User login
CDC revises COVID-19 test kits, broadens ‘person under investigation’ definition
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .
The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .
The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
In a telebriefing on the COVID-19 outbreak, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at .
The definition has been revised “to meet the needs of this rapidly evolving situation,” she said. The new PUI definition includes travel to more geographic areas to reflect this past week’s marked uptick in coronavirus activity in Italy and Iran. In addition to these countries and China, recent travel to Japan or South Korea also constitutes an epidemiologic risk factor which, in conjunction with clinical features, warrant an individual being classified as a PUI. These five countries each now have widespread person-to-person transmission of the virus.
Dr. Messonnier left open the possibility that the PUI definition would continue to evolve if such transmission within communities becomes more common. Asked whether the small number of U.S. cases thus might be an artifact of low test volumes, she said, “We aggressively controlled our borders to slow the spread. This was an intentional U.S. strategy. The CDC has always had the capacity to test rapidly from the time the sequence was available. ...We have been testing aggressively.”
The original PUI definition, she explained, emphasized individuals with fever, cough, or trouble breathing who had traveled recently from areas with COVID-19 activity, in particular China’s Hubei province. “We have been most focused on symptomatic people who are closely linked to, or who had, travel history, but our criteria also allow for clinical discretion,” she said. “There is no substitute for an astute clinician on the front lines of patient care.”
The first COVID-19 case from person-to-person spread was reported on Feb. 27. “At this time, we don’t know how or where this person became infected,” said Dr. Messonnier, although investigations are still underway. She responded to a question about whether the CDC delayed allowing COVID-19 testing for the patient for several days, as was reported in some media accounts. “According to CDC records, the first call we got was Feb. 23,” when public health officials in California reported a severely ill person with no travel abroad and no known contacts with individuals that would trigger suspicions for coronavirus. The CDC recommended COVID-19 testing on that day, she said.
Dr. Messonnