User login
Second-generation long-acting injectable antipsychotics: A practical guide
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
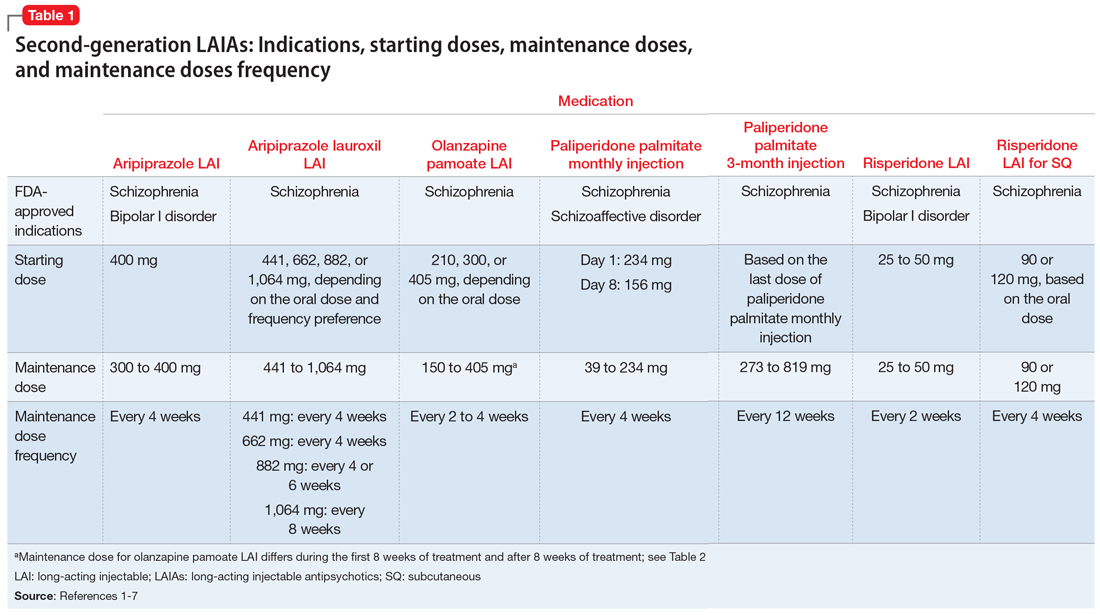
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
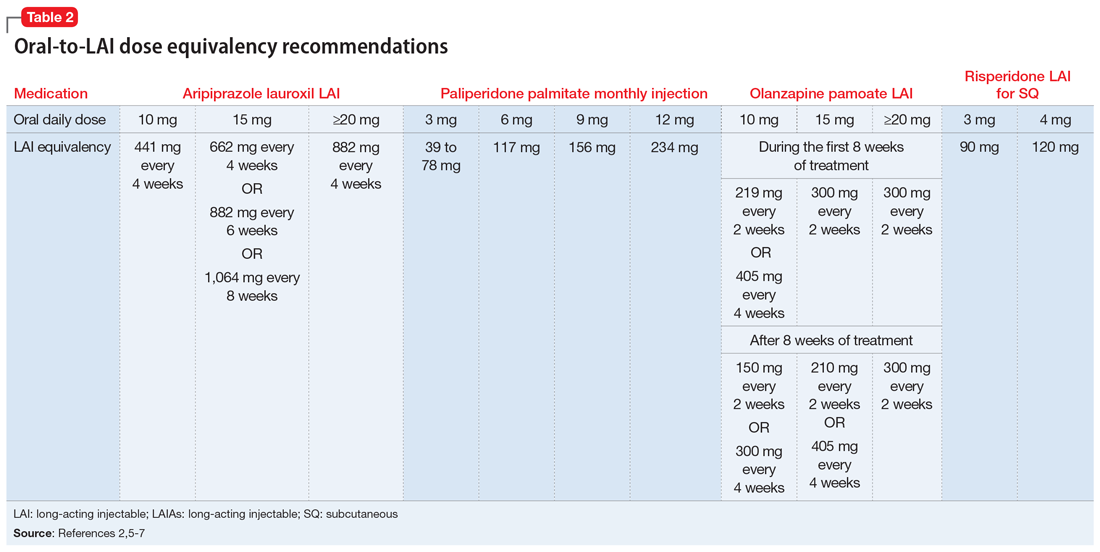
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
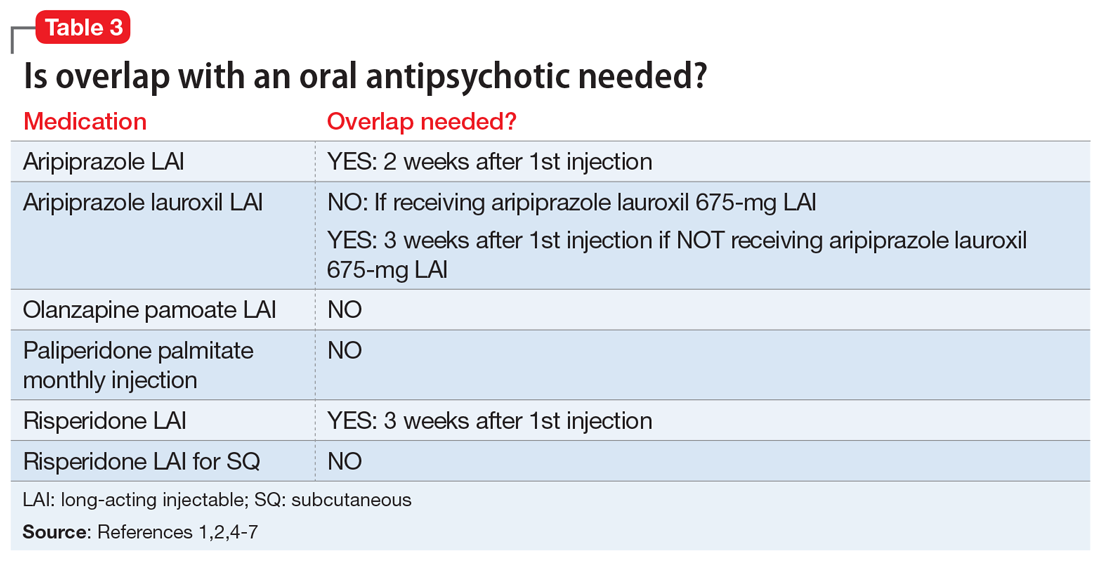
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
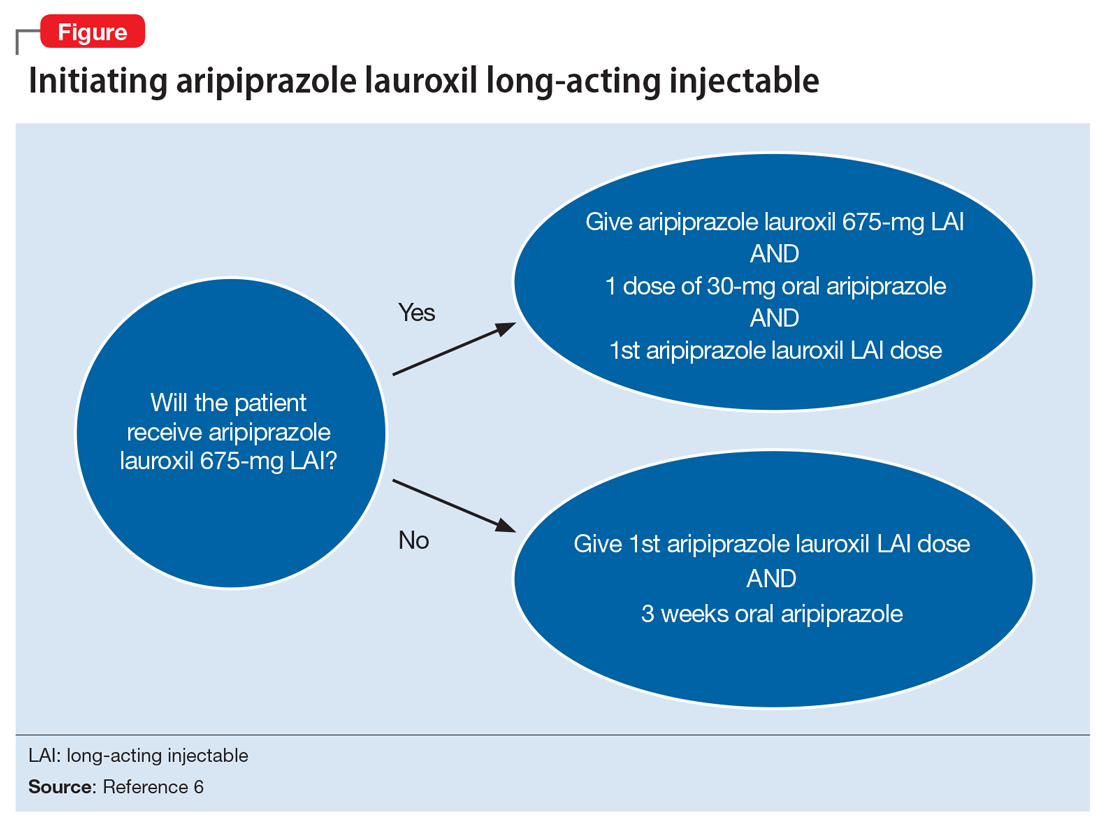
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
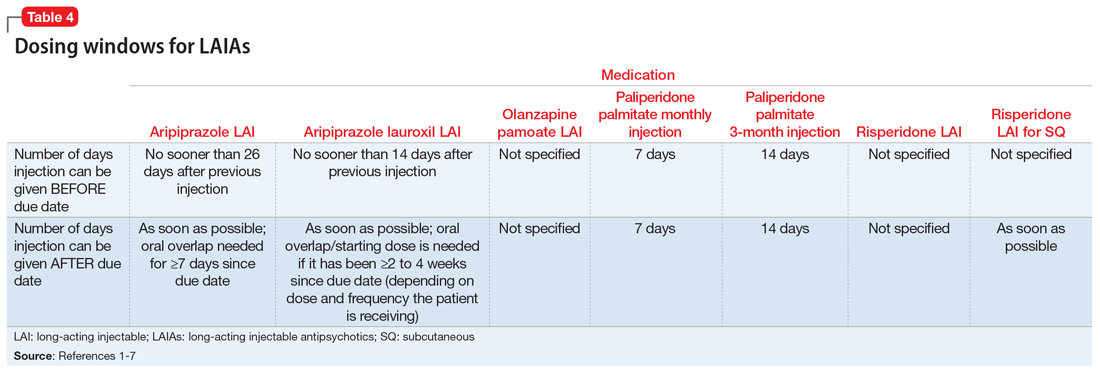
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
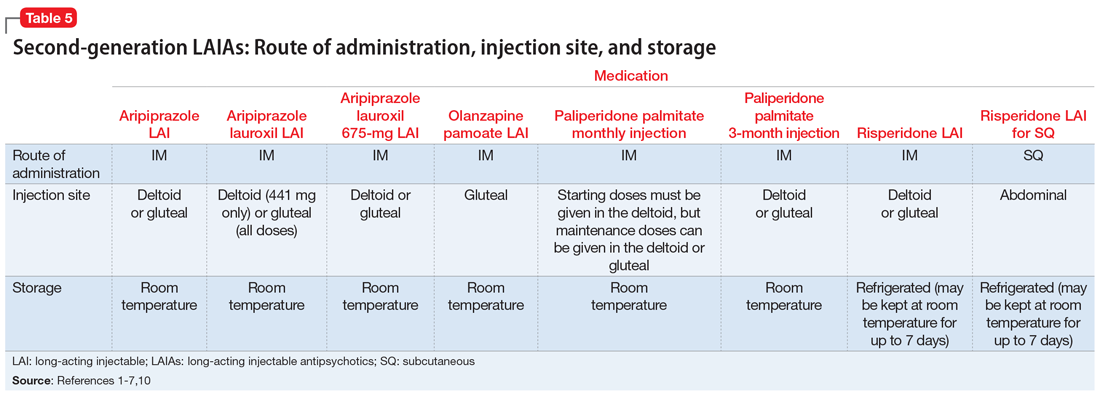
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
Kratom: What we know, what to tell your patients
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
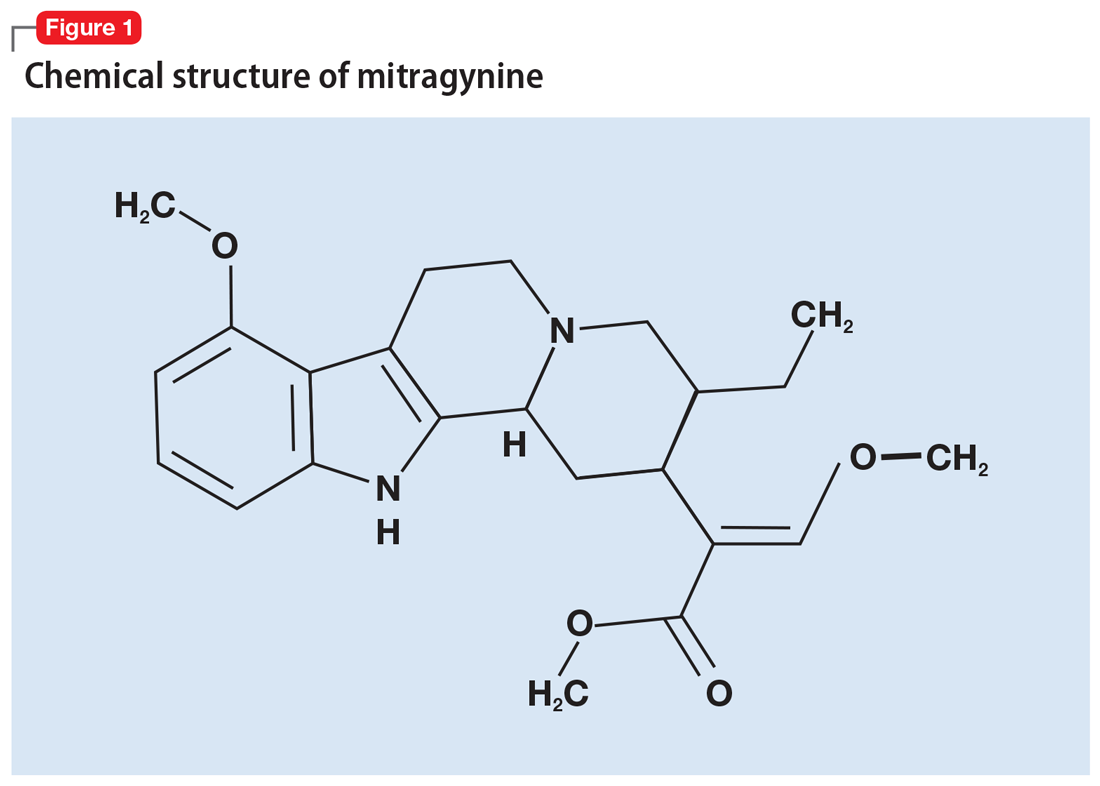
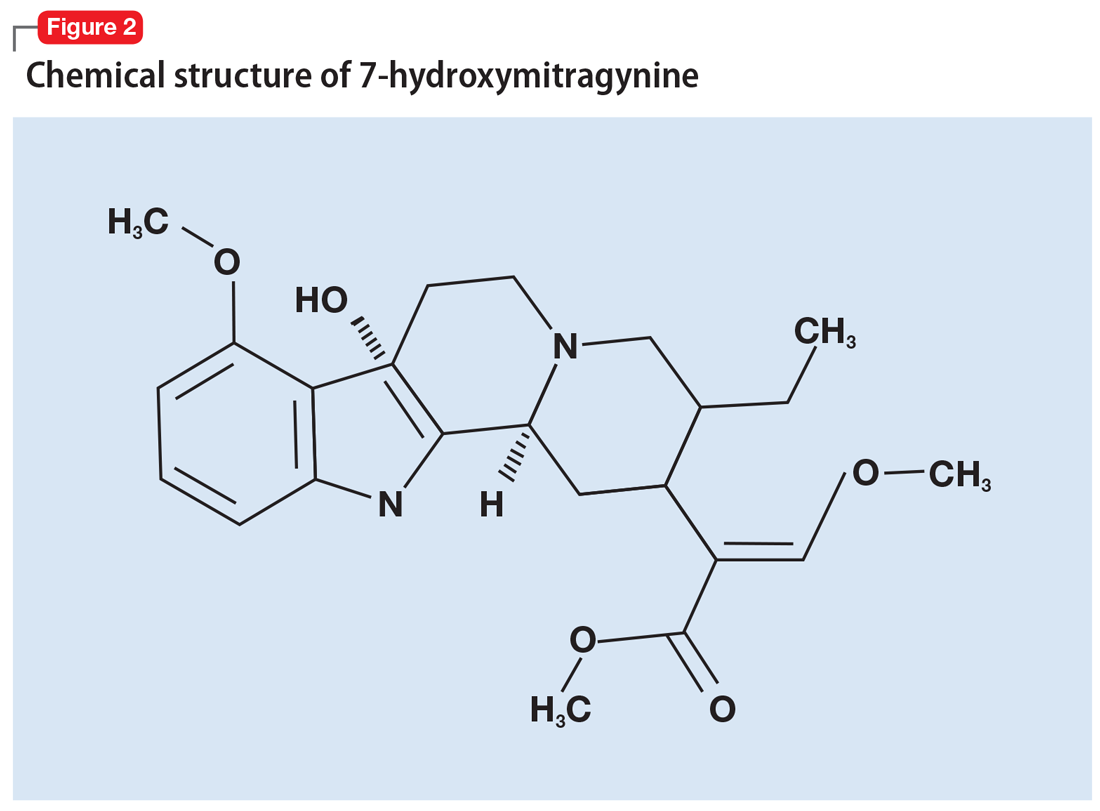
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Ketamine and serotonin syndrome: A case report
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
Paradise lost: Life, liberty, and the pursuit of happiness among psychiatric patients
The United States Declaration of Independence is widely known for the words that begin its second paragraph:
We hold these truths to be self-evident, that all men are created equal, that they are endowed by their Creator with certain unalienable Rights, that among these are Life, Liberty and the pursuit of Happiness.
Those basic rights are accessible and exercised by all healthy US citizens, but for many individuals with psychiatric disorders, those inalienable rights may be elusive. Consider how they are compromised by untreated psychiatric illness.
Life. This is the most basic right. In the United States, healthy individuals cherish being alive, and many take it for granted, unlike the residents of nondemocratic countries, where persons may be killed by dictators for political or other reasons (Stalin and Hitler murdered millions of innocent people). In the past, persons with mental illness were considered possessed by demons and were killed or burned at the stake (as in the Middle Ages). But unfortunately, the current major risk for the loss of life among psychiatric patients is the patients themselves. Suicidal urges, attempts, and completions are of epidemic proportions and continue to rise every year. Our patients end their own lives because their illness prompts them to relinquish their life and to embrace untimely death. And once life is lost, all other rights are abdicated. Suicide attempts are common among patients who are diagnosed with bipolar disorder, major depressive disorder, schizophrenia, anxiety, obsessive-compulsive disorder (OCD), posttraumatic stress disorder, and borderline personality disorder. Sometimes, suicide is unintentional, such as when a patient with a substance use disorder inadvertently overdoses (as in the contemporary opioid epidemic) or ingests drugs laced with a deadly substance. For many untreated patients, life can be so fragile, tenuous, and tragically brief.
Liberty. Healthy citizens in the United States (and other democratic countries) have many liberties: where to live, what to do, where to move, what to say, what to believe, who to assemble with, what to eat or drink, whom to befriend, whom to marry, whether or not to procreate, and what to wear. They can choose to be an activist for any cause, no matter how quaint, or to disfigure their bodies with tattoos or piercings.
In contrast, the liberties of individuals with a psychiatric disorder can be compromised. In fact, patients’ liberties can be seriously shackled by their illness. A person with untreated schizophrenia can be enslaved by fixed irrational beliefs that may constrain their choices or determine how they live or relate to others. Command hallucinations can dictate what a patient should or mustn’t do. Poor reality testing detrimentally limits the options of a person with psychosis. A lack of insight deprives a patient with schizophrenia from rational decision-making. Self-neglect leads to physical, mental, and social deterioration.
For persons with depression, the range of liberties is shattered by social withdrawal, overwhelming guilt, sense of worthlessness, dismal hopelessness, doleful ruminations, and loss of appetite or sleep. The only rights that people with depression may exercise is to injure their body or end their life.
Think also of patients with OCD, who are subjugated by their ongoing obsessions or compulsive rituals; think of those with panic disorder who are unable to leave their home due to agoraphobia or
Continue to: Happiness
Happiness. I often wonder if most Americans these days are pursuing pleasure rather than happiness, seeking the momentary thrill and gratification instead of long-lasting happiness and joy. But persons with psychiatric brain disorders have great difficulty pursuing either pleasure or happiness. Anhedonia is a common symptom in schizophrenia and depression, depriving patients from experiencing enjoyable activities (ie, having fun) as they used to do before they got sick. Persons with anxiety have such emotional turmoil, it is hard for them to experience pleasure or happiness when feelings of impending doom permeates their souls. Persons with an addictive disorder are coerced to seek their substance for a momentary reward, only to spend a much longer time craving and seeking their substance of choice again and again. On the other end of the spectrum, for persons with mania, the excessive pursuit of high-risk pleasures can have grave consequences or embarrassment after they recover.
Happiness for patients with mental illness is possible only when they emerge from their illness and are “liberated” from the symptoms that disrupt their lives. As psychiatrists, we don’t just evaluate and treat patients with psychiatric illness—we restore their liberties and ability to pursue happiness and enjoy small pleasures.
The motto on the seal of the American University of Beirut, which I attended in my youth, is “That they may have life, and to have it abundantly.” As I have grown older and wiser, I have come to realize the true meaning of that motto. Life is a right we take for granted, but without it, we cannot exercise the various liberties, or be able to pursue happiness. I exercised my right to become a psychiatrist, and that provided me with lifelong happiness and satisfaction, especially when I prevent the loss of life of my patients, restore their liberty by ridding them of illness, and resurrect their ability to experience pleasure and pursue happiness.
The United States Declaration of Independence is widely known for the words that begin its second paragraph:
We hold these truths to be self-evident, that all men are created equal, that they are endowed by their Creator with certain unalienable Rights, that among these are Life, Liberty and the pursuit of Happiness.
Those basic rights are accessible and exercised by all healthy US citizens, but for many individuals with psychiatric disorders, those inalienable rights may be elusive. Consider how they are compromised by untreated psychiatric illness.
Life. This is the most basic right. In the United States, healthy individuals cherish being alive, and many take it for granted, unlike the residents of nondemocratic countries, where persons may be killed by dictators for political or other reasons (Stalin and Hitler murdered millions of innocent people). In the past, persons with mental illness were considered possessed by demons and were killed or burned at the stake (as in the Middle Ages). But unfortunately, the current major risk for the loss of life among psychiatric patients is the patients themselves. Suicidal urges, attempts, and completions are of epidemic proportions and continue to rise every year. Our patients end their own lives because their illness prompts them to relinquish their life and to embrace untimely death. And once life is lost, all other rights are abdicated. Suicide attempts are common among patients who are diagnosed with bipolar disorder, major depressive disorder, schizophrenia, anxiety, obsessive-compulsive disorder (OCD), posttraumatic stress disorder, and borderline personality disorder. Sometimes, suicide is unintentional, such as when a patient with a substance use disorder inadvertently overdoses (as in the contemporary opioid epidemic) or ingests drugs laced with a deadly substance. For many untreated patients, life can be so fragile, tenuous, and tragically brief.
Liberty. Healthy citizens in the United States (and other democratic countries) have many liberties: where to live, what to do, where to move, what to say, what to believe, who to assemble with, what to eat or drink, whom to befriend, whom to marry, whether or not to procreate, and what to wear. They can choose to be an activist for any cause, no matter how quaint, or to disfigure their bodies with tattoos or piercings.
In contrast, the liberties of individuals with a psychiatric disorder can be compromised. In fact, patients’ liberties can be seriously shackled by their illness. A person with untreated schizophrenia can be enslaved by fixed irrational beliefs that may constrain their choices or determine how they live or relate to others. Command hallucinations can dictate what a patient should or mustn’t do. Poor reality testing detrimentally limits the options of a person with psychosis. A lack of insight deprives a patient with schizophrenia from rational decision-making. Self-neglect leads to physical, mental, and social deterioration.
For persons with depression, the range of liberties is shattered by social withdrawal, overwhelming guilt, sense of worthlessness, dismal hopelessness, doleful ruminations, and loss of appetite or sleep. The only rights that people with depression may exercise is to injure their body or end their life.
Think also of patients with OCD, who are subjugated by their ongoing obsessions or compulsive rituals; think of those with panic disorder who are unable to leave their home due to agoraphobia or
Continue to: Happiness
Happiness. I often wonder if most Americans these days are pursuing pleasure rather than happiness, seeking the momentary thrill and gratification instead of long-lasting happiness and joy. But persons with psychiatric brain disorders have great difficulty pursuing either pleasure or happiness. Anhedonia is a common symptom in schizophrenia and depression, depriving patients from experiencing enjoyable activities (ie, having fun) as they used to do before they got sick. Persons with anxiety have such emotional turmoil, it is hard for them to experience pleasure or happiness when feelings of impending doom permeates their souls. Persons with an addictive disorder are coerced to seek their substance for a momentary reward, only to spend a much longer time craving and seeking their substance of choice again and again. On the other end of the spectrum, for persons with mania, the excessive pursuit of high-risk pleasures can have grave consequences or embarrassment after they recover.
Happiness for patients with mental illness is possible only when they emerge from their illness and are “liberated” from the symptoms that disrupt their lives. As psychiatrists, we don’t just evaluate and treat patients with psychiatric illness—we restore their liberties and ability to pursue happiness and enjoy small pleasures.
The motto on the seal of the American University of Beirut, which I attended in my youth, is “That they may have life, and to have it abundantly.” As I have grown older and wiser, I have come to realize the true meaning of that motto. Life is a right we take for granted, but without it, we cannot exercise the various liberties, or be able to pursue happiness. I exercised my right to become a psychiatrist, and that provided me with lifelong happiness and satisfaction, especially when I prevent the loss of life of my patients, restore their liberty by ridding them of illness, and resurrect their ability to experience pleasure and pursue happiness.
The United States Declaration of Independence is widely known for the words that begin its second paragraph:
We hold these truths to be self-evident, that all men are created equal, that they are endowed by their Creator with certain unalienable Rights, that among these are Life, Liberty and the pursuit of Happiness.
Those basic rights are accessible and exercised by all healthy US citizens, but for many individuals with psychiatric disorders, those inalienable rights may be elusive. Consider how they are compromised by untreated psychiatric illness.
Life. This is the most basic right. In the United States, healthy individuals cherish being alive, and many take it for granted, unlike the residents of nondemocratic countries, where persons may be killed by dictators for political or other reasons (Stalin and Hitler murdered millions of innocent people). In the past, persons with mental illness were considered possessed by demons and were killed or burned at the stake (as in the Middle Ages). But unfortunately, the current major risk for the loss of life among psychiatric patients is the patients themselves. Suicidal urges, attempts, and completions are of epidemic proportions and continue to rise every year. Our patients end their own lives because their illness prompts them to relinquish their life and to embrace untimely death. And once life is lost, all other rights are abdicated. Suicide attempts are common among patients who are diagnosed with bipolar disorder, major depressive disorder, schizophrenia, anxiety, obsessive-compulsive disorder (OCD), posttraumatic stress disorder, and borderline personality disorder. Sometimes, suicide is unintentional, such as when a patient with a substance use disorder inadvertently overdoses (as in the contemporary opioid epidemic) or ingests drugs laced with a deadly substance. For many untreated patients, life can be so fragile, tenuous, and tragically brief.
Liberty. Healthy citizens in the United States (and other democratic countries) have many liberties: where to live, what to do, where to move, what to say, what to believe, who to assemble with, what to eat or drink, whom to befriend, whom to marry, whether or not to procreate, and what to wear. They can choose to be an activist for any cause, no matter how quaint, or to disfigure their bodies with tattoos or piercings.
In contrast, the liberties of individuals with a psychiatric disorder can be compromised. In fact, patients’ liberties can be seriously shackled by their illness. A person with untreated schizophrenia can be enslaved by fixed irrational beliefs that may constrain their choices or determine how they live or relate to others. Command hallucinations can dictate what a patient should or mustn’t do. Poor reality testing detrimentally limits the options of a person with psychosis. A lack of insight deprives a patient with schizophrenia from rational decision-making. Self-neglect leads to physical, mental, and social deterioration.
For persons with depression, the range of liberties is shattered by social withdrawal, overwhelming guilt, sense of worthlessness, dismal hopelessness, doleful ruminations, and loss of appetite or sleep. The only rights that people with depression may exercise is to injure their body or end their life.
Think also of patients with OCD, who are subjugated by their ongoing obsessions or compulsive rituals; think of those with panic disorder who are unable to leave their home due to agoraphobia or
Continue to: Happiness
Happiness. I often wonder if most Americans these days are pursuing pleasure rather than happiness, seeking the momentary thrill and gratification instead of long-lasting happiness and joy. But persons with psychiatric brain disorders have great difficulty pursuing either pleasure or happiness. Anhedonia is a common symptom in schizophrenia and depression, depriving patients from experiencing enjoyable activities (ie, having fun) as they used to do before they got sick. Persons with anxiety have such emotional turmoil, it is hard for them to experience pleasure or happiness when feelings of impending doom permeates their souls. Persons with an addictive disorder are coerced to seek their substance for a momentary reward, only to spend a much longer time craving and seeking their substance of choice again and again. On the other end of the spectrum, for persons with mania, the excessive pursuit of high-risk pleasures can have grave consequences or embarrassment after they recover.
Happiness for patients with mental illness is possible only when they emerge from their illness and are “liberated” from the symptoms that disrupt their lives. As psychiatrists, we don’t just evaluate and treat patients with psychiatric illness—we restore their liberties and ability to pursue happiness and enjoy small pleasures.
The motto on the seal of the American University of Beirut, which I attended in my youth, is “That they may have life, and to have it abundantly.” As I have grown older and wiser, I have come to realize the true meaning of that motto. Life is a right we take for granted, but without it, we cannot exercise the various liberties, or be able to pursue happiness. I exercised my right to become a psychiatrist, and that provided me with lifelong happiness and satisfaction, especially when I prevent the loss of life of my patients, restore their liberty by ridding them of illness, and resurrect their ability to experience pleasure and pursue happiness.
Depression, or something else?
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
CASE Suicidal behavior, severe headaches
Ms. A, age 60, presents to the emergency department (ED) with depression, suicidal behavior, and 3 days of severe headaches. Neurology is consulted and an MRI is ordered, which shows a 3.0-cm mass lesion in the left temporal lobe with associated vasogenic edema that is suspicious for metastatic disease (Figure).
Ms. A is admitted to the hospital for further workup of her brain lesion. She is started on IV dexamethasone, 10 mg every 6 hours, a glucocorticosteroid, for brain edema, and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
Upon admission, in addition to oncology and neurosurgery, psychiatry is also consulted to evaluate Ms. A for depression and suicidality.
EVALUATION Mood changes and poor judgment
Ms. A has a psychiatric history of depression and alcohol use disorder but says she has not consumed any alcohol in years. Her medical history includes hypertension, diabetes, and stage 4 non-small–cell lung cancer, for which she received surgery and adjuvant chemoradiotherapy 1 year ago.
On initial intake, Ms. A reports that in addition to the headaches, she has also been experiencing worsening depression and suicidal behavior. For the past 2 months, she has had a severely depressed mood, with notable anhedonia, poor appetite, insomnia, low energy, and decreased concentration. The changes in her mental health were triggered by her mother’s death. Three days prior to admission, the patient planned to overdose on antihypertensive pills, but her suicide attempt was interrupted when her family called. She denies any current suicidal ideation, intent, or plan.
According to her family, Ms. A has been increasingly irritable and her personality has changed in the past month. She also has been repeatedly sorting through her neighbors’ garbage.
Ms. A’s current psychiatric medications are duloxetine, 30 mg/d; quetiapine, 50 mg every night at bedtime; and buspirone, 10 mg/d. However, it is unclear if she is consistently taking these medications.
Continue to: On mental status examination...
On mental status examination, Ms. A is calm and she has no abnormal movements. She says she is depressed. Her affect is reactive and labile. She is alert and oriented to person, place, and time. Her attention, registration, and recall are intact. Her executive function is not tested. However, Ms. A’s insight and judgment seem poor.
To address Ms. A’s worsening depression, the psychiatry team increases her duloxetine from 30 to 60 mg/d, and she continues quetiapine, 50 mg every night at bedtime, for mood lability. Buspirone is not continued because she was not taking a therapeutic dosage in the community.
Within 4 days, Ms. A shows improvement in sleep, appetite, and mood. She has no further suicidal ideation.
[polldaddy:10511743]
The authors’ observations
Ms. A had a recurrence of what was presumed to be major depressive disorder (MDD) in the context of her mother’s death. However, she also exhibited irritability, mood lability, and impulsivity, all of which could be part of her depression, or a separate problem related to her brain tumor. Because Ms. A had never displayed bizarre behavior before the past few weeks, it is likely that her CNS lesion was directly affecting her personality and possibly underlying her planned suicide attempt.
Fifty to 80% of patients with CNS tumors, either primary or metastatic, present with psychiatric symptoms.1 Table 11-3 lists common psychiatric symptoms of brain tumors. Unfortunately, there is little reliable evidence that directly correlates tumor location with specific psychiatric symptoms. A 2010 meta-analysis found a statistically significant link between anorexia nervosa and hypothalamic tumors.1 However, for other brain regions, there is only an increased likelihood that any given tumor location will produce psychiatric symptoms.1,4 For instance, compared to patients with tumors in other locations, those with temporal lobe tumors are more likely to present with mood disorders, personality changes, and memory problems.1 In contrast, patients with frontal lobe tumors have an increased likelihood of psychosis, mood disorders, and personality changes.1 Patients with tumors in the pituitary region often present with anxiety.1
Continue to: When considering treatment options...
When considering treatment options for Ms. A, alcohol withdrawal was unlikely given the remote history of alcohol use, low alcohol blood level, and lack of evidence of unstable vital signs or tremor. Although she might have benefited from inpatient psychiatric treatment, this needed to wait until there was a definitive treatment plan for her brain tumor. Finally, although a paraneoplastic syndrome, such as limbic encephalitis, could be causing her psychiatric symptoms, this scenario is less likely with non-small–cell lung cancer.
Although uncommon, CNS tumors can present with psychiatric symptoms as the only manifestation. This is more likely when a patient exhibits new-onset or atypical symptoms, or fails to respond to standard psychiatric treatment.4 Case reports have described patients with brain tumors being misdiagnosed as having a primary psychiatric condition, which delays treatment of their CNS cancer.2 Additionally, frontal and limbic tumors are more likely to present with psychiatric manifestations; up to 90% of patients exhibit altered mental status or personality changes, as did Ms. A.1,4 Clearly, it is easier to identify patients with psychiatric symptoms resulting from a brain tumor when they also present with focal neurologic deficits or systemic symptoms, such as headache or nausea and vomiting. Ms. A presented with severe headaches, which is what led to her early imaging and prompt diagnosis.
Numerous proposed mechanisms might account for the psychiatric symptoms that occur during the course of a brain tumor, including direct injury to neuronal cells, secretion of hormones or other tumor-derived substances, and peri-ictal phenomena.3
TREATMENT Tumor is removed, but memory is impaired
Ms. A is scheduled for craniotomy and surgical resection of the frontal mass. Prior to surgery, Ms. A shows interest in improving her health, cooperates with staff, and seeks her daughter’s input on treatment. One week after admission, Ms. A has her mass resected, which is confirmed on biopsy to be a lung metastasis. Post-surgery, Ms. A receives codeine, 30 mg every 6 hours as needed, for pain; she continues dexamethasone, 4 mg IV every 6 hours, for brain edema and levetiracetam, 500 mg twice a day, for seizure prophylaxis.
On Day 2 after surgery, Ms. A attempts to elope. When she is approached by a psychiatrist on the treatment team, she does not recognize him. Although her long-term memory seems intact, she is unable to remember the details of recent events, including her medical and surgical treatments.
[polldaddy:10511745]
Continue to: The authors' observations
The authors’ observations
Ms. A’s memory impairment may be secondary to a surgically acquired neurocognitive deficit. In the United States, brain metastases represent a significant public health issue, affecting >100,000 patients per year.5 Metastatic lesions are the most common brain tumors. Lung cancer, breast cancer, and melanoma are the leading solid tumors to spread to the CNS.5 In cases of single brain metastasis, similar to Ms. A’s solitary left temporal lobe lesion, surgical resection plays a critical role in treatment. It provides histological confirmation of metastatic disease and can relieve mass effect if present. Studies have shown that combined surgical resection with radiation improves survival relative to patients who undergo radiation therapy alone.6,7
However, the benefits of surgical resection need to be balanced with preservation of neurologic function. Emerging evidence suggests that a majority of patients have surgically-acquired cognitive deficits due to damage of normal surrounding tissues, and these deficits are associated with reduced quality of life.8,9 Further, a study examining glioma surgical resections found that patients with left temporal lobe tumors exhibit more frequent and severe neurocognitive decline than patients with right temporal lobe tumors, especially in domains such as verbal memory.8 Ms. A’s memory impairment was persistent during her postoperative course, which suggests that it was not just an immediate post-surgical phenomenon, but a longer-lasting cognitive change directly related to the resection.
It is also possible that Ms. A had a prior neurocognitive disorder that manifested to a greater degree as a result of the CNS tumor. Ms. A might have had early-onset Alzheimer’s disease, although her intact memory before surgery makes this less likely. Alternatively, she could have had vascular dementia, especially given her long-standing hypertension and diabetes. This might have been missed in the initial evaluation because executive function was not tested. However, the relatively abrupt onset of memory problems after surgery suggests that she had no underlying neurocognitive disorder.
Ms. A’s presumed episode of MDD might also explain her memory changes. Major depressive disorder is increasingly common among geriatric patients, affecting approximately 5% of community-dwelling older adults.10 Its incidence increases with medical comorbidities, as suggested by depression rates of 5% to 10% in the primary care setting vs 37% in patients after critical-care hospitalizations.10 Late-life depression (LLD) occurs in adults age ≥60. Unlike depression in younger patients, LLD is more likely to be associated with cognitive impairment, specifically impairment of executive function and memory.11 The incidence of cognitive impairment in LLD is higher in patients with a history of depression, such as Ms. A.11,12 However, in general, patients who are depressed have memory complaints out of proportion to the clinical findings, and they show poor effort on cognitive testing. Ms. A exhibited neither of these, which makes it less likely that LLD was the exclusive cause of her memory loss.13 Table 214 outlines the management of cognitive deficits in a patient with a brain tumor.
EVALUATION Increasingly agitated and paranoid
After the tumor resection, Ms. A becomes increasingly irritable, uncooperative, and agitated. She repeatedly demands to be discharged. She insists she is fine and refuses medications and further laboratory workup. She becomes paranoid about the nursing staff and believes they are trying to kill her.
Continue to: On psychiatric re-evaluation...
On psychiatric re-evaluation, Ms. A demonstrates pressured speech, perseveration about going home, paranoid delusions, and anger at her family and physicians.
[polldaddy:10511747]
The authors’ observations
Ms. A’s refusal of medications and agitation may be explained by postoperative delirium, a surgical complication that is increasingly common among geriatric patients and is associated with poor clinical outcomes. Delirium is characterized by an acute onset and fluctuating course of symptoms that include inattention, motoric hypo- or hyperactivity, inappropriate behavior, emotional lability, cognitive dysfunction, and psychotic symptoms.15 Risk factors that contribute to postoperative delirium include older age, alcohol use, and poor baseline functional and cognitive status.16 The pathophysiology of delirium is not fully understood, but accumulating evidence suggests that different sets of interacting biologic factors (ie, neurotransmitters and inflammation) contribute to a disruption of large-scale neuronal networks in the brain, resulting in cognitive dysfunction.15 Patients who develop postoperative delirium are more likely to develop long-term cognitive dysfunction and have an increased risk of dementia.16
Another potential source of Ms. A’s agitation is steroid use. Ms. A received IV dexamethasone, 8 to 16 mg/d, around the time of her surgery. Steroids are commonly used to treat brain tumors, particularly when there is vasogenic edema. Steroid psychosis is a term loosely used to describe a wide range of psychiatric symptoms induced by corticosteroids that includes, but is not limited to, depression, mania, psychosis, delirium, and cognitive impairment.17 Steroid-induced psychiatric adverse effects occur in 5% to 18% of patients receiving corticosteroids and often happen early in treatment, although they can occur at any point.18 Corticosteroids influence brain activity via glucocorticoid and mineralocorticoid receptors. These receptors are widely distributed throughout the brain and affect neurotransmitter systems, such as the serotonergic system, that are associated with changes in mood, behavior, and cognition.17 While the adverse psychiatric manifestations of steroid use vary, higher dosages are associated with an increased risk of psychiatric complications; mania is more prevalent early in the course of treatment, and depression is more common with long-term use.17,19 Table 317,18 outlines the evidence-based treatment of corticosteroid-induced adverse psychiatric effects.
Although there are no clinical guidelines or FDA-approved medications for treating steroid-induced psychiatric adverse events, these are best managed by tapering and discontinuing steroids when possible and simultaneously using psychotropic medications to treat psychiatric symptoms. Case reports and limited evidence-based literature have demonstrated that steroid-induced mania responds to mood stabilizers or antipsychotics, while depression can be managed with antidepressants or lithium.17
Additionally, patients with CNS tumors are at risk for seizures and often are prescribed antiepileptics. Because it is easy to administer and does not need to be titrated, levetiracetam is a commonly used agent. However, levetiracetam can cause psychiatric adverse effects, including behavior changes and frank psychosis.20
Continue to: Finally, Ms. A's altered mental status...
Finally, Ms. A’s altered mental status could have been related to opioid intoxication. Opioids are used to manage postsurgical pain, and studies have shown these medications can be a precipitating factor for delirium in geriatric patients.21
TREATMENT Medication adjustments
At the request of the psychiatry team, levetiracetam is discontinued due to its potential for psychiatric adverse effects. The neurosurgery team replaces it with valproic acid, 500 mg every 12 hours. Ms. A is also tapered off steroids fairly rapidly because of the potential for steroid-induced psychiatric adverse effects. Her quetiapine is titrated from 50 to 150 mg every night at bedtime, and duloxetine is discontinued.
OUTCOME Agitation improves dramatically
Ms. A’s new medication regimen dramatically improves her agitation, which allows Ms. A, her family, and the medical team to work together to establish treatment goals. Ms. A ultimately returns home with the assistance of her family. She continues to have memory issues, but with improved emotion regulation. Several months later, Ms. A is readmitted to the hospital because her cancer has progressed despite treatment.
Bottom Line
Brain tumors may present with various psychiatric manifestations that can change during the course of the patient’s treatment. A comprehensive psychiatric evaluation should parse out the interplay between direct effects of the tumor and any adverse effects that are the result of medical and/or surgical interventions to determine the cause of psychiatric symptoms and their appropriate management.
Related Resource
Madhusoodanan S, Ting MB, Farah T, et al. Psychiatric aspects of brain tumors: a review. World J Psychiatry. 2015;5(3):273-285.
Drug Brand Names
Aripiprazole • Abilify
Buspirone • Buspar
Chlorpromazine • Thorazine
Codeine • Codeine systemic
Dexamethasone • Decadron
Duloxetine • Cymbalta
Haloperidol • Haldol
Levetiracetam • Keppra
Lorazepam • Ativan
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakene
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
1. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
2. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
3. Pearl ML, Talgat G, Valea FA, et al. Psychiatric symptoms due to brain metastases. Med Update Psychiatr. 1998;3(4):91-94.
4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343-349.
5. Ferguson SD, Wagner KM, Prabhu SS, et al. Neurosurgical management of brain metastases. Clin Exp Metastasis. 2017;34(6-7):377-389.
6. Husain ZA, Regine WF, Kwok Y, et al. Brain metastases: contemporary management and future directions. Eur J Clin Med Oncol. 2011;3(3):38-45.
7. Vecht CJ, Haaxmareiche H, Noordijk EM, et al. Treatment of single brain metastasis - radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993;33(6):583-590.
8. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87-95.
9. Wu AS, Witgert ME, Lang FF, et al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125.
10. Taylor WD. Depression in the elderly. N Engl J Med. 2014;371(13):1228-1236.
11. Liguori C, Pierantozzi M, Chiaravalloti A, et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front Aging Neurosci. 2018;10:38.
12. Luijendijk HJ, van den Berg JF, Dekker MJHJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394-1401.
13. Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13(3):105-117.
14. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168.
15. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
16. Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323.
17. Kusljic S, Manias E, Gogos A. Corticosteroid-induced psychiatric disturbances: it is time for pharmacists to take notice. Res Soc Adm Pharm. 2016;12(2):355-360.
18. Cerullo MA. Corticosteroid-induced mania: prepare for the unpredictable. Current Psychiatry. 2006;5(6):43-50.
19. Dubovsky AN, Arvikar S, Stern TA, et al. Steroid psychosis revisited. Psychosomatics. 2012;53(2):103-115.
20. Habets JGV, Leentjens AFG, Schijns OEMG. Serious and reversible levetiracetam-induced psychiatric symptoms after resection of frontal low-grade glioma: two case histories. Br J Neurosurg. 2017;31(4):471-473.
21
Meeting the mental health needs of American Muslim patients
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
Interacting with colleagues on social media: Tips for avoiding trouble
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
In gestational diabetes, early postpartum glucose testing is a winner
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
REPORTING FROM THE PREGNANCY MEETING