User login
Angiosarcoma Imitating a Morpheaform Basal Cell Carcinoma
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
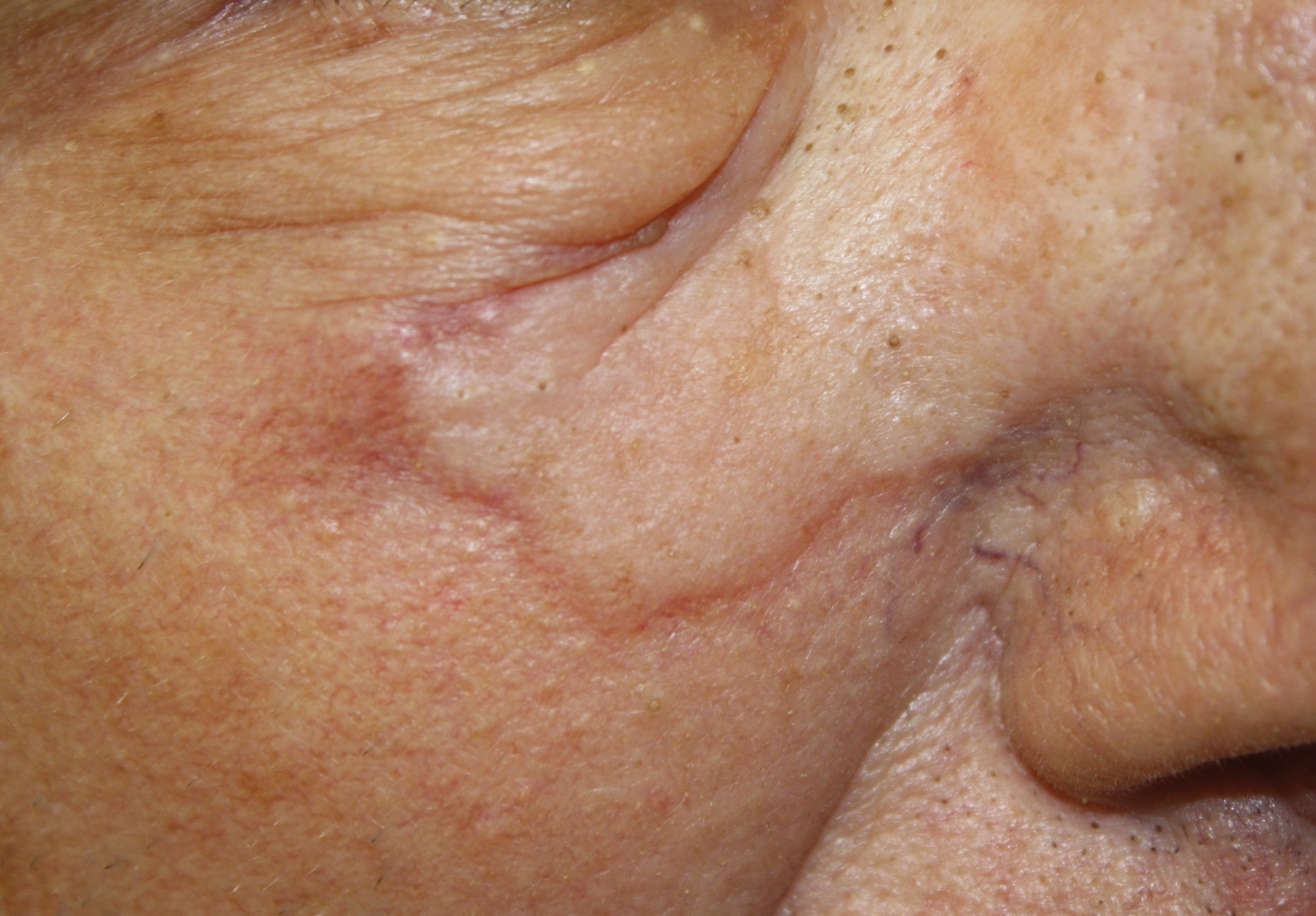
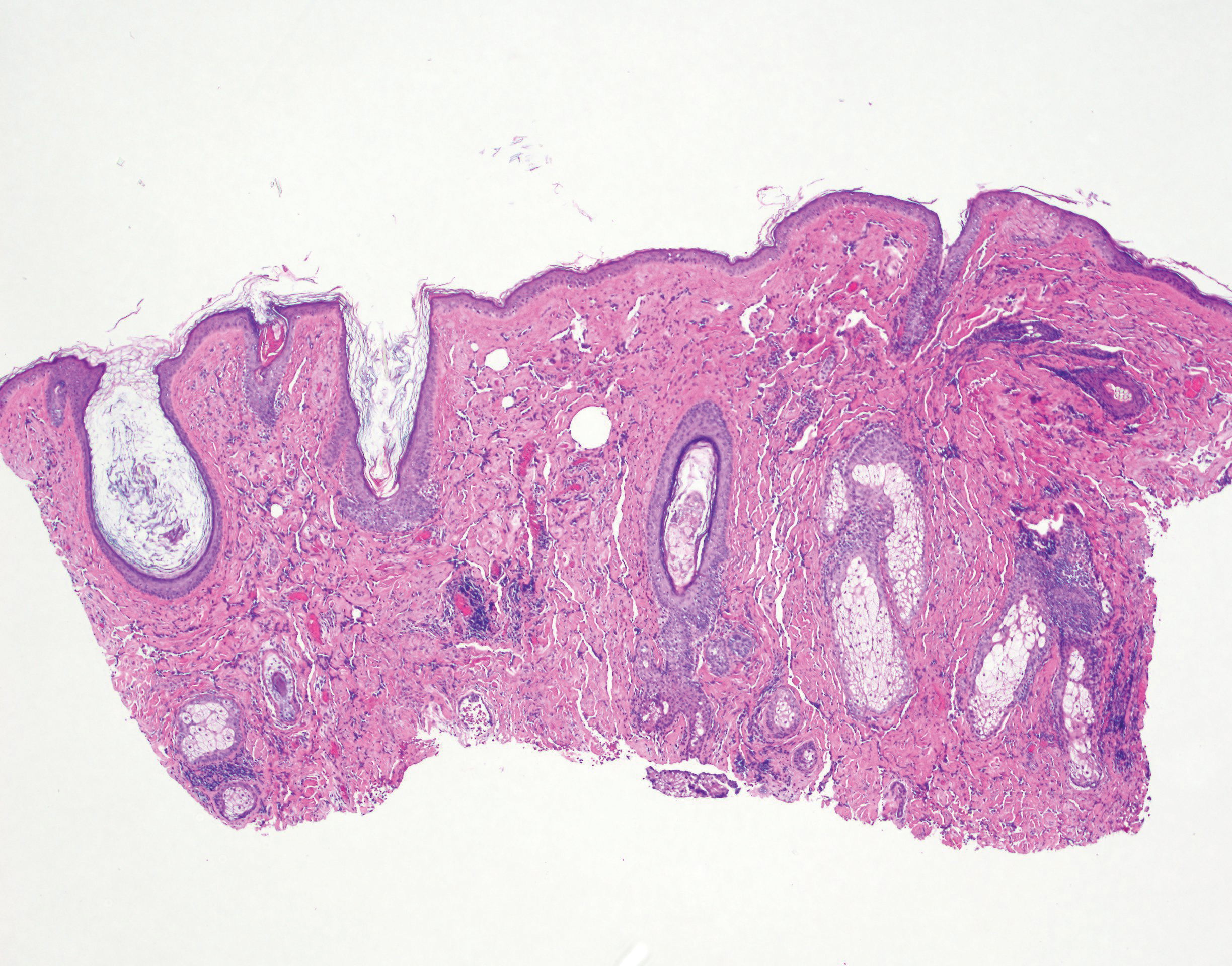
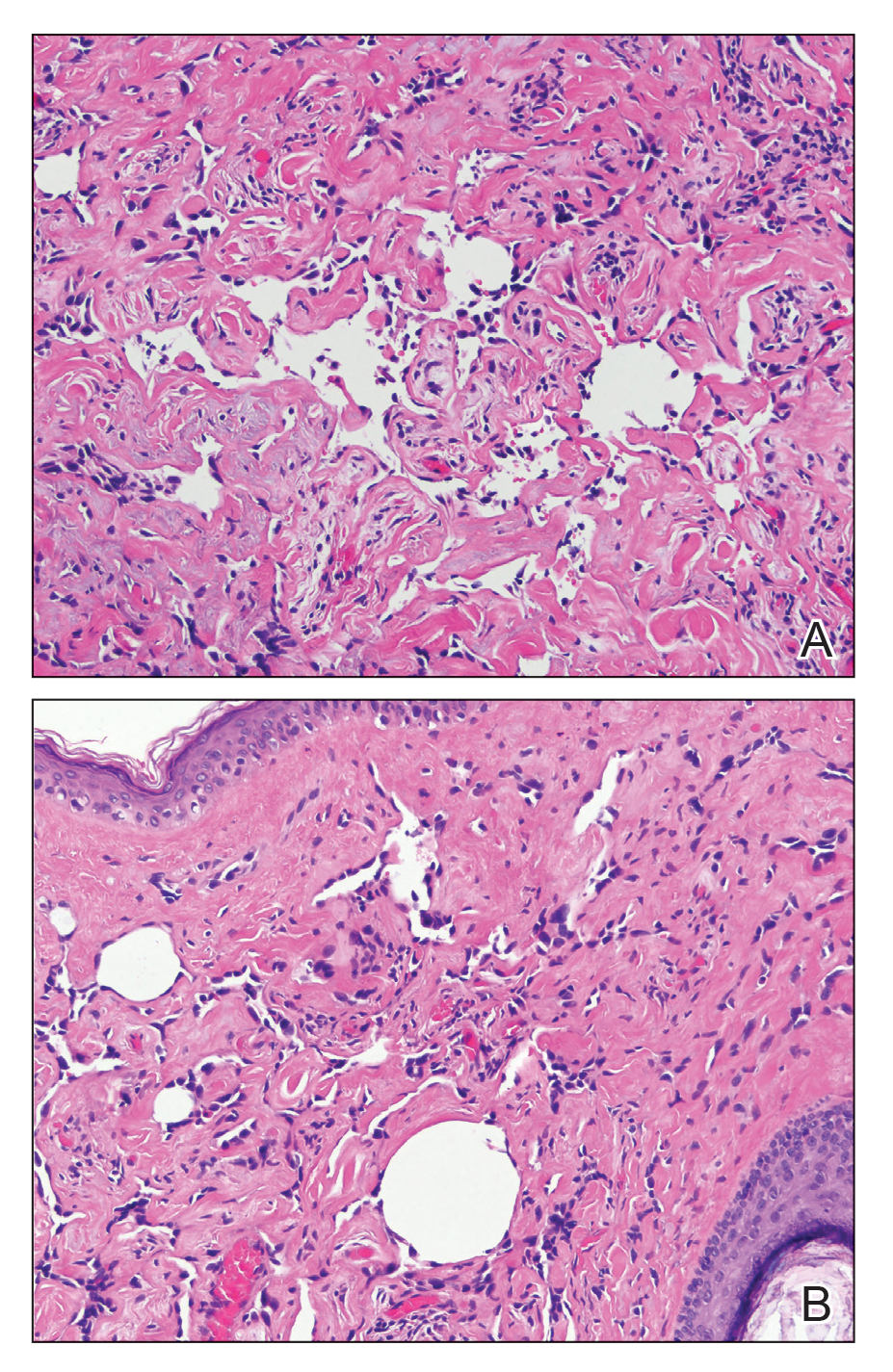
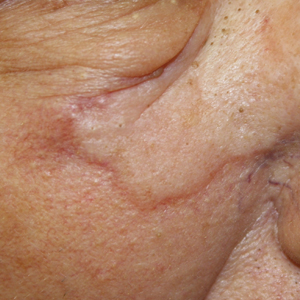
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
Practice Points
- Angiosarcoma is an aggressive vascular tumor with a poor prognosis.
- Angiosarcomas can arise in the setting of chronic lymphedema or prior radiation therapy or can arise spontaneously.
- Classically, angiosarcoma presents as a violaceous patch or plaque but occasionally can exhibit atypical clinical features. Angiosarcomas should be considered on the differential for any changing plaque on the head or neck.
Sharp climb in weight gain after breast cancer diagnosis
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Significant weight gain after a diagnosis of breast cancer may be a bigger problem than previously thought, and clinicians need to do more to help patients manage it, say the authors of a national survey conducted in Australia.
“We found that two-thirds of our respondents were currently overweight or obese,” report Carolyn Ee, MBBS, PhD, of the NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, and colleagues.
“Because weight gain after breast cancer may lead to poorer outcomes, efforts to prevent and manage weight gain must be prioritized and accelerated particularly in the first year after diagnosis,” they comment.
