User login
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population (FULL)
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
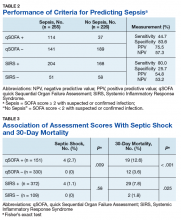
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
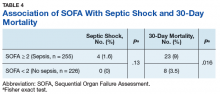
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
Crohn’s & Colitis Congress has passed, DDW ahead
In late January, the Crohn’s & Colitis Foundation teamed with AGA to present the Crohn’s & Colitis Congress® in Austin, Tex. Each year, this is the premier gathering for IBD experts and the rest of us to catch up on the substantial progress we are making in treating patients with IBD. This month, we highlight a number of articles from the Congress, including results showing how a focused IBD quality initiative reduced emergency department visits, an article about the effects of IBD on fertility, and the link between stress and ulcerative colitis flares. All of these articles are worth reading, since they can help our care of patients. On agau.gastro.org, you can access slides from the Congress.
Several more articles deserve mention. Three articles from the AGA journals highlight new information about colorectal cancer prevention and the U.S. Multi-Society Task Force on Colorectal Cancer has updated colonoscopy follow-up guidance. In our practice management section, we provide a step-by-step guide to changes in evaluation and management (E/M) coding – these changes are the most impactful since the Medicare E/M documentation specifications first appeared.
We have 2 months left before Digestive Disease Week® (DDW). Each year, DDW marks the end of our AGA Institute President’s term and the beginning of another’s epoch. Hashem B. El-Serag will pass the gavel to Bishr Omary – both great friends and great gastroenterologists. I am happy to see that Gail Hecht follows me as this year’s AGA Julius Friedenwald Medal recipient (AGA’s highest honor). She, too, is a great friend and role model for me and many others. DDW returns to Chicago in early May, and once again will be the world’s best gathering of physicians and scientists dedicated to digestive diseases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
In late January, the Crohn’s & Colitis Foundation teamed with AGA to present the Crohn’s & Colitis Congress® in Austin, Tex. Each year, this is the premier gathering for IBD experts and the rest of us to catch up on the substantial progress we are making in treating patients with IBD. This month, we highlight a number of articles from the Congress, including results showing how a focused IBD quality initiative reduced emergency department visits, an article about the effects of IBD on fertility, and the link between stress and ulcerative colitis flares. All of these articles are worth reading, since they can help our care of patients. On agau.gastro.org, you can access slides from the Congress.
Several more articles deserve mention. Three articles from the AGA journals highlight new information about colorectal cancer prevention and the U.S. Multi-Society Task Force on Colorectal Cancer has updated colonoscopy follow-up guidance. In our practice management section, we provide a step-by-step guide to changes in evaluation and management (E/M) coding – these changes are the most impactful since the Medicare E/M documentation specifications first appeared.
We have 2 months left before Digestive Disease Week® (DDW). Each year, DDW marks the end of our AGA Institute President’s term and the beginning of another’s epoch. Hashem B. El-Serag will pass the gavel to Bishr Omary – both great friends and great gastroenterologists. I am happy to see that Gail Hecht follows me as this year’s AGA Julius Friedenwald Medal recipient (AGA’s highest honor). She, too, is a great friend and role model for me and many others. DDW returns to Chicago in early May, and once again will be the world’s best gathering of physicians and scientists dedicated to digestive diseases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
In late January, the Crohn’s & Colitis Foundation teamed with AGA to present the Crohn’s & Colitis Congress® in Austin, Tex. Each year, this is the premier gathering for IBD experts and the rest of us to catch up on the substantial progress we are making in treating patients with IBD. This month, we highlight a number of articles from the Congress, including results showing how a focused IBD quality initiative reduced emergency department visits, an article about the effects of IBD on fertility, and the link between stress and ulcerative colitis flares. All of these articles are worth reading, since they can help our care of patients. On agau.gastro.org, you can access slides from the Congress.
Several more articles deserve mention. Three articles from the AGA journals highlight new information about colorectal cancer prevention and the U.S. Multi-Society Task Force on Colorectal Cancer has updated colonoscopy follow-up guidance. In our practice management section, we provide a step-by-step guide to changes in evaluation and management (E/M) coding – these changes are the most impactful since the Medicare E/M documentation specifications first appeared.
We have 2 months left before Digestive Disease Week® (DDW). Each year, DDW marks the end of our AGA Institute President’s term and the beginning of another’s epoch. Hashem B. El-Serag will pass the gavel to Bishr Omary – both great friends and great gastroenterologists. I am happy to see that Gail Hecht follows me as this year’s AGA Julius Friedenwald Medal recipient (AGA’s highest honor). She, too, is a great friend and role model for me and many others. DDW returns to Chicago in early May, and once again will be the world’s best gathering of physicians and scientists dedicated to digestive diseases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
U.S. reports first death from COVID-19, possible outbreak at long-term care facility
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.
The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.
The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
The first death in the United States from the novel coronavirus (COVID-19) was a Washington state man in his 50s who had underlying health conditions, state health officials announced on Feb 29. At the same time, officials there are investigating a possible COVID-19 outbreak at a long-term care facility.
Washington state officials reported two other presumptive positive cases of COVID-19, both of whom are associated with LifeCare of Kirkland, Washington. One is a woman in her 70s who is a resident at the facility and the other is a woman in her 40s who is a health care worker at the facility.
Additionally, many residents and staff members at the facility have reported respiratory symptoms, according to Jeff Duchin, MD, health officer for public health in Seattle and King County. Among the more than 100 residents at the facility, 27 have respiratory symptoms; while among the 180 staff members, 25 have reported symptoms.
Overall, these reports bring the total number of U.S. COVID-19 cases detected by the public health system to 22, though that number is expected to climb as these investigations continue.
The general risk to the American public is still low, including residents in long-term care facilities, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during the Feb. 29 press briefing. Older people are are higher risk, however, and long-term care facilities should emphasize handwashing and the early identification of individuals with symptoms.
Dr. Duchin added that health care workers who are sick should stay home and that visitors should be screened for symptoms, the same advice offered to limit the spread of influenza at long-term care facilities.
The CDC briefing comes after President Trump held his own press conference at the White House where he identified the person who had died as being a woman in her 50s who was medically at risk.
During that press conference, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said that the current pattern of disease with COVID-19 suggests that 75%-80% of patients will have mild illness and recover, while 15%-20% will require advanced medical care.
For the most part, the more serious cases will occur in those who are elderly or have underlying medical conditions. There is “no indication” that individuals who recover from the virus are becoming re-infected, Dr. Fauci said.
The administration also announced a series of actions aimed at slowing the spread of the virus and responding to it. On March 2, President Trump will meet with leaders in the pharmaceutical industry at the White House to discuss vaccine development. The administration is also working to ensure an adequate supply of face masks. Vice President Mike Pence said there are currently more than 40 million masks available, but that the administration has received promises of 35 million more masks per month from manufacturers. Access to masks will be prioritized for high-risk health care workers, Vice President Pence said. “The average American does not need to go out and buy a mask,” he added.
