User login
Fever, abdominal pain, and adnexal mass
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
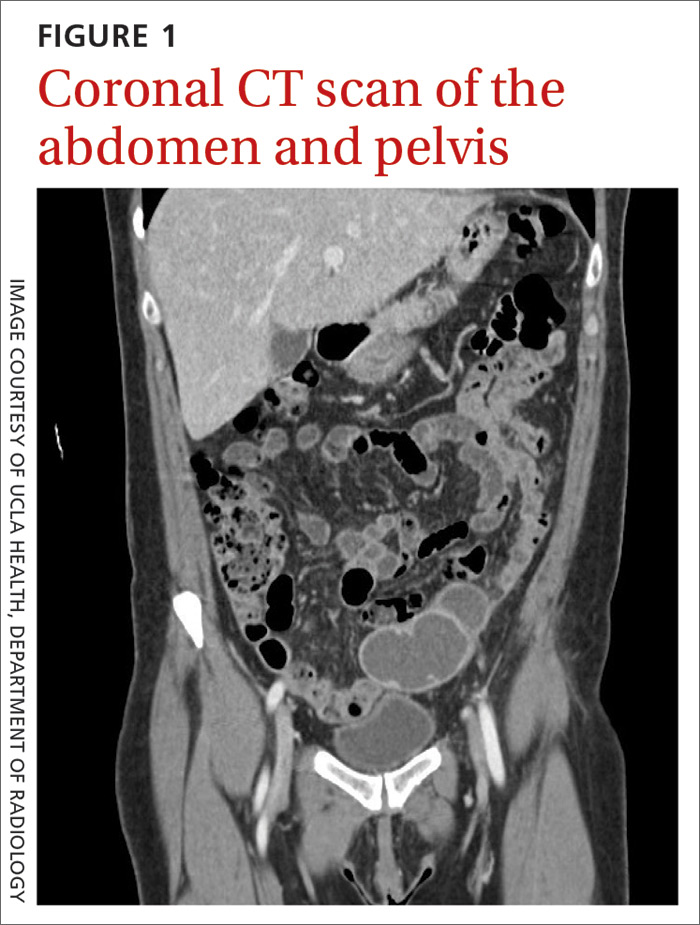
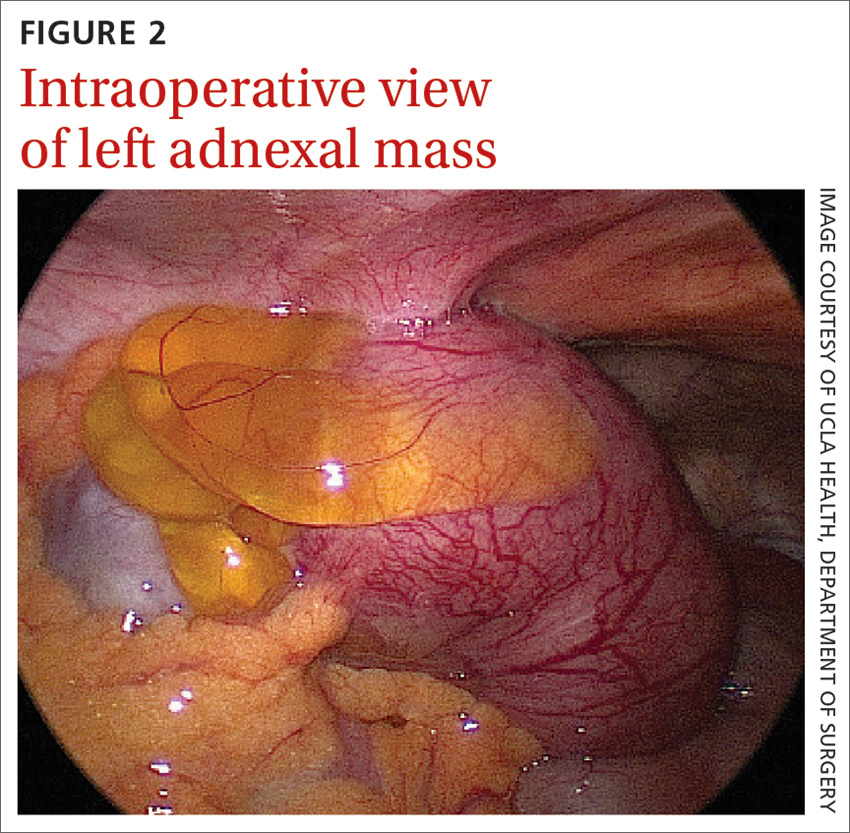
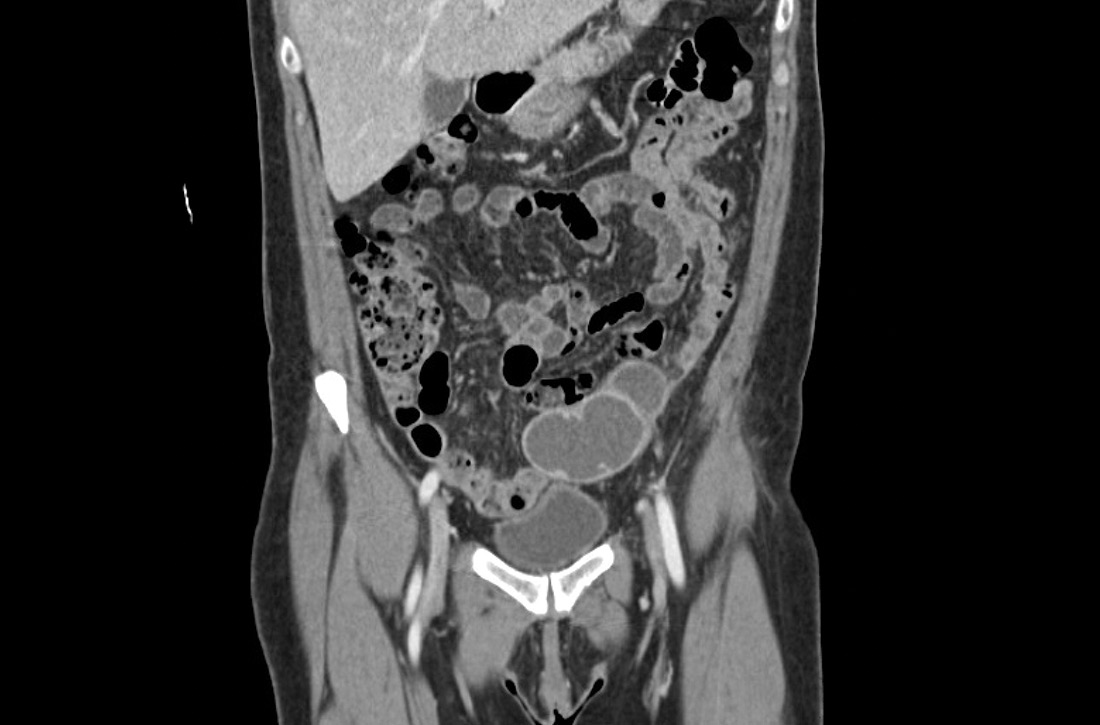
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
Conservative care or surgery for rotator cuff tears?
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
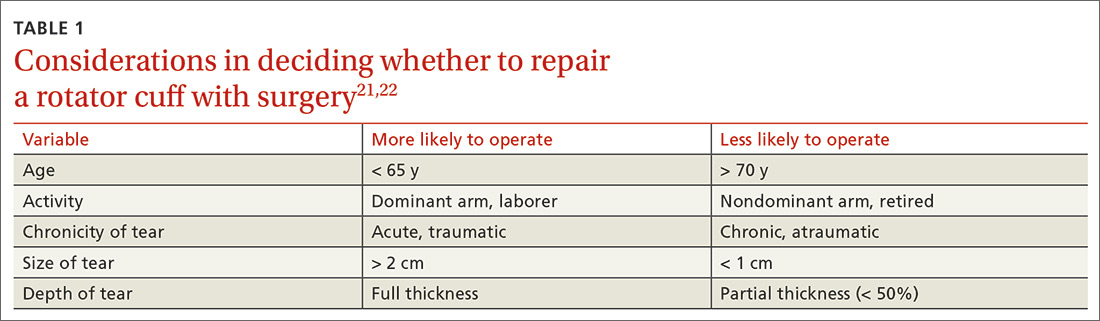
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
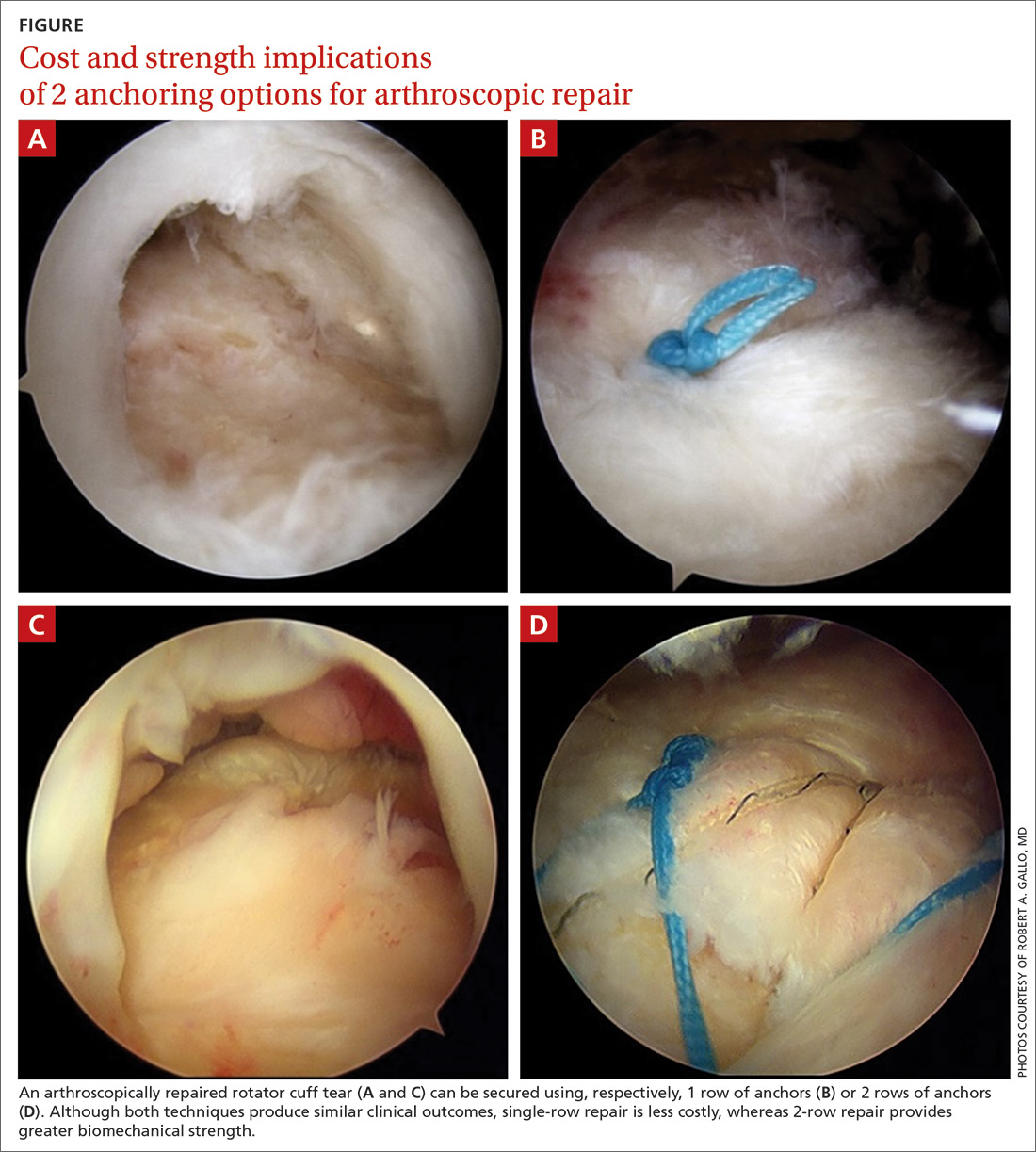
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
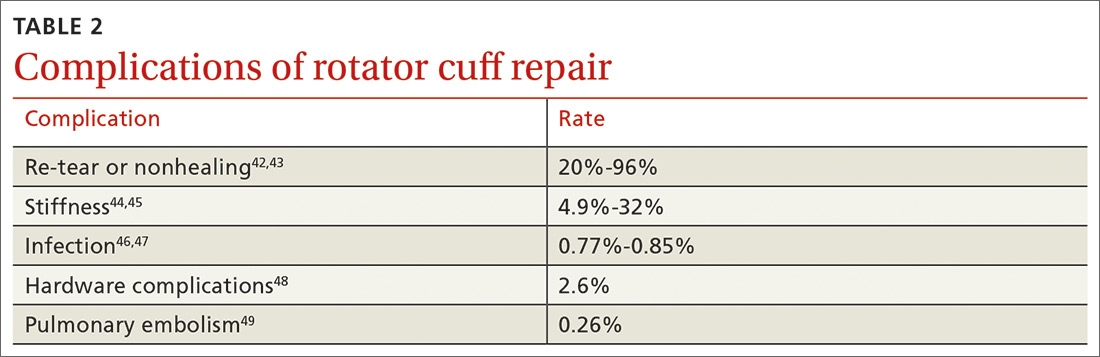
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
PRACTICE RECOMMENDATIONS
› Offer a trial of conservative management to patients with chronic, nontraumatic, or partial-thickness rotator cuff injury and to those who are poor surgical candidates. B
› Counsel patients that the rate of surgical complications is low and outcomes are favorable in properly selected patients for operative repair of rotator cuff tear. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Food insecurity: How to recognize & address it
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and
According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.
Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.
For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; lillian_amezquita@brown.edu.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and
According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.
Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.
For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; lillian_amezquita@brown.edu.
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and
According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.
Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.
For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; lillian_amezquita@brown.edu.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
PRACTICE RECOMMENDATIONS
› Consistently use the American Academy of Pediatrics 2-question survey to screen for food insecurity (all populations). A
› Identify and treat maternal depression during pregnancy and the postpartum period, and beyond A and screen for depression in older adults A because depression can reduce motivation to accomplish daily activities, such as obtaining and preparing food.
› Ask older adults about declines in performing activities of daily living C and how food is eaten or prepared . C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
HLA gene variant predicts anti-TNF antibodies in Crohn’s
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
REPORTING FROM GUILD 2020
Screen all adults for hepatitis C, says USPSTF
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
FROM JAMA
Consider toys as culprits in children with contact allergies
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
FROM CONTACT DERMATITIS
Gender imbalance seen in authorship of rheumatology guidelines
Less than one-third of first authors on rheumatology guidelines or recommendations are women, according to a research letter published in Annals of the Rheumatic Diseases.
Giovanni Adami, MD, from the department of medicine at the University of Verona (Italy), and coauthors examined 366 English-language guidelines and recommendations published between 2004 and 2019 around the world.
They found that only 32% featured a female first author. However, they did observe a significant trend toward increasing female first authorship over the study period, with parity first being achieved for guidelines and recommendations published in 2017.
Male-dominated first authorship was seen almost across the disease subject matter. For RA, only 18.8% of the 96 guidelines or recommendations examined had a female first author, and of the 12 documents on polymyalgia rheumatica and giant cell arteritis, none featured a female first author.
Among the 73 guidelines and recommendations relating to psoriatic arthritis and spondyloarthritis, only 23.3% featured a female first author. However, three of the six documents on polymyositis and dermatomyositis had a female lead author, the only area where parity was achieved.
The authors noted the recent establishment of the EULAR Task Force on Gender Equity in Academic Rheumatology, which they said was an important first step toward gender equity in rheumatology guidelines authorship.
“Indeed, in the last 15 years we have witnessed an increase in female representativeness,” they wrote. “Notwithstanding, efforts should be made to improve the representation of female authors nationally and internationally.”
Commenting on the findings, rheumatologist Jean Liew, MD, said an interesting thing is that, in the United States at least, rheumatology is not a male-dominated field.
“Even though the practicing clinicians in rheumatology, most of them are women ... at the top of things it’s not as equitable as what it should be,” said Dr. Liew, acting instructor and senior fellow in the division of rheumatology at the University of Washington, Seattle.
Dr. Liew, who coauthored another study showing a significant gender gap in speakers at American College of Rheumatology meetings, said there was evidence suggesting men were more likely to be promoted, get grants, and get positive reviews, which made it harder for women to advance to senior research and leadership positions.
She noted that the ACR has been making a concerted effort to improve gender balance in the choice of speakers for meetings, but said that the problem of gender inequity in rheumatology required more widespread initiatives to address.
“It really takes people being aware of the problem and being good sponsors and promoting women who are qualified,” she said in an interview. “There should be more mentorship and sponsorship for women, otherwise this will never change.”
She also commented that pursuing research careers in rheumatology was difficult enough without the additional pressures of family life. “It’s years and years of sacrifice, it’s hard to get funding, which already makes it harder, especially for women with families who feel like they have to also be there at home.”
The study had no outside funding, and the authors declared no competing interests.
SOURCE: Adami G et al. Ann Rheum Dis. 2020 Feb 26. doi: 10.1136/annrheumdis-2020-217119.
Less than one-third of first authors on rheumatology guidelines or recommendations are women, according to a research letter published in Annals of the Rheumatic Diseases.
Giovanni Adami, MD, from the department of medicine at the University of Verona (Italy), and coauthors examined 366 English-language guidelines and recommendations published between 2004 and 2019 around the world.
They found that only 32% featured a female first author. However, they did observe a significant trend toward increasing female first authorship over the study period, with parity first being achieved for guidelines and recommendations published in 2017.
Male-dominated first authorship was seen almost across the disease subject matter. For RA, only 18.8% of the 96 guidelines or recommendations examined had a female first author, and of the 12 documents on polymyalgia rheumatica and giant cell arteritis, none featured a female first author.
Among the 73 guidelines and recommendations relating to psoriatic arthritis and spondyloarthritis, only 23.3% featured a female first author. However, three of the six documents on polymyositis and dermatomyositis had a female lead author, the only area where parity was achieved.
The authors noted the recent establishment of the EULAR Task Force on Gender Equity in Academic Rheumatology, which they said was an important first step toward gender equity in rheumatology guidelines authorship.
“Indeed, in the last 15 years we have witnessed an increase in female representativeness,” they wrote. “Notwithstanding, efforts should be made to improve the representation of female authors nationally and internationally.”
Commenting on the findings, rheumatologist Jean Liew, MD, said an interesting thing is that, in the United States at least, rheumatology is not a male-dominated field.
“Even though the practicing clinicians in rheumatology, most of them are women ... at the top of things it’s not as equitable as what it should be,” said Dr. Liew, acting instructor and senior fellow in the division of rheumatology at the University of Washington, Seattle.
Dr. Liew, who coauthored another study showing a significant gender gap in speakers at American College of Rheumatology meetings, said there was evidence suggesting men were more likely to be promoted, get grants, and get positive reviews, which made it harder for women to advance to senior research and leadership positions.
She noted that the ACR has been making a concerted effort to improve gender balance in the choice of speakers for meetings, but said that the problem of gender inequity in rheumatology required more widespread initiatives to address.
“It really takes people being aware of the problem and being good sponsors and promoting women who are qualified,” she said in an interview. “There should be more mentorship and sponsorship for women, otherwise this will never change.”
She also commented that pursuing research careers in rheumatology was difficult enough without the additional pressures of family life. “It’s years and years of sacrifice, it’s hard to get funding, which already makes it harder, especially for women with families who feel like they have to also be there at home.”
The study had no outside funding, and the authors declared no competing interests.
SOURCE: Adami G et al. Ann Rheum Dis. 2020 Feb 26. doi: 10.1136/annrheumdis-2020-217119.
Less than one-third of first authors on rheumatology guidelines or recommendations are women, according to a research letter published in Annals of the Rheumatic Diseases.
Giovanni Adami, MD, from the department of medicine at the University of Verona (Italy), and coauthors examined 366 English-language guidelines and recommendations published between 2004 and 2019 around the world.
They found that only 32% featured a female first author. However, they did observe a significant trend toward increasing female first authorship over the study period, with parity first being achieved for guidelines and recommendations published in 2017.
Male-dominated first authorship was seen almost across the disease subject matter. For RA, only 18.8% of the 96 guidelines or recommendations examined had a female first author, and of the 12 documents on polymyalgia rheumatica and giant cell arteritis, none featured a female first author.
Among the 73 guidelines and recommendations relating to psoriatic arthritis and spondyloarthritis, only 23.3% featured a female first author. However, three of the six documents on polymyositis and dermatomyositis had a female lead author, the only area where parity was achieved.
The authors noted the recent establishment of the EULAR Task Force on Gender Equity in Academic Rheumatology, which they said was an important first step toward gender equity in rheumatology guidelines authorship.
“Indeed, in the last 15 years we have witnessed an increase in female representativeness,” they wrote. “Notwithstanding, efforts should be made to improve the representation of female authors nationally and internationally.”
Commenting on the findings, rheumatologist Jean Liew, MD, said an interesting thing is that, in the United States at least, rheumatology is not a male-dominated field.
“Even though the practicing clinicians in rheumatology, most of them are women ... at the top of things it’s not as equitable as what it should be,” said Dr. Liew, acting instructor and senior fellow in the division of rheumatology at the University of Washington, Seattle.
Dr. Liew, who coauthored another study showing a significant gender gap in speakers at American College of Rheumatology meetings, said there was evidence suggesting men were more likely to be promoted, get grants, and get positive reviews, which made it harder for women to advance to senior research and leadership positions.
She noted that the ACR has been making a concerted effort to improve gender balance in the choice of speakers for meetings, but said that the problem of gender inequity in rheumatology required more widespread initiatives to address.
“It really takes people being aware of the problem and being good sponsors and promoting women who are qualified,” she said in an interview. “There should be more mentorship and sponsorship for women, otherwise this will never change.”
She also commented that pursuing research careers in rheumatology was difficult enough without the additional pressures of family life. “It’s years and years of sacrifice, it’s hard to get funding, which already makes it harder, especially for women with families who feel like they have to also be there at home.”
The study had no outside funding, and the authors declared no competing interests.
SOURCE: Adami G et al. Ann Rheum Dis. 2020 Feb 26. doi: 10.1136/annrheumdis-2020-217119.
FROM ANNALS OF THE RHEUMATIC DISEASES
Glucocorticoid use linked to mortality in RA with diabetes
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
REPORTING FROM BMC RHEUMATOLOGY
New guideline offers recommendations for reproductive health in patients with rheumatic diseases
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
FROM ARTHRITIS & RHEUMATOLOGY
E. coli strain directly linked with CRC mutational signature
Individuals exposed to pks+ Escherichia coli may have an increased risk of colorectal cancer (CRC), which suggests that treating this genotoxic strain could potentially reduce risk of CRC, according to investigators.
While previous studies have demonstrated associations between various intestinal bacteria and CRC, this is the first study to show a direct link between exposure to a particular strain of bacteria and a unique mutational signature, reported lead author Cayetano Pleguezuelos-Manzano, of the Hubrecht Institute in Utrecht, Netherlands.
Recent studies showed that colibactin, a toxin produced by pks+ E. coli, causes a specific type of DNA damage, although the outcome of this damage remained unclear, the investigators wrote in Nature.
To look for a possible mutational signature resulting from this damage, the investigators used human intestinal organoids, which were established from primary crypt stem cells. A pks+ E. coli strain was microinjected into one group of organoids, while another E. coli strain (pks∆clbQ), which does not produce colibactin, was injected into a second group.
Immunofluorescence showed that the organoids exposed to the pks+ E. coli strain developed characteristic DNA damage, whereas the control group did not.
Next, the investigators repeatedly injected organoids with either pks+ E. coli, pks∆clbQ, or dye only. This experiment was conducted for 5 months to achieve long-term exposure. Whole genome sequencing showed that the pks+ E. coli group developed two unique mutational signatures: a single-base substitution (SBS-pks) and a small indel signature (ID-pks). Neither of the other two groups developed these signatures, which suggests that they were a direct consequence of exposure to pks+ E. coli.
To determine the prevalence of such mutational signatures in human patients, the investigators looked for the SBS-pks and ID-pks signatures in 5,876 human cancer genomes. One analysis involving 496 CRC metastases showed strong enrichment of both signatures, compared with other cancer types (P less than .0001). Another analysis involving 2,208 CRC tumors found that 5.0% and 4.4% of patients had SBS-pks and ID-pks enrichment, respectively.
“This study implies that detection and removal of pks+ E. coli, as well as re-evaluation of probiotic strains harboring the pks island, could decrease the risk of cancer in a large group of individuals,” the investigators concluded.
The study was funded by the Ministry of Education, Culture and Science of the government of the Netherlands. The investigators reported additional relationships with OrigiMed, Bayer, Janssen, and others.
SOURCE: Pleguezuelos-Manzano C et al. Nature. 2020 Feb 27. doi: 10.1038/s41586-020-2080-8.
Individuals exposed to pks+ Escherichia coli may have an increased risk of colorectal cancer (CRC), which suggests that treating this genotoxic strain could potentially reduce risk of CRC, according to investigators.
While previous studies have demonstrated associations between various intestinal bacteria and CRC, this is the first study to show a direct link between exposure to a particular strain of bacteria and a unique mutational signature, reported lead author Cayetano Pleguezuelos-Manzano, of the Hubrecht Institute in Utrecht, Netherlands.
Recent studies showed that colibactin, a toxin produced by pks+ E. coli, causes a specific type of DNA damage, although the outcome of this damage remained unclear, the investigators wrote in Nature.
To look for a possible mutational signature resulting from this damage, the investigators used human intestinal organoids, which were established from primary crypt stem cells. A pks+ E. coli strain was microinjected into one group of organoids, while another E. coli strain (pks∆clbQ), which does not produce colibactin, was injected into a second group.
Immunofluorescence showed that the organoids exposed to the pks+ E. coli strain developed characteristic DNA damage, whereas the control group did not.
Next, the investigators repeatedly injected organoids with either pks+ E. coli, pks∆clbQ, or dye only. This experiment was conducted for 5 months to achieve long-term exposure. Whole genome sequencing showed that the pks+ E. coli group developed two unique mutational signatures: a single-base substitution (SBS-pks) and a small indel signature (ID-pks). Neither of the other two groups developed these signatures, which suggests that they were a direct consequence of exposure to pks+ E. coli.
To determine the prevalence of such mutational signatures in human patients, the investigators looked for the SBS-pks and ID-pks signatures in 5,876 human cancer genomes. One analysis involving 496 CRC metastases showed strong enrichment of both signatures, compared with other cancer types (P less than .0001). Another analysis involving 2,208 CRC tumors found that 5.0% and 4.4% of patients had SBS-pks and ID-pks enrichment, respectively.
“This study implies that detection and removal of pks+ E. coli, as well as re-evaluation of probiotic strains harboring the pks island, could decrease the risk of cancer in a large group of individuals,” the investigators concluded.
The study was funded by the Ministry of Education, Culture and Science of the government of the Netherlands. The investigators reported additional relationships with OrigiMed, Bayer, Janssen, and others.
SOURCE: Pleguezuelos-Manzano C et al. Nature. 2020 Feb 27. doi: 10.1038/s41586-020-2080-8.
Individuals exposed to pks+ Escherichia coli may have an increased risk of colorectal cancer (CRC), which suggests that treating this genotoxic strain could potentially reduce risk of CRC, according to investigators.
While previous studies have demonstrated associations between various intestinal bacteria and CRC, this is the first study to show a direct link between exposure to a particular strain of bacteria and a unique mutational signature, reported lead author Cayetano Pleguezuelos-Manzano, of the Hubrecht Institute in Utrecht, Netherlands.
Recent studies showed that colibactin, a toxin produced by pks+ E. coli, causes a specific type of DNA damage, although the outcome of this damage remained unclear, the investigators wrote in Nature.
To look for a possible mutational signature resulting from this damage, the investigators used human intestinal organoids, which were established from primary crypt stem cells. A pks+ E. coli strain was microinjected into one group of organoids, while another E. coli strain (pks∆clbQ), which does not produce colibactin, was injected into a second group.
Immunofluorescence showed that the organoids exposed to the pks+ E. coli strain developed characteristic DNA damage, whereas the control group did not.
Next, the investigators repeatedly injected organoids with either pks+ E. coli, pks∆clbQ, or dye only. This experiment was conducted for 5 months to achieve long-term exposure. Whole genome sequencing showed that the pks+ E. coli group developed two unique mutational signatures: a single-base substitution (SBS-pks) and a small indel signature (ID-pks). Neither of the other two groups developed these signatures, which suggests that they were a direct consequence of exposure to pks+ E. coli.
To determine the prevalence of such mutational signatures in human patients, the investigators looked for the SBS-pks and ID-pks signatures in 5,876 human cancer genomes. One analysis involving 496 CRC metastases showed strong enrichment of both signatures, compared with other cancer types (P less than .0001). Another analysis involving 2,208 CRC tumors found that 5.0% and 4.4% of patients had SBS-pks and ID-pks enrichment, respectively.
“This study implies that detection and removal of pks+ E. coli, as well as re-evaluation of probiotic strains harboring the pks island, could decrease the risk of cancer in a large group of individuals,” the investigators concluded.
The study was funded by the Ministry of Education, Culture and Science of the government of the Netherlands. The investigators reported additional relationships with OrigiMed, Bayer, Janssen, and others.
SOURCE: Pleguezuelos-Manzano C et al. Nature. 2020 Feb 27. doi: 10.1038/s41586-020-2080-8.
FROM NATURE