SK et al. JAMA Oncol. 2020 Feb 20. doi: 10.1001/jamaoncol.2019.6731.
FROM JAMA ONCOLOGY
Secondary bile acid deficiency may be culprit in UC inflammation
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
FROM CELL HOST & MICROBE
Abbreviated MRI bests digital breast tomosynthesis in finding cancer in dense breasts
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
FROM JAMA