Additionally, Vice President Pence announced new travel restrictions with Iran that would bar entry to the United States for any foreign national who visited Iran in the last 14 days. The federal government is also advising Americans not to travel to the regions in Italy and South Korea that have been most affected by COVID-19. The government is also working with officials in Italy and South Korea to conduct medical screening of anyone coming into the United States from those countries.
Depression in MS predicted worsening of neurologic function
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
WEST PALM BEACH, FLA. – Among patients with relapsing-remitting multiple sclerosis (MS), depression increases the likelihood of having worse neurologic function one year later, according to a study presented at ACTRIMS Forum 2020. Patients’ subjective descriptions of disease activity did not significantly change during that time, which “suggests that depression is not merely a reactive phenomenon, but rather an independent contributor to clinical worsening in the long term,” said Jenny Feng, MD, a neuroimmunology fellow at the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
The researchers hypothesize that depression’s influence on psychomotor function may contribute to clinical worsening in MS.
More than half of patients with MS have depression, and there is a higher prevalence of depression in relapsing-remitting MS than in progressive disease. “Depression is associated with systemic inflammation,” Dr. Feng said at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We know that depressed individuals tend to have slower walking speeds, slower processing speeds, and worse quality of life measures.” But neurologists do not know whether patients feel depressed because the disease is getting worse, or whether depression is an independent contributing factor to MS, Dr. Feng said.
To examine whether depression affects neurologic performance and disease activity in patients with MS, Dr. Feng and colleagues analyzed real-world data from about 2,400 patients in MS PATHS (Multiple Sclerosis Partners Advancing Technology and Health Solutions), a network of centers in the United States and Europe. The researchers assessed the longitudinal relationship between depression, measures of neurologic function, and MRI metrics.
The researchers included patients with relapsing-remitting MS who had clinical and imaging data available at baseline and about 1 year later. Patients completed tests of manual dexterity, walking speed, and processing speed that are based on the Multiple Sclerosis Functional Composite. A worsening of 20% on any measure is considered clinically significant.
Patients had a mean age of about 45 years and mean disease duration of about 14 years. Patients with a T score greater than 45 on the Neuro-QoL depression questionnaire were classified as having depression, and approximately half of the population had depression. Patients with depression were more likely to have an employment status of disabled and to receive infusion medications.
The investigators used propensity score analysis to adjust for baseline differences between patients with and without depression and evaluated the effect of depression on year 1 outcome measures using logistic regression for categorical variables and linear regression for continuous variables.
“After propensity weighting for baseline covariates including neuroperformance scores, individuals with depression continued to worsen,” Dr. Feng said. Patients with depression were more likely to have a 20% worsening in at least one measure of neurologic performance at year 1 (odds ratio, 1.31). “There was a trend for increased odds of interval relapses, increased T2 lesion burden, and contrast-enhancing lesions at year 1” in patients with depression, but the results were not statistically significant. “Despite worsening neuroperformance at year 1 in individuals with baseline depression, their [patient-reported outcomes] at year 1 were not significantly worse.”
The researcher lacked information about the date of depression onset and medication compliance, Dr. Feng said. In addition, propensity weighting does not account for potential bias due to missing data.
The findings support the existing practice of actively screening for and treating depression in patients with MS, Dr. Feng said.
Dr. Feng had no disclosures. Coauthors have consulted for and received research support from pharmaceutical companies. MS PATHS is supported by Biogen.
SOURCE: Feng JJ et al. ACTRIMS Forum 2020. Abstract P226.
REPORTING FROM ACTRIMS Forum 2020
How often do neurologists escalate MS therapy after detecting MRI activity?
WEST PALM BEACH, FLA. – About a third of patients with multiple sclerosis (MS) switch to a more potent disease-modifying therapy (DMT) within 1 year of disease activity being detected on MRI, according to a study of prescribing practices. The number of T2 lesions on MRI may be associated with the likelihood of switching DMTs, said Ryan Canissario, MD, a neurology resident at University of Rochester (New York) Medical Center, and colleagues.
The researchers had hypothesized that “the majority of patients would undergo a change in DMT in response to MRI activity,” they said. Delays in follow-up or therapy start times may partly explain the relatively low rates of switching during the first few months. “We speculate that other reasons ... include clinician or patient risk tolerance, patient age, prior longstanding stability on existing therapy, recent therapy change prior to MRI, or high baseline DMT potency,” the researchers said. Future studies will try to clarify the findings and assess outcomes related to prescribing practices.
Preventing new lesions on MRI is a primary treatment target in MS. “Following this principle, change in [DMT] should be considered in the setting of MRI evidence of disease activity,” but prescribing practices have not been well characterized, Dr. Canissario and colleagues said.
To identify and characterize patients who underwent a DMT change after the detection of brain MRI disease activity, Dr. Canissario and colleagues analyzed data from more than 1,300 patients in MS PATHS (MS Partners Advancing Technology and Health Solutions), a research network of 10 health care institutions. The investigators presented their results at ACTRIMS Forum 2020, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
By consensus, the investigators classified DMTs as low potency (for example, interferons, immunoglobulin G, glatiramer acetate, and teriflunomide), medium potency (azathioprine, cladribine, daclizumab, dimethyl fumarate, fingolimod, methotrexate, and mycophenolate mofetil), or high potency (alemtuzumab, cyclophosphamide, mitoxantrone, natalizumab, ocrelizumab, ofatumumab, and rituximab).
The researchers reviewed available imaging data from Apr. 2015 to Aug. 2019 to identify patients with new T2 or gadolinium-enhancing lesions. They determined whether these patients had an escalation in DMT potency or a lateral switch at 3, 6, 9, and 12 months after a radiologist reviewed the MRI.
The number of patients with MRI evidence of disease activity and complete DMT data ranged from 1,364 at 3 months to 952 at 12 months. The proportion of patients who had an escalation in therapy was 17.4% at 3 months, 25.5% at 6 months, 30.4% at 9 months, and 34.3% at 12 months. The proportion with a lateral change was 2% at 3 months, 3.4% at 6 months, 4.3% at 9 months, and 6% at 12 months.
The percentage of patients with DMT escalation or lateral change at 9 months increased with an increasing number of new T2 lesions. About 27% of patients with one new lesion switched therapy, compared with 43.5% of those with more than three new lesions.
Dr. Canissario had no disclosures. Coauthors disclosed research support from and consulting for pharmaceutical companies. MS PATHS is funded by Biogen.
SOURCE: Canissario R et al. ACTRIMS Forum 2020. Abstract P112.
WEST PALM BEACH, FLA. – About a third of patients with multiple sclerosis (MS) switch to a more potent disease-modifying therapy (DMT) within 1 year of disease activity being detected on MRI, according to a study of prescribing practices. The number of T2 lesions on MRI may be associated with the likelihood of switching DMTs, said Ryan Canissario, MD, a neurology resident at University of Rochester (New York) Medical Center, and colleagues.