Their article was published online February 20 in BMC Cancer.
The 60-item anonymous online survey was sent to 1835 members of the Breast Cancer Network Australia Review and Survey Group.
Although the response rate for the online survey was only 15%, most women reported a “high” level of concern about weight gain – and with good reason. The results showed that 64% of women gained an average of 9 kg (20 lb), and 17% gained >20 kg (44 lb).
In the 2-year period following a breast cancer diagnosis, the rate of overweight and obesity increased from 48% to 67%. The rate of obesity alone almost doubled, from 17% to 32%.
Overweight and obesity are strongly implicated in the development of breast cancer, the authors note. Weight gain after diagnosis is associated with morbidity and all-cause mortality and may increase the risk for breast cancer recurrence by 30% to 40%.
The survey, which also collected data from 26 women who were participants in online women’s health and breast cancer support groups, did not include information on quality of life and levels of distress. “Additional research in this area appears to be warranted,” the study authors suggest.
In a press statement, Ee noted that 77% of women reported that weight gain had occurred in the 12- to 18-month period after diagnosis. This could provide a “window of opportunity” for intervention, she said.
“Timing may be the key in helping women to manage weight after a diagnosis of breast cancer,” she added. “Cancer services and general practitioners play an important role in having early conversations with women and referring them to a team of qualified healthcare professionals such as dieticians and exercise physiologists with experience in cancer.”
Coauthor John Boyages, MBBS, PhD, a radiation oncologist at the Icon Cancer Center, Sydney Adventist Hospital, Wahroonga, emphasized that all women should be prescribed exercise after being diagnosed with breast cancer. “Prescribing a healthy lifestyle is just as important as prescribing tablets,” he said.
“As doctors, we really need to actively think about weight, nutrition, and exercise and advise about possible interventions. [Among patients with breast cancer,] weight gain adds to self-esteem problems, increases the risk of heart disease and other cancers, and several reports suggest it may affect prognosis and also increases the risk of lymphedema,” he added
“More effort needs to be geared to treating the whole body, as we know obesity is a risk factor for poorer outcomes when dealing with breast cancer,” commented Stephanie Bernik, MD, chief of breast service at Mount Sinai West and associate professor of surgery at the Icahn School of Medicine at Mount Sinai in New York City. She was not involved in the study and was approached for comment.
Berwick also agreed that meeting with a nutritionist or receiving weight loss support is helpful for patients with cancer, but she added that not all cancer centers have the resources to provide these services.
Survey Details
Of 309 women who responded to the survey, complete data for pre- and post-diagnosis body mass index (BMI) were collected from 277 respondents, representing 15% of those surveyed.
Of these women, 254 had been diagnosed with stage I-III breast cancer; 33 had been diagnosed with ductal carcinoma in situ. The mean age of the patients was 59 years.
The results showed that for 20% of women, BMI increased from a healthy weight range at the time of diagnosis (BMI <25) to an unhealthy weight range (BMI >25). In addition, for 4.8% of patients, BMI increased from an overweight range (BMI 25 to <30) to obesity (BMI >30), and 60.7% reported an increase in BMI >1 kg/m2. Conversely, only a small proportion of women lost weight – 6% experienced a decrease of more than one BMI category.
Weight gain occurred within the first 2 years of diagnosis in 87% of women and within the first 12 months in 58%. In women who gained >10 kg (22 lb), 78% said they were highly concerned about it, as did 59% of women who gained >5 kg (11 lb).
Among all age groups (35 to 74 years), 69% experienced excess weight gain that was 0.48 kg higher each year compared with age-matched control persons who had not been diagnosed with breast cancer. Over 5 years, this represented an additional weight gain of 2 kg (5 lb) among women with breast cancer.
When approached for comment, Bernick agreed with the authors that these results should be interpreted with caution.
She pointed to the self-reporting bias and the fact that only 15% of women responded to the survey. “Perhaps it was only women who had gained weight who found it worthwhile reporting their experience with weight gain after a breast cancer diagnosis,” she suggested.
Even so, there are many reasons why weight gain during treatment for breast cancer presents a problem for women in the United States as well as Australia, Bernik told Medscape Medical News.
“Women undergoing chemotherapy may not have the energy to keep up with exercise regimens and may find eating food comforting,” she pointed out. “Because chemotherapy delivery and the after effects may take up a few days out of every 2 to 3 weeks, women have less time and energy to eat correctly or exercise. Furthermore, women sometimes get steroids while receiving chemotherapy, and this is known to drive up one’s appetite.”
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
‘Promising’ responses with preoperative immunotherapy in oral cavity cancer
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
Preoperative nivolumab, with or without ipilimumab, appeared safe and effective in a phase 2 trial of patients with oral cavity squamous cell carcinoma (OCSCC).
“We found that nivolumab, with or without ipilimumab, was feasible prior to surgery in patients with oral cavity cancers, with no delays in surgery observed,” said Jonathan D. Schoenfeld, MD, of the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“We did observe promising rates of volumetric and pathologic response, with near-complete and complete responses observed, particularly in the nivo-ipi arm.”
Dr. Schoenfeld presented these results at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
“The rationale behind evaluation of neoadjuvant immunotherapy is the potential of tumor downstaging, converting unresectable disease to resectable and inducing durable immunological memory as a result of exposure to the full breadth of tumor antigens preoperatively,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
“This randomized, phase 2 window study ... found that treatment with two neoadjuvant cycles of nivolumab alone or along with ipilimumab during the first cycle was feasible from an adverse events perspective and led to volumetric responses in approximately 50% of patients.”
Patients and treatment
The trial (NCT02919683) enrolled 30 patients with OCSCC, but 1 patient was excluded due to metastases at baseline. Patients had T2 (n = 20) or greater (n = 9) disease at baseline, and 58% of patients (n = 17) had node-positive disease.
The patients were randomized to two cycles of nivolumab (3 mg/kg) or nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg with the first cycle). Patients underwent surgery 3-7 days after completing cycle 2.
In the nivolumab monotherapy arm (n = 14), the median age was 64.4 years (range, 39.1-81 years), and 71.4% of patients were men. Oral tongue was the primary tumor site in 50% of patients, and 50% of patients had stage IV disease.
In the nivolumab-ipilimumab arm (n = 15), the median age was 65.2 years (range, 32.5-78.4 years), and 53.3% of patients were men. Oral tongue was the primary tumor site in 60% of patients, and 73.3% of patients had stage IV disease.
Safety and tolerability
Six patients did not receive the full cycle 2 dose of immunotherapy, two in the nivolumab arm and four in the nivolumab-ipilimumab arm. This was most commonly due to an infusion reaction during cycle 2, Dr. Schoenfeld said.
There were no cases in which surgery was delayed. However, one patient did have surgery moved to an earlier date after cycle 1 because of concerns about progression.
There were three severe immune-related adverse events. In the nivolumab-ipilimumab arm, there was a case of grade 3 pneumonitis and a case of grade 3 colitis. Both of these events were reversible with treatment.
In the nivolumab monotherapy arm, one patient had grade 4 new-onset diabetes with diabetic ketoacidosis. This patient is still insulin dependent.
Perioperative adverse events in both arms included pulmonary embolism, postoperative hematoma, and flap failures (n = 2). One patient with flap failure also had a perioperative stroke, experienced progressive clinical decline, and ultimately died.
Response and survival
In the nivolumab monotherapy arm, 50% of patients (n = 7) had a volumetric response, and 8% (n = 1) had a pathologic complete response.
In the nivolumab-ipilimumab arm, 53% of patients (n = 8) had a volumetric response, and 20% (n = 3) had a pathologic complete response.
“In general, we found that our response metrics were concordant; that is, patients with volumetric responses tended more frequently to have pathologic responses,” Dr. Schoenfeld said. “There were a couple notable cases where there were volumetric increases and significant pathologic responses.”
To identify factors associated with response, Dr. Schoenfeld and colleagues performed correlative multiplex immunofluorescence on 21 patient specimens prior to treatment.
“We did not identify any differences in baseline levels of PD-L1 expression in tumor cells between the two arms,” Dr. Schoenfeld noted. “We found that CD4-positive T cells in the pretreatment specimens correlated with pathologic response [P = .016]. Interestingly, this association was only significant in patients treated with nivo-ipi [P = .008] but not nivolumab alone [P = .83].”
Ten patients went on to receive radiation, and nine received chemoradiation. One patient presented to the operating room but did not undergo surgery because he was thought to require total glossectomy. This patient received chemoradiotherapy, achieved a complete response, and is still disease free after more than 3 years of follow-up.
The median follow-up for the entire cohort was 14 months. At 12 months, the progression-free survival rate was 85%, and the overall survival rate was 89%.
Dr. Schoenfeld noted that this study was not powered to assess survival or to directly compare nivolumab monotherapy and nivolumab plus ipilimumab.
‘Encouraging’ results, but what’s next?
“We were very encouraged by the toxicity data ... [and] the impressive pathologic responses in both arms, but particularly in the nivo-ipi arm,” Dr. Schoenfeld said. “I think the real question is, ‘Does this translate into a significant progression-free or overall survival advantage?’ So I think that would be something worthy of further study.”
“These results are encouraging for management of patients with oral cavity cancers who remain at high risk for recurrence with the current standard of care but will need validation in larger prospective studies,” Dr. Sehgal noted. “Multiple clinical trials are currently ongoing to evaluate the role of neoadjuvant immunotherapy for disease-specific outcomes, notable being phase 2 NCT02296684 and phase 3 KEYNOTE-689 (NCT03765918) with pembrolizumab and phase 3 IMSTAR-HN (NCT03700905) with nivolumab alone or along with ipilimumab.”
The current study was funded by Bristol-Myers Squibb. Dr. Schoenfeld disclosed relationships with Bristol-Myers Squibb and other companies. Dr. Sehgal had no relevant conflicts to disclose.
SOURCE: Schoenfeld J et al. Head and Neck Cancers Symposium 2020, Abstract 1.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
Can this patient get IV contrast?
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
Mortality sevenfold higher post TAVR with severe kidney injury
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Acute kidney injury (AKI), a potentially modifiable risk factor in some cases, predicts increased mortality within the first year after transcatheter aortic valve transplantation (TAVR), according to an analysis of a U.S. registry presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“After adjustment, there are higher rates of all-cause mortality regardless of the severity of AKI,” reported Howard M. Julien, MD, of the University of Pennsylvania, Philadelphia.
Relative to the absence of AKI (stage 0), the hazard ratio for death at 1 year was more than threefold greater (HR, 3.26), even for those with stage 1 AKI. When unadjusted for covariates, it remained more than twice as high (HR, 2.67; P less than .001), Dr. Julien reported.
For stage 3 AKI, the unadjusted risk was more than nine times higher and remained roughly seven times greater after adjustment (HR, 7.04; P less than .001). Stage 2 AKI was linked with an adjusted risk of about the same magnitude.
Drawn from the National Cardiovascular TAVR Registry, which is maintained jointly by the Society of Thoracic Surgeons and the American College of Cardiology, data were analyzed on more than 100,000 TAVRs performed during 2012-2018. A subset of TAVRs performed between January 2016 and June 2018 served as a source of trends in what Dr. Julien described as the “modern era” of this procedure.
The incidence of AKI overall was about 10%, but rates were higher at the earliest time point in the analysis and fell modestly over the study period for all three stages. In a logistic regression analysis, the factors associated with the greatest odds ratio of developing AKI in patients following TAVR were conversion to open heart surgery (OR, 10.84, P less than .001), nonfemoral access (OR, 2.33; P less than .001), anemia (OR, 1.90; P less than .001), general versus moderate sedation (OR, 1.62; P less than .001), diabetes (OR, 1.61; P less than .001), and cardiogenic shock within 24 hours (OR, 1.60; P less than .023).
Other factors with a significant but lower relative risk association with AKI included a high contrast volume (OR, 1.004; P less than .001), use of a self-expanding valve (HR, 1.22; P = .009), severe lung disease (OR, 1.21; P = .043) and prior peripheral artery disease (HR, 1.20; P = .043).
“The message from these data is that there appears to be a cluster of patients who are unstable at the time of their procedure and are more likely to develop the most severe forms of AKI,” Dr. Julien reported.
The higher rate of AKI in patients who have diabetes is “not surprising,” but several of the factors associated with AKI are potentially modifiable. This includes choices in regard to sedation and arterial access. The value of modifying the amount of contrast is less clear, because the volume of contrast was no longer significant after an adjustment with multivariate analysis.
In fact, all of these factors require validation. Dr. Julien warned that neither the cause of AKI nor its temporal relationship to TAVR could be consistently determined from the registry data. In addition, retrospective analyses always include the potential for unrecognized residual confounders.
Still, these data are useful for drawing attention to the fact that AKI is a common complication of TAVR and one that is associated with adverse outcomes, including reduced survival at 1 year.
“The factors taken from these data might be useful to help identify patients who are at risk of the most severe forms of AKI and, hopefully, lead to prevention strategies that take these characteristics into consideration,” Dr. Julien said.
Dr. Julien reported no potential financial conflicts of interest.
REPORTING FROM CRT 2020
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
HLA-B27 status predicts radiographic phenotype of axSpA
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
The presence of HLA-B27 may predict the radiographic phenotype of patients with axial spondyloarthritis (axSpA), according to recent research.
The findings suggest HLA-B27-positive patients have worse radiographic damage, more typical marginal syndesmophytes, and a greater number of bilateral fused sacroiliac joints in the spine, reported Laura C. Coates, MBChB, PhD, of the University of Oxford (England) and colleagues. Their report was published in Arthritis Care & Research.
“In order to achieve phenotypic diversity, we studied patients with PsA [psoriatic arthritis] and axial involvement (a group of patients recognized to have less frequent carriage of HLA-B27), and AS [ankylosing spondylitis],” they wrote.
The researchers conducted a multicenter, cross-sectional cohort study involving 198 patients with AS and 244 with PsA. Various clinical, radiographic, and laboratory data were collected from databases in Ireland, Spain, Germany, Russia, Canada, and Italy.
HLA-B27-positive patients were older (mean 49.1 years vs. 53.8 years), were more often male (73% vs. 59%), and had longer disease duration (mean 13.6 years vs. 11.0 years).
The team compared HLA-B27 carriers and noncarriers on syndesmophyte morphology, the symmetry of the sacroiliac joints and syndesmophytes, in addition to radiographic damage, as measured by the modified Stoke Ankylosing spondylitis spinal score (mSASSS) and PsA Spondylitis Radiology Index (PASRI).
After analysis, the researchers found that HLA-B27 positivity was associated with higher median mSASSS (6 vs. 2; P = .04) and PASRI scores (12 vs. 6; P less than .0001), marginal syndesmophytes (odds ratio, 1.97; 95% confidence interval, 1.16-3.36), and syndesmophyte symmetry (OR, 3.02; 95% CI, 1.38-6.61).
“[Our] study [showed] no difference in sacroiliac symmetry, and no difference in nonmarginal syndesmophytes, according to HLA-B27 status,” they reported.
In addition, they reported that male sex (OR, 1.66; 95% CI, 1.04-2.66) and age (OR, 1.08; 95% CI, 1.05-1.10) were positive predictors of marginal syndesmophytes.
In contrast, only male sex (OR, 2.55; 95% CI, 1.46-4.64) and age (OR, 1.05; 95% CI, 1.03-1.07) predicted the presence of nonmarginal syndesmophytes.
The researchers acknowledged that two key limitations of the study were the absence of disease-group matching and lack of independent central reading of radiographs.
“This analysis suggests less difference in radiographic phenotype between AS and axial PsA than previously found but emphasizes the importance of HLA-B27 status in severity and the phenotypic expression of disease radiographically,” they concluded.
The study was funded by the Academy of Medical Sciences (U.K.). The authors reported having no conflicts of interest.
SOURCE: Coates LC et al. Arthritis Care Res. 2020 Feb 26. doi: 10.1002/acr.24174.
FROM ARTHRITIS CARE & RESEARCH
Meta-analysis highlights safety concerns with interleukin inhibition
MAUI, HAWAII – The use of interleukin inhibitors for treatment of rheumatologic diseases doubles a patient’s risk of serious infections, according to a comprehensive systematic review and meta-analysis of 74 randomized, placebo-controlled trials presented by Jawad Bilal, MBBS, at the 2020 Rheumatology Winter Clinical Symposium.
The meta-analysis, which incorporated 29,214 patients with a variety of rheumatic diseases, demonstrated that patients receiving interleukin (IL) inhibitors had a 1.97-fold increased risk of serious infections, a finding accompanied by a high degree of statistical certainty. The number-needed-to-harm was 67 patients treated for a median of 24 weeks in order to generate one additional serious infection.
“That number-needed-to-harm is a significant finding because having a serious infection means by definition you’re getting admitted to the hospital and receiving IV antibiotics,” Dr. Bilal observed in an interview.
The meta-analysis also found that IL inhibition was associated with a 2.35-fold increased risk of opportunistic infections and a 1.52-fold higher risk of developing cancer, both findings with statistical significance (P =.03) but only moderate certainty because fewer of those events were captured in the trials compared to the numbers of serious infections, explained Dr. Bilal of the University of Arizona, Tucson.
For opportunistic infections, the number-needed-to-harm was 250 patients treated with an IL inhibitor for a median of 54 weeks in order to result in one additional opportunistic infection. For cancer, the number-needed-to-harm was 250 for a median of 24 weeks.
Dr. Bilal noted that while the IL inhibitors are drugs of established efficacy in rheumatologic diseases, their safety has not previously undergone anything approaching the comprehensive scrutiny carried out in this meta-analysis. The meta-analysis, which included all published placebo-controlled randomized trials and their extension studies, employed rigorous methodology in accord with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement and the GRADE approach to data analysis. Studies of IL inhibitors in patients with dermatologic and GI diseases were excluded from the meta-analysis.
He offered a caveat regarding the cancer risk findings: “Our analysis showed that the cancer risk is increased, but the results are not conclusive because we only had a few years of data. With cancer, you really need at least 8-10 years of data. So the real-world experience with the interleukin inhibitors in the large registries is what’s going to tell if the cancer risk is really increased or not. In the meantime, we all have to be cautious.”
The number of serious infections collected in the meta-analysis afforded sufficient statistical power for the investigators to break down differential risks based on individual drugs and indications. Among the drugs associated with significantly increased risk of serious infections were anakinra, with an odds ratio of 2.67, compared with placebo; secukinumab with an OR of 2.43; and tocilizumab with an OR or 1.76. Ustekinumab and ixekizumab were associated with 2.57- and 3.89-fold increased risks, respectively, but the number of rheumatology patients treated with those two biologics wasn’t large enough for those findings to achieve statistical significance.
Rheumatoid arthritis patients who received an IL inhibitor rather than placebo had a 1.98-fold increased risk of serious infection, while those with psoriatic arthritis had a 2.21-fold increased risk. Patients treated for SLE had a 6.44-fold increased risk, and those with juvenile idiopathic arthritis had a 5.37-fold higher risk, but the margins for error were such that those results weren’t statistically significant.
“I think this study is going to help clinicians and patients when they’re trying to weigh the risks and benefits of IL inhibitors, especially if they already have risk factors, like a recent history of serious infection or a history of cancer or of opportunistic infection,” Dr. Bilal commented.
A study limitation was that he and his coinvestigators had to lump together the various IL inhibitors in order to gain statistical power, even though the drugs work differently, he noted.
Dr. Bilal reported having no financial conflicts regarding his study, the full details of which have been published (JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.13102).
MAUI, HAWAII – The use of interleukin inhibitors for treatment of rheumatologic diseases doubles a patient’s risk of serious infections, according to a comprehensive systematic review and meta-analysis of 74 randomized, placebo-controlled trials presented by Jawad Bilal, MBBS, at the 2020 Rheumatology Winter Clinical Symposium.
The meta-analysis, which incorporated 29,214 patients with a variety of rheumatic diseases, demonstrated that patients receiving interleukin (IL) inhibitors had a 1.97-fold increased risk of serious infections, a finding accompanied by a high degree of statistical certainty. The number-needed-to-harm was 67 patients treated for a median of 24 weeks in order to generate one additional serious infection.
“That number-needed-to-harm is a significant finding because having a serious infection means by definition you’re getting admitted to the hospital and receiving IV antibiotics,” Dr. Bilal observed in an interview.
The meta-analysis also found that IL inhibition was associated with a 2.35-fold increased risk of opportunistic infections and a 1.52-fold higher risk of developing cancer, both findings with statistical significance (P =.03) but only moderate certainty because fewer of those events were captured in the trials compared to the numbers of serious infections, explained Dr. Bilal of the University of Arizona, Tucson.
For opportunistic infections, the number-needed-to-harm was 250 patients treated with an IL inhibitor for a median of 54 weeks in order to result in one additional opportunistic infection. For cancer, the number-needed-to-harm was 250 for a median of 24 weeks.
Dr. Bilal noted that while the IL inhibitors are drugs of established efficacy in rheumatologic diseases, their safety has not previously undergone anything approaching the comprehensive scrutiny carried out in this meta-analysis. The meta-analysis, which included all published placebo-controlled randomized trials and their extension studies, employed rigorous methodology in accord with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement and the GRADE approach to data analysis. Studies of IL inhibitors in patients with dermatologic and GI diseases were excluded from the meta-analysis.
He offered a caveat regarding the cancer risk findings: “Our analysis showed that the cancer risk is increased, but the results are not conclusive because we only had a few years of data. With cancer, you really need at least 8-10 years of data. So the real-world experience with the interleukin inhibitors in the large registries is what’s going to tell if the cancer risk is really increased or not. In the meantime, we all have to be cautious.”
The number of serious infections collected in the meta-analysis afforded sufficient statistical power for the investigators to break down differential risks based on individual drugs and indications. Among the drugs associated with significantly increased risk of serious infections were anakinra, with an odds ratio of 2.67, compared with placebo; secukinumab with an OR of 2.43; and tocilizumab with an OR or 1.76. Ustekinumab and ixekizumab were associated with 2.57- and 3.89-fold increased risks, respectively, but the number of rheumatology patients treated with those two biologics wasn’t large enough for those findings to achieve statistical significance.
Rheumatoid arthritis patients who received an IL inhibitor rather than placebo had a 1.98-fold increased risk of serious infection, while those with psoriatic arthritis had a 2.21-fold increased risk. Patients treated for SLE had a 6.44-fold increased risk, and those with juvenile idiopathic arthritis had a 5.37-fold higher risk, but the margins for error were such that those results weren’t statistically significant.
“I think this study is going to help clinicians and patients when they’re trying to weigh the risks and benefits of IL inhibitors, especially if they already have risk factors, like a recent history of serious infection or a history of cancer or of opportunistic infection,” Dr. Bilal commented.
A study limitation was that he and his coinvestigators had to lump together the various IL inhibitors in order to gain statistical power, even though the drugs work differently, he noted.
Dr. Bilal reported having no financial conflicts regarding his study, the full details of which have been published (JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.13102).
MAUI, HAWAII – The use of interleukin inhibitors for treatment of rheumatologic diseases doubles a patient’s risk of serious infections, according to a comprehensive systematic review and meta-analysis of 74 randomized, placebo-controlled trials presented by Jawad Bilal, MBBS, at the 2020 Rheumatology Winter Clinical Symposium.
The meta-analysis, which incorporated 29,214 patients with a variety of rheumatic diseases, demonstrated that patients receiving interleukin (IL) inhibitors had a 1.97-fold increased risk of serious infections, a finding accompanied by a high degree of statistical certainty. The number-needed-to-harm was 67 patients treated for a median of 24 weeks in order to generate one additional serious infection.
“That number-needed-to-harm is a significant finding because having a serious infection means by definition you’re getting admitted to the hospital and receiving IV antibiotics,” Dr. Bilal observed in an interview.
The meta-analysis also found that IL inhibition was associated with a 2.35-fold increased risk of opportunistic infections and a 1.52-fold higher risk of developing cancer, both findings with statistical significance (P =.03) but only moderate certainty because fewer of those events were captured in the trials compared to the numbers of serious infections, explained Dr. Bilal of the University of Arizona, Tucson.
For opportunistic infections, the number-needed-to-harm was 250 patients treated with an IL inhibitor for a median of 54 weeks in order to result in one additional opportunistic infection. For cancer, the number-needed-to-harm was 250 for a median of 24 weeks.
Dr. Bilal noted that while the IL inhibitors are drugs of established efficacy in rheumatologic diseases, their safety has not previously undergone anything approaching the comprehensive scrutiny carried out in this meta-analysis. The meta-analysis, which included all published placebo-controlled randomized trials and their extension studies, employed rigorous methodology in accord with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement and the GRADE approach to data analysis. Studies of IL inhibitors in patients with dermatologic and GI diseases were excluded from the meta-analysis.
He offered a caveat regarding the cancer risk findings: “Our analysis showed that the cancer risk is increased, but the results are not conclusive because we only had a few years of data. With cancer, you really need at least 8-10 years of data. So the real-world experience with the interleukin inhibitors in the large registries is what’s going to tell if the cancer risk is really increased or not. In the meantime, we all have to be cautious.”
The number of serious infections collected in the meta-analysis afforded sufficient statistical power for the investigators to break down differential risks based on individual drugs and indications. Among the drugs associated with significantly increased risk of serious infections were anakinra, with an odds ratio of 2.67, compared with placebo; secukinumab with an OR of 2.43; and tocilizumab with an OR or 1.76. Ustekinumab and ixekizumab were associated with 2.57- and 3.89-fold increased risks, respectively, but the number of rheumatology patients treated with those two biologics wasn’t large enough for those findings to achieve statistical significance.
Rheumatoid arthritis patients who received an IL inhibitor rather than placebo had a 1.98-fold increased risk of serious infection, while those with psoriatic arthritis had a 2.21-fold increased risk. Patients treated for SLE had a 6.44-fold increased risk, and those with juvenile idiopathic arthritis had a 5.37-fold higher risk, but the margins for error were such that those results weren’t statistically significant.
“I think this study is going to help clinicians and patients when they’re trying to weigh the risks and benefits of IL inhibitors, especially if they already have risk factors, like a recent history of serious infection or a history of cancer or of opportunistic infection,” Dr. Bilal commented.
A study limitation was that he and his coinvestigators had to lump together the various IL inhibitors in order to gain statistical power, even though the drugs work differently, he noted.
Dr. Bilal reported having no financial conflicts regarding his study, the full details of which have been published (JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.13102).
REPORTING FROM RWCS 2020
No sedation fails to improve mortality in mechanically ventilated patients
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
REPORTING FROM CCC49
Avoiding missteps in BP measurement
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.