The researchers had hypothesized that “the majority of patients would undergo a change in DMT in response to MRI activity,” they said. Delays in follow-up or therapy start times may partly explain the relatively low rates of switching during the first few months. “We speculate that other reasons ... include clinician or patient risk tolerance, patient age, prior longstanding stability on existing therapy, recent therapy change prior to MRI, or high baseline DMT potency,” the researchers said. Future studies will try to clarify the findings and assess outcomes related to prescribing practices.
Preventing new lesions on MRI is a primary treatment target in MS. “Following this principle, change in [DMT] should be considered in the setting of MRI evidence of disease activity,” but prescribing practices have not been well characterized, Dr. Canissario and colleagues said.
To identify and characterize patients who underwent a DMT change after the detection of brain MRI disease activity, Dr. Canissario and colleagues analyzed data from more than 1,300 patients in MS PATHS (MS Partners Advancing Technology and Health Solutions), a research network of 10 health care institutions. The investigators presented their results at ACTRIMS Forum 2020, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
By consensus, the investigators classified DMTs as low potency (for example, interferons, immunoglobulin G, glatiramer acetate, and teriflunomide), medium potency (azathioprine, cladribine, daclizumab, dimethyl fumarate, fingolimod, methotrexate, and mycophenolate mofetil), or high potency (alemtuzumab, cyclophosphamide, mitoxantrone, natalizumab, ocrelizumab, ofatumumab, and rituximab).
The researchers reviewed available imaging data from Apr. 2015 to Aug. 2019 to identify patients with new T2 or gadolinium-enhancing lesions. They determined whether these patients had an escalation in DMT potency or a lateral switch at 3, 6, 9, and 12 months after a radiologist reviewed the MRI.
The number of patients with MRI evidence of disease activity and complete DMT data ranged from 1,364 at 3 months to 952 at 12 months. The proportion of patients who had an escalation in therapy was 17.4% at 3 months, 25.5% at 6 months, 30.4% at 9 months, and 34.3% at 12 months. The proportion with a lateral change was 2% at 3 months, 3.4% at 6 months, 4.3% at 9 months, and 6% at 12 months.
The percentage of patients with DMT escalation or lateral change at 9 months increased with an increasing number of new T2 lesions. About 27% of patients with one new lesion switched therapy, compared with 43.5% of those with more than three new lesions.
Dr. Canissario had no disclosures. Coauthors disclosed research support from and consulting for pharmaceutical companies. MS PATHS is funded by Biogen.
SOURCE: Canissario R et al. ACTRIMS Forum 2020. Abstract P112.
WEST PALM BEACH, FLA. – About a third of patients with multiple sclerosis (MS) switch to a more potent disease-modifying therapy (DMT) within 1 year of disease activity being detected on MRI, according to a study of prescribing practices. The number of T2 lesions on MRI may be associated with the likelihood of switching DMTs, said Ryan Canissario, MD, a neurology resident at University of Rochester (New York) Medical Center, and colleagues.
The researchers had hypothesized that “the majority of patients would undergo a change in DMT in response to MRI activity,” they said. Delays in follow-up or therapy start times may partly explain the relatively low rates of switching during the first few months. “We speculate that other reasons ... include clinician or patient risk tolerance, patient age, prior longstanding stability on existing therapy, recent therapy change prior to MRI, or high baseline DMT potency,” the researchers said. Future studies will try to clarify the findings and assess outcomes related to prescribing practices.
Preventing new lesions on MRI is a primary treatment target in MS. “Following this principle, change in [DMT] should be considered in the setting of MRI evidence of disease activity,” but prescribing practices have not been well characterized, Dr. Canissario and colleagues said.
To identify and characterize patients who underwent a DMT change after the detection of brain MRI disease activity, Dr. Canissario and colleagues analyzed data from more than 1,300 patients in MS PATHS (MS Partners Advancing Technology and Health Solutions), a research network of 10 health care institutions. The investigators presented their results at ACTRIMS Forum 2020, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
By consensus, the investigators classified DMTs as low potency (for example, interferons, immunoglobulin G, glatiramer acetate, and teriflunomide), medium potency (azathioprine, cladribine, daclizumab, dimethyl fumarate, fingolimod, methotrexate, and mycophenolate mofetil), or high potency (alemtuzumab, cyclophosphamide, mitoxantrone, natalizumab, ocrelizumab, ofatumumab, and rituximab).
The researchers reviewed available imaging data from Apr. 2015 to Aug. 2019 to identify patients with new T2 or gadolinium-enhancing lesions. They determined whether these patients had an escalation in DMT potency or a lateral switch at 3, 6, 9, and 12 months after a radiologist reviewed the MRI.
The number of patients with MRI evidence of disease activity and complete DMT data ranged from 1,364 at 3 months to 952 at 12 months. The proportion of patients who had an escalation in therapy was 17.4% at 3 months, 25.5% at 6 months, 30.4% at 9 months, and 34.3% at 12 months. The proportion with a lateral change was 2% at 3 months, 3.4% at 6 months, 4.3% at 9 months, and 6% at 12 months.
The percentage of patients with DMT escalation or lateral change at 9 months increased with an increasing number of new T2 lesions. About 27% of patients with one new lesion switched therapy, compared with 43.5% of those with more than three new lesions.
Dr. Canissario had no disclosures. Coauthors disclosed research support from and consulting for pharmaceutical companies. MS PATHS is funded by Biogen.
SOURCE: Canissario R et al. ACTRIMS Forum 2020. Abstract P112.
REPORTING FROM ACTRIMS FORUM 2020
Incidence of cardiovascular events is doubled in patients with MS
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
WEST PALM BEACH, FLA. – The incidence rate of many cardiovascular events is more than doubled in patients with multiple sclerosis (MS), compared with matched controls without MS, according to a study presented at ACTRIMS Forum 2020. The risk of a major adverse cardiac event (MACE) – that is, a first myocardial infarction, stroke, or cardiac arrest – is approximately twofold higher. Venous thromboembolism and peripheral vascular disease also occur at notably increased rates, reported Rebecca Persson, MPH, and colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Ms. Persson is an epidemiologist at the Boston Collaborative Drug Surveillance Program in Lexington, Mass.
Vascular comorbidities are more prevalent in patients with MS than in the general population, but few studies have reported on the incidence of cardiovascular disease after MS diagnosis. To describe rates of incident cardiovascular disease after MS diagnosis and compare them with rates in a matched population without MS, the researchers analyzed data from a U.S. Department of Defense database.
The study included a cohort of 6,406 patients with MS diagnosed and treated during Jan. 2004–Aug. 2017 who had at least one prescription for an MS disease-modifying treatment.
A cohort of 66,281 patients without MS were matched to the patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. The researchers excluded patients with a history of cardiovascular disease or select comorbidities such as dyslipidemia, atrial fibrillation, or a disorder related to peripheral vascular disease. They also excluded patients with a history of treated hypertension or treated type 2 diabetes, defined as diagnosis and treatment within 90 days of each other.
Researchers considered a patient to have a cardiovascular disease outcome – including MI, stroke, cardiac arrest, heart failure, angina or unspecified ischemic heart disease, transient ischemic attack or unspecified cerebrovascular disease, venous thromboembolism, peripheral vascular disease, pericardial disease, bradycardia or heart block, or arrhythmia other than atrial fibrillation or atrial flutter – if the disease was recorded five or more times.
The researchers followed patients from cohort entry until study outcome (separate for each outcome), loss of eligibility, death, or end of data collection. Ms. Persson and colleagues calculated incidence rates (IRs) using the Byar method and incidence rate ratios (IRRs) using Poisson regression for each outcome.
The median age at MS diagnosis or at the matched date was 38 years, and 71% were female. The median duration of record after patients entered the cohort was 7.2 years for patients with MS and 5.3 years for patients without MS.
The IRs of all cardiovascular disease types, with the exception of bradycardia or heart block, were higher for patients with MS, compared with non-MS patients, the researchers reported. Many cardiovascular disease outcomes had IRRs greater than 2. “The incidence of MI was higher among MS patients than among non-MS patients,” the researchers said (IR, 12.4 vs. 5.9 per 10,000 person-years; IRR, 2.11).
“Risk of MACE and risk of stroke were higher among MS patients than among non-MS patients,” the researchers said. Relative risks also were higher among women than among men (2.47 vs. 1.55 for MACE, and 2.19 vs. 1.71 for stroke). When the investigators performed a sensitivity analysis to address the possibility that physicians might misdiagnosis MS symptoms as stroke, the rate of stroke was attenuated among patients with MS, but remained elevated relative to the rate among patients without MS (IRR, 1.63).
The IR of venous thromboembolism was more than 2 times higher among patients with MS than among non-MS patients (38.4 vs. 15.1 per 10,000 person-years; IRR, 2.54), as was the risk of peripheral vascular disease (14.9 vs. 6.0 per 10,000 person-years; IRR, 2.49). The relative risk of peripheral vascular disease was higher in women than men, and the risk in patients with MS increased after age 40 years.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four of Ms. Persson’s coauthors are employees of BMS, and one works for a company that has a business relationship with Celgene.
SOURCE: Persson R et al. ACTRIMS Forum 2020. Abstract P082.
REPORTING FROM ACTRIMS FORUM 2020
Functional connectivity model identifies MS impairment
WEST PALM BEACH, FLA. – A machine learning model that combines data on the brain’s functional connectivity with clinical information such as age, sex and disease duration shows the potential to provide an accurate assessment of clinical impairment in patients with multiple sclerosis (MS).
“This is the first study to show that dynamic functional connectivity is useful to identify the impairment level in MS, and can be used for personalized treatment by clinicians,” first author Ceren Tozlu, PhD, of Weill Cornell Medicine, New York, said in an interview.
“We found out that structural connectivity is the most important feature that distinguishes MS patients from healthy controls, while dynamic functional connectivity was more discriminative compared to the static functional connectivity in MS patient classification regarding their impairment level.”
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Statistical assessment of the clinical impairment of MS using MRI is hindered by a relatively weak correlation between the impairment and disease burden, such as lesion load.
However, the brain’s functional connectivity network, which is indicative of the disruption of the transmission of signals of gray matter regions, could provide a deeper understanding of connectome-level mechanisms that underlie variability in MS-related impairments, Dr. Tozlu and colleagues say.
With no previous study pulling together multimodal imaging data including static and dynamic functional connectivity to classify MS patients with a clinically significant impairment versus non–clinically significant impairment, Dr. Tozlu and the team sought to build a machine-learning–based model to do so.
For the study, they enrolled 79 patients with MS, including 42 with Expanded Disability Status scores of 2 or higher, representing clinically significant impairment at baseline.
The patients, who had a mean age of 45 years, were 66% female and had a mean disease duration of 12.48 years. The ensemble model that was used incorporated functional connectivity and a clinical dataset of age, sex, and disease duration. Functional connectivity was measured by evaluating blood oxygen level dependent (BOLD) signal activity between 86 FreeSurfer-based gray matter regions.
“Functional connectivity is a statistical correlation (Pearson’s correlation coefficient) between two time series of BOLD signals measured on two distinct region of interest of the brain during MRI scan,” Dr. Tozlu explained. “In our study, BOLD time series were measured using resting-state functional MRI technique that last 7 minutes.”
The ensemble model was able to classify low-adapting MS patients with an area under ROC curve (AUC) of 0.638 and a balanced accuracy of 0.659. The model performed well in accurately classifying the MS patients with clinically significant impairment with a sensitivity of 0.719.
“The models in which we applied functional and structural connectivity showed a high performance in classifying MS patients regarding their impairment level,” Dr. Tozlu said.
She noted that “these models may be extended to predict change in impairment level in a longitudinal study, for instance, identifying MS patients who may have a clinically significant impairment.”
In further evaluating which particular functional connections were most related to MS disease activity, Dr. Tozlu and colleagues found the most discriminative areas were between the right superior parietal and right inferior temporal, between right lateral occipital and left pericalcarine, and between right pericalcarine and right side of frontal pole.
If further validated, the approach could have important, broader clinical implications, Dr. Tozlu said.
“If the validation of these models on a larger dataset is successful, this model may be used to decide for personalized treatment,” Dr. Tozlu added. “The model could offer guidance in providing more powerful treatment for MS patients who may have a clinically significant impairment and less powerful treatment for MS patients who may not have a clinically significant impairment in order to avoid the side effects of treatments.
“Therefore, we believe that dynamics in functional connectivity should be taken into account in the next studies in MS.”
In commenting on the research, Eric Klawiter, MD, associate professor of neurology, Harvard Medical School and associate neurologist at Massachusetts General Hospital, both in Boston, said the findings offer valuable insights in the use of machine learning and MS imaging.
“This research shows very nicely the power of machine learning and connectivity techniques to differentiate MS phenotypes based on disability level,” he said in an interview.
“The future direction of this work is to develop predictive markers for disability progression and this would have significant impact in how we evaluate newly diagnosed patients and counsel their treatment decisions.”
Dr. Tozlu and Dr. Klawiter had no disclosures to report.
SOURCE: Tozlu C et al. ACTRIMS Forum 2020. Abstract P025.
WEST PALM BEACH, FLA. – A machine learning model that combines data on the brain’s functional connectivity with clinical information such as age, sex and disease duration shows the potential to provide an accurate assessment of clinical impairment in patients with multiple sclerosis (MS).
“This is the first study to show that dynamic functional connectivity is useful to identify the impairment level in MS, and can be used for personalized treatment by clinicians,” first author Ceren Tozlu, PhD, of Weill Cornell Medicine, New York, said in an interview.
“We found out that structural connectivity is the most important feature that distinguishes MS patients from healthy controls, while dynamic functional connectivity was more discriminative compared to the static functional connectivity in MS patient classification regarding their impairment level.”
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Statistical assessment of the clinical impairment of MS using MRI is hindered by a relatively weak correlation between the impairment and disease burden, such as lesion load.
However, the brain’s functional connectivity network, which is indicative of the disruption of the transmission of signals of gray matter regions, could provide a deeper understanding of connectome-level mechanisms that underlie variability in MS-related impairments, Dr. Tozlu and colleagues say.
With no previous study pulling together multimodal imaging data including static and dynamic functional connectivity to classify MS patients with a clinically significant impairment versus non–clinically significant impairment, Dr. Tozlu and the team sought to build a machine-learning–based model to do so.
For the study, they enrolled 79 patients with MS, including 42 with Expanded Disability Status scores of 2 or higher, representing clinically significant impairment at baseline.
The patients, who had a mean age of 45 years, were 66% female and had a mean disease duration of 12.48 years. The ensemble model that was used incorporated functional connectivity and a clinical dataset of age, sex, and disease duration. Functional connectivity was measured by evaluating blood oxygen level dependent (BOLD) signal activity between 86 FreeSurfer-based gray matter regions.
“Functional connectivity is a statistical correlation (Pearson’s correlation coefficient) between two time series of BOLD signals measured on two distinct region of interest of the brain during MRI scan,” Dr. Tozlu explained. “In our study, BOLD time series were measured using resting-state functional MRI technique that last 7 minutes.”
The ensemble model was able to classify low-adapting MS patients with an area under ROC curve (AUC) of 0.638 and a balanced accuracy of 0.659. The model performed well in accurately classifying the MS patients with clinically significant impairment with a sensitivity of 0.719.
“The models in which we applied functional and structural connectivity showed a high performance in classifying MS patients regarding their impairment level,” Dr. Tozlu said.
She noted that “these models may be extended to predict change in impairment level in a longitudinal study, for instance, identifying MS patients who may have a clinically significant impairment.”
In further evaluating which particular functional connections were most related to MS disease activity, Dr. Tozlu and colleagues found the most discriminative areas were between the right superior parietal and right inferior temporal, between right lateral occipital and left pericalcarine, and between right pericalcarine and right side of frontal pole.
If further validated, the approach could have important, broader clinical implications, Dr. Tozlu said.
“If the validation of these models on a larger dataset is successful, this model may be used to decide for personalized treatment,” Dr. Tozlu added. “The model could offer guidance in providing more powerful treatment for MS patients who may have a clinically significant impairment and less powerful treatment for MS patients who may not have a clinically significant impairment in order to avoid the side effects of treatments.
“Therefore, we believe that dynamics in functional connectivity should be taken into account in the next studies in MS.”
In commenting on the research, Eric Klawiter, MD, associate professor of neurology, Harvard Medical School and associate neurologist at Massachusetts General Hospital, both in Boston, said the findings offer valuable insights in the use of machine learning and MS imaging.
“This research shows very nicely the power of machine learning and connectivity techniques to differentiate MS phenotypes based on disability level,” he said in an interview.
“The future direction of this work is to develop predictive markers for disability progression and this would have significant impact in how we evaluate newly diagnosed patients and counsel their treatment decisions.”
Dr. Tozlu and Dr. Klawiter had no disclosures to report.
SOURCE: Tozlu C et al. ACTRIMS Forum 2020. Abstract P025.
WEST PALM BEACH, FLA. – A machine learning model that combines data on the brain’s functional connectivity with clinical information such as age, sex and disease duration shows the potential to provide an accurate assessment of clinical impairment in patients with multiple sclerosis (MS).
“This is the first study to show that dynamic functional connectivity is useful to identify the impairment level in MS, and can be used for personalized treatment by clinicians,” first author Ceren Tozlu, PhD, of Weill Cornell Medicine, New York, said in an interview.
“We found out that structural connectivity is the most important feature that distinguishes MS patients from healthy controls, while dynamic functional connectivity was more discriminative compared to the static functional connectivity in MS patient classification regarding their impairment level.”
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Statistical assessment of the clinical impairment of MS using MRI is hindered by a relatively weak correlation between the impairment and disease burden, such as lesion load.
However, the brain’s functional connectivity network, which is indicative of the disruption of the transmission of signals of gray matter regions, could provide a deeper understanding of connectome-level mechanisms that underlie variability in MS-related impairments, Dr. Tozlu and colleagues say.
With no previous study pulling together multimodal imaging data including static and dynamic functional connectivity to classify MS patients with a clinically significant impairment versus non–clinically significant impairment, Dr. Tozlu and the team sought to build a machine-learning–based model to do so.
For the study, they enrolled 79 patients with MS, including 42 with Expanded Disability Status scores of 2 or higher, representing clinically significant impairment at baseline.
The patients, who had a mean age of 45 years, were 66% female and had a mean disease duration of 12.48 years. The ensemble model that was used incorporated functional connectivity and a clinical dataset of age, sex, and disease duration. Functional connectivity was measured by evaluating blood oxygen level dependent (BOLD) signal activity between 86 FreeSurfer-based gray matter regions.
“Functional connectivity is a statistical correlation (Pearson’s correlation coefficient) between two time series of BOLD signals measured on two distinct region of interest of the brain during MRI scan,” Dr. Tozlu explained. “In our study, BOLD time series were measured using resting-state functional MRI technique that last 7 minutes.”
The ensemble model was able to classify low-adapting MS patients with an area under ROC curve (AUC) of 0.638 and a balanced accuracy of 0.659. The model performed well in accurately classifying the MS patients with clinically significant impairment with a sensitivity of 0.719.
“The models in which we applied functional and structural connectivity showed a high performance in classifying MS patients regarding their impairment level,” Dr. Tozlu said.
She noted that “these models may be extended to predict change in impairment level in a longitudinal study, for instance, identifying MS patients who may have a clinically significant impairment.”
In further evaluating which particular functional connections were most related to MS disease activity, Dr. Tozlu and colleagues found the most discriminative areas were between the right superior parietal and right inferior temporal, between right lateral occipital and left pericalcarine, and between right pericalcarine and right side of frontal pole.
If further validated, the approach could have important, broader clinical implications, Dr. Tozlu said.
“If the validation of these models on a larger dataset is successful, this model may be used to decide for personalized treatment,” Dr. Tozlu added. “The model could offer guidance in providing more powerful treatment for MS patients who may have a clinically significant impairment and less powerful treatment for MS patients who may not have a clinically significant impairment in order to avoid the side effects of treatments.
“Therefore, we believe that dynamics in functional connectivity should be taken into account in the next studies in MS.”
In commenting on the research, Eric Klawiter, MD, associate professor of neurology, Harvard Medical School and associate neurologist at Massachusetts General Hospital, both in Boston, said the findings offer valuable insights in the use of machine learning and MS imaging.
“This research shows very nicely the power of machine learning and connectivity techniques to differentiate MS phenotypes based on disability level,” he said in an interview.
“The future direction of this work is to develop predictive markers for disability progression and this would have significant impact in how we evaluate newly diagnosed patients and counsel their treatment decisions.”
Dr. Tozlu and Dr. Klawiter had no disclosures to report.
SOURCE: Tozlu C et al. ACTRIMS Forum 2020. Abstract P025.
REPORTING FROM ACTRIMS FORUM 2020
Key clinical point:
Major finding: The model classified low-adapting MS patients with an ROC curve (AUC) of 0.638 and a balanced accuracy of 0.659.
Study details: Modeling study based on 79 patients with MS, including low adapters.
Disclosures: Dr. Tozlu and Dr. Klawiter had no disclosures to report.
Source: Tozlu C et al. ACTRIMS Forum 2020. Abstract P025.
Inebilizumab benefits patients with NMOSD
WEST PALM BEACH, FLA. – Compared with placebo, inebilizumab reduces the risk of attacks, the risk of disability worsening, the number of hospitalizations, and the number of new MRI lesions in patients with neuromyelitis optica spectrum disorder (NMOSD), according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The drug’s efficacy was sustained for one year in the study, and the treatment had an acceptable safety profile.
“Multiple lines of evidence suggest that NMO is predominantly a B-cell–mediated disorder,” said Bruce Cree, MD, PhD, professor of neurology at the University of California, San Francisco, Weill Institute for Neurosciences. Inebilizumab depletes B cells and reduces inflammatory disease activity in NMO potentially by altering immune networks that are dependent on B cells for cytokine production or antigen presentation.” Inebilizumab is an anti-CD19 monoclonal antibody.
Dr. Cree and colleagues conducted a randomized, controlled trial called N-MOmentum to characterize the long-term efficacy and safety of inebilizumab in patients with NMOSD. The investigators randomized patients with NMOSD to inebilizumab or placebo monotherapy in a 3:1 ratio for 6.5 months. The study’s primary outcome was the time to the first adjudicated attack. Patients who had an adjudicated attack or completed the trial could receive inebilizumab in an ongoing open-label extension.
The study was conducted at 99 sites in 25 countries. In all, 230 patients were randomized and dosed (174 received inebilizumab, and 56 received placebo). About 91% of the population was aquaporin-4-IgG–positive (AQP4-IgG+), and 91% was female. The population’s mean age at baseline was 43 years. The population’s median baseline Expanded Disability Status Scale score was approximately 3.5, and the range was from 0 to 8.0. Approximately 50% of participants were white, 20% were Asian, and 9% were of African descent. “The demographic profile is similar to that of many published studies,” said Dr. Cree.
Because of clear evidence of efficacy, the independent data monitoring committee recommended that the study be stopped early. In the randomized, controlled trial, inebilizumab reduced the risk of attack by 77.3% among AQP4-IgG+ patients and by 72.8% in the total population. The number needed to treat for 6.5 months to prevent one attack was 3.2 for the AQP4-IgG+ group and 3.7 for the total population.
Furthermore, inebilizumab significantly reduced the risk of worsening disability, the number of new MRI lesions, and NMOSD-related hospitalizations. After 1 year on inebilizumab, 85% of patients were free of an NMOSD attack. In safety analyses that combined data from the randomized, controlled trial and interim data from the open-label extension, the mean duration of inebilizumab treatment was 1.5 years.
“The rapid effect of inebilizumab on attack prevention is not mediated by decreasing AQP4-IgG, although it is possible that long-term inebilizumab treatment might eventually reduce AQP4-IgG production,” said Dr. Cree.
The most common adverse events (AEs) were urinary tract infection (UTI, 19.6%), nasopharyngitis (12.9%), and infusion-related reactions (IRRs, 11.6%). IRRs were most common with the first infusion. The proportion of inebilizumab-treated patients with IgG levels below the lower limit of normal was 7.5% at 1 year and 13.4% at 2 years. Serious AEs occurred in 12% of patients, and UTI was the most common (2.2%). Two patients died in the open-label extension; one death resulted from NMOSD, and one from new presumed inflammatory brain lesions of undetermined etiology.
“The open-label results show a striking durability of treatment effect,” said Dr. Cree. “Most of the attacks that occurred during the open-label extension occurred early on, suggesting that the risk of attack decreases with duration of B-cell depletion. The open-label study also is important for assessing the longer-term AE profile of inebilizumab treatment. One potentially important observation from the open-label extension is that the extent of B-cell depletion correlates with reduced attack risk. Approximately 95% of participants with deep B-cell depletion were free of attacks. Participants who either incompletely depleted B cells or who began to reconstitute B cells more rapidly were at increased risk of attack. Therefore, by monitoring B-cell counts in inebilizumab-treated patients, it may be possible to further reduce the risk of attack in patients who partially deplete, or replete B cells early, with an extra inebilizumab treatment.”
Viela Bio, which is developing inebilizumab, and MedImmune sponsored the study. Dr. Cree has received compensation for consulting services that he provided to Alexion, Atara, Biogen, EMD Serono, Novartis, and TG Therapeutics.
SOURCE: Cree BA et al. ACTRIMS 2020. Abstract P207.
WEST PALM BEACH, FLA. – Compared with placebo, inebilizumab reduces the risk of attacks, the risk of disability worsening, the number of hospitalizations, and the number of new MRI lesions in patients with neuromyelitis optica spectrum disorder (NMOSD), according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The drug’s efficacy was sustained for one year in the study, and the treatment had an acceptable safety profile.
“Multiple lines of evidence suggest that NMO is predominantly a B-cell–mediated disorder,” said Bruce Cree, MD, PhD, professor of neurology at the University of California, San Francisco, Weill Institute for Neurosciences. Inebilizumab depletes B cells and reduces inflammatory disease activity in NMO potentially by altering immune networks that are dependent on B cells for cytokine production or antigen presentation.” Inebilizumab is an anti-CD19 monoclonal antibody.
Dr. Cree and colleagues conducted a randomized, controlled trial called N-MOmentum to characterize the long-term efficacy and safety of inebilizumab in patients with NMOSD. The investigators randomized patients with NMOSD to inebilizumab or placebo monotherapy in a 3:1 ratio for 6.5 months. The study’s primary outcome was the time to the first adjudicated attack. Patients who had an adjudicated attack or completed the trial could receive inebilizumab in an ongoing open-label extension.
The study was conducted at 99 sites in 25 countries. In all, 230 patients were randomized and dosed (174 received inebilizumab, and 56 received placebo). About 91% of the population was aquaporin-4-IgG–positive (AQP4-IgG+), and 91% was female. The population’s mean age at baseline was 43 years. The population’s median baseline Expanded Disability Status Scale score was approximately 3.5, and the range was from 0 to 8.0. Approximately 50% of participants were white, 20% were Asian, and 9% were of African descent. “The demographic profile is similar to that of many published studies,” said Dr. Cree.
Because of clear evidence of efficacy, the independent data monitoring committee recommended that the study be stopped early. In the randomized, controlled trial, inebilizumab reduced the risk of attack by 77.3% among AQP4-IgG+ patients and by 72.8% in the total population. The number needed to treat for 6.5 months to prevent one attack was 3.2 for the AQP4-IgG+ group and 3.7 for the total population.
Furthermore, inebilizumab significantly reduced the risk of worsening disability, the number of new MRI lesions, and NMOSD-related hospitalizations. After 1 year on inebilizumab, 85% of patients were free of an NMOSD attack. In safety analyses that combined data from the randomized, controlled trial and interim data from the open-label extension, the mean duration of inebilizumab treatment was 1.5 years.
“The rapid effect of inebilizumab on attack prevention is not mediated by decreasing AQP4-IgG, although it is possible that long-term inebilizumab treatment might eventually reduce AQP4-IgG production,” said Dr. Cree.
The most common adverse events (AEs) were urinary tract infection (UTI, 19.6%), nasopharyngitis (12.9%), and infusion-related reactions (IRRs, 11.6%). IRRs were most common with the first infusion. The proportion of inebilizumab-treated patients with IgG levels below the lower limit of normal was 7.5% at 1 year and 13.4% at 2 years. Serious AEs occurred in 12% of patients, and UTI was the most common (2.2%). Two patients died in the open-label extension; one death resulted from NMOSD, and one from new presumed inflammatory brain lesions of undetermined etiology.
“The open-label results show a striking durability of treatment effect,” said Dr. Cree. “Most of the attacks that occurred during the open-label extension occurred early on, suggesting that the risk of attack decreases with duration of B-cell depletion. The open-label study also is important for assessing the longer-term AE profile of inebilizumab treatment. One potentially important observation from the open-label extension is that the extent of B-cell depletion correlates with reduced attack risk. Approximately 95% of participants with deep B-cell depletion were free of attacks. Participants who either incompletely depleted B cells or who began to reconstitute B cells more rapidly were at increased risk of attack. Therefore, by monitoring B-cell counts in inebilizumab-treated patients, it may be possible to further reduce the risk of attack in patients who partially deplete, or replete B cells early, with an extra inebilizumab treatment.”
Viela Bio, which is developing inebilizumab, and MedImmune sponsored the study. Dr. Cree has received compensation for consulting services that he provided to Alexion, Atara, Biogen, EMD Serono, Novartis, and TG Therapeutics.
SOURCE: Cree BA et al. ACTRIMS 2020. Abstract P207.
WEST PALM BEACH, FLA. – Compared with placebo, inebilizumab reduces the risk of attacks, the risk of disability worsening, the number of hospitalizations, and the number of new MRI lesions in patients with neuromyelitis optica spectrum disorder (NMOSD), according to a study presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The drug’s efficacy was sustained for one year in the study, and the treatment had an acceptable safety profile.
“Multiple lines of evidence suggest that NMO is predominantly a B-cell–mediated disorder,” said Bruce Cree, MD, PhD, professor of neurology at the University of California, San Francisco, Weill Institute for Neurosciences. Inebilizumab depletes B cells and reduces inflammatory disease activity in NMO potentially by altering immune networks that are dependent on B cells for cytokine production or antigen presentation.” Inebilizumab is an anti-CD19 monoclonal antibody.
Dr. Cree and colleagues conducted a randomized, controlled trial called N-MOmentum to characterize the long-term efficacy and safety of inebilizumab in patients with NMOSD. The investigators randomized patients with NMOSD to inebilizumab or placebo monotherapy in a 3:1 ratio for 6.5 months. The study’s primary outcome was the time to the first adjudicated attack. Patients who had an adjudicated attack or completed the trial could receive inebilizumab in an ongoing open-label extension.
The study was conducted at 99 sites in 25 countries. In all, 230 patients were randomized and dosed (174 received inebilizumab, and 56 received placebo). About 91% of the population was aquaporin-4-IgG–positive (AQP4-IgG+), and 91% was female. The population’s mean age at baseline was 43 years. The population’s median baseline Expanded Disability Status Scale score was approximately 3.5, and the range was from 0 to 8.0. Approximately 50% of participants were white, 20% were Asian, and 9% were of African descent. “The demographic profile is similar to that of many published studies,” said Dr. Cree.
Because of clear evidence of efficacy, the independent data monitoring committee recommended that the study be stopped early. In the randomized, controlled trial, inebilizumab reduced the risk of attack by 77.3% among AQP4-IgG+ patients and by 72.8% in the total population. The number needed to treat for 6.5 months to prevent one attack was 3.2 for the AQP4-IgG+ group and 3.7 for the total population.
Furthermore, inebilizumab significantly reduced the risk of worsening disability, the number of new MRI lesions, and NMOSD-related hospitalizations. After 1 year on inebilizumab, 85% of patients were free of an NMOSD attack. In safety analyses that combined data from the randomized, controlled trial and interim data from the open-label extension, the mean duration of inebilizumab treatment was 1.5 years.
“The rapid effect of inebilizumab on attack prevention is not mediated by decreasing AQP4-IgG, although it is possible that long-term inebilizumab treatment might eventually reduce AQP4-IgG production,” said Dr. Cree.
The most common adverse events (AEs) were urinary tract infection (UTI, 19.6%), nasopharyngitis (12.9%), and infusion-related reactions (IRRs, 11.6%). IRRs were most common with the first infusion. The proportion of inebilizumab-treated patients with IgG levels below the lower limit of normal was 7.5% at 1 year and 13.4% at 2 years. Serious AEs occurred in 12% of patients, and UTI was the most common (2.2%). Two patients died in the open-label extension; one death resulted from NMOSD, and one from new presumed inflammatory brain lesions of undetermined etiology.
“The open-label results show a striking durability of treatment effect,” said Dr. Cree. “Most of the attacks that occurred during the open-label extension occurred early on, suggesting that the risk of attack decreases with duration of B-cell depletion. The open-label study also is important for assessing the longer-term AE profile of inebilizumab treatment. One potentially important observation from the open-label extension is that the extent of B-cell depletion correlates with reduced attack risk. Approximately 95% of participants with deep B-cell depletion were free of attacks. Participants who either incompletely depleted B cells or who began to reconstitute B cells more rapidly were at increased risk of attack. Therefore, by monitoring B-cell counts in inebilizumab-treated patients, it may be possible to further reduce the risk of attack in patients who partially deplete, or replete B cells early, with an extra inebilizumab treatment.”
Viela Bio, which is developing inebilizumab, and MedImmune sponsored the study. Dr. Cree has received compensation for consulting services that he provided to Alexion, Atara, Biogen, EMD Serono, Novartis, and TG Therapeutics.
SOURCE: Cree BA et al. ACTRIMS 2020. Abstract P207.
REPORTING FROM ACTRIMS FORUM 2020
Eculizumab reduces relapse-related hospitalizations in patients with NMOSD
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that eculizumab may have a favorable effect on health-resource utilization.
Many patients with NMOSD, a rare autoimmune inflammatory disease, have relapses that result in hospitalization. Eculizumab (Soliris)is a humanized monoclonal antibody that inhibits the terminal complement protein C5. In the randomized, double-blind, placebo-controlled PREVENT study, Dean M. Wingerchuk, MD, chair of neurology at Mayo Clinic in Phoenix, , and colleagues found that eculizumab was associated with a 94% reduction in the risk of relapse, compared with placebo, in AQP4-IgG-positive patients with NMOSD.
In a new analysis of the PREVENT data, the investigators sought to evaluate rates of relapse-related hospitalization and associated treatment among study participants. The researchers chose time to first adjudicated relapse as their primary endpoint.
In the PREVENT study, Dr. Wingerchuk and colleagues randomized patients with AQP4-IgG-positive NMOSD to eculizumab (1,200 mg/2 weeks) or placebo. The annualized relapse-related hospitalization and treatment rates were calculated as the number of relapses requiring hospitalization or treatment divided by the total number of patient-years during the study.
Approximately 91% of participants were female. Mean age at initial clinical presentation was about 37 years. Participants’ median Expanded Disability Status Scale score at baseline was 4, and their mean annualized relapse rate in the 24 months before screening was 2. In all, 96 patients received eculizumab, and 47 received placebo. The median length of exposure to treatment was 89.4 weeks in the eculizumab group and 41.3 weeks among controls.
The rate of adverse events requiring hospitalization was 29% in the eculizumab group and 53% in the placebo group. The most common events requiring hospitalization were physician-determined relapses. Infections were the next most common events requiring hospitalization.
The overall annualized hospitalization rate was 0.26 in the eculizumab group and 0.78 in the placebo group. The difference between groups was statistically significant. In addition, the annualized relapse-related hospitalization rate was lower in the eculizumab group than in the placebo group (0.04 vs. 0.31, respectively).
The annualized relapse-related use of intravenous methylprednisolone for the eculizumab and placebo groups were 0.07 and 0.42, respectively; for use of plasma exchange, 0.02 and 0.19; and for use of high-dose oral corticosteroids, 0.04 and 0.11. The differences between groups in use of intravenous methylprednisolone and plasma exchange were statistically significant.
Alexion Pharmaceuticals, which markets eculizumab, sponsored the study. Dr. Wingerchuk received grants from Alexion during the study. He also received personal fees from Biogen, BrainStorm Therapeutics, Celgene, MedImmune, Novartis, and ONO Pharmaceutical.
SOURCE: Kim H et al. ACTRIMS 2020. Abstract P197.
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that eculizumab may have a favorable effect on health-resource utilization.
Many patients with NMOSD, a rare autoimmune inflammatory disease, have relapses that result in hospitalization. Eculizumab (Soliris)is a humanized monoclonal antibody that inhibits the terminal complement protein C5. In the randomized, double-blind, placebo-controlled PREVENT study, Dean M. Wingerchuk, MD, chair of neurology at Mayo Clinic in Phoenix, , and colleagues found that eculizumab was associated with a 94% reduction in the risk of relapse, compared with placebo, in AQP4-IgG-positive patients with NMOSD.
In a new analysis of the PREVENT data, the investigators sought to evaluate rates of relapse-related hospitalization and associated treatment among study participants. The researchers chose time to first adjudicated relapse as their primary endpoint.
In the PREVENT study, Dr. Wingerchuk and colleagues randomized patients with AQP4-IgG-positive NMOSD to eculizumab (1,200 mg/2 weeks) or placebo. The annualized relapse-related hospitalization and treatment rates were calculated as the number of relapses requiring hospitalization or treatment divided by the total number of patient-years during the study.
Approximately 91% of participants were female. Mean age at initial clinical presentation was about 37 years. Participants’ median Expanded Disability Status Scale score at baseline was 4, and their mean annualized relapse rate in the 24 months before screening was 2. In all, 96 patients received eculizumab, and 47 received placebo. The median length of exposure to treatment was 89.4 weeks in the eculizumab group and 41.3 weeks among controls.
The rate of adverse events requiring hospitalization was 29% in the eculizumab group and 53% in the placebo group. The most common events requiring hospitalization were physician-determined relapses. Infections were the next most common events requiring hospitalization.
The overall annualized hospitalization rate was 0.26 in the eculizumab group and 0.78 in the placebo group. The difference between groups was statistically significant. In addition, the annualized relapse-related hospitalization rate was lower in the eculizumab group than in the placebo group (0.04 vs. 0.31, respectively).
The annualized relapse-related use of intravenous methylprednisolone for the eculizumab and placebo groups were 0.07 and 0.42, respectively; for use of plasma exchange, 0.02 and 0.19; and for use of high-dose oral corticosteroids, 0.04 and 0.11. The differences between groups in use of intravenous methylprednisolone and plasma exchange were statistically significant.
Alexion Pharmaceuticals, which markets eculizumab, sponsored the study. Dr. Wingerchuk received grants from Alexion during the study. He also received personal fees from Biogen, BrainStorm Therapeutics, Celgene, MedImmune, Novartis, and ONO Pharmaceutical.
SOURCE: Kim H et al. ACTRIMS 2020. Abstract P197.
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The results suggest that eculizumab may have a favorable effect on health-resource utilization.
Many patients with NMOSD, a rare autoimmune inflammatory disease, have relapses that result in hospitalization. Eculizumab (Soliris)is a humanized monoclonal antibody that inhibits the terminal complement protein C5. In the randomized, double-blind, placebo-controlled PREVENT study, Dean M. Wingerchuk, MD, chair of neurology at Mayo Clinic in Phoenix, , and colleagues found that eculizumab was associated with a 94% reduction in the risk of relapse, compared with placebo, in AQP4-IgG-positive patients with NMOSD.
In a new analysis of the PREVENT data, the investigators sought to evaluate rates of relapse-related hospitalization and associated treatment among study participants. The researchers chose time to first adjudicated relapse as their primary endpoint.
In the PREVENT study, Dr. Wingerchuk and colleagues randomized patients with AQP4-IgG-positive NMOSD to eculizumab (1,200 mg/2 weeks) or placebo. The annualized relapse-related hospitalization and treatment rates were calculated as the number of relapses requiring hospitalization or treatment divided by the total number of patient-years during the study.
Approximately 91% of participants were female. Mean age at initial clinical presentation was about 37 years. Participants’ median Expanded Disability Status Scale score at baseline was 4, and their mean annualized relapse rate in the 24 months before screening was 2. In all, 96 patients received eculizumab, and 47 received placebo. The median length of exposure to treatment was 89.4 weeks in the eculizumab group and 41.3 weeks among controls.
The rate of adverse events requiring hospitalization was 29% in the eculizumab group and 53% in the placebo group. The most common events requiring hospitalization were physician-determined relapses. Infections were the next most common events requiring hospitalization.
The overall annualized hospitalization rate was 0.26 in the eculizumab group and 0.78 in the placebo group. The difference between groups was statistically significant. In addition, the annualized relapse-related hospitalization rate was lower in the eculizumab group than in the placebo group (0.04 vs. 0.31, respectively).
The annualized relapse-related use of intravenous methylprednisolone for the eculizumab and placebo groups were 0.07 and 0.42, respectively; for use of plasma exchange, 0.02 and 0.19; and for use of high-dose oral corticosteroids, 0.04 and 0.11. The differences between groups in use of intravenous methylprednisolone and plasma exchange were statistically significant.
Alexion Pharmaceuticals, which markets eculizumab, sponsored the study. Dr. Wingerchuk received grants from Alexion during the study. He also received personal fees from Biogen, BrainStorm Therapeutics, Celgene, MedImmune, Novartis, and ONO Pharmaceutical.
SOURCE: Kim H et al. ACTRIMS 2020. Abstract P197.
REPORTING FROM ACTRIMS FORUM 2020
Isotretinoin data provide postmeal absorption guidance
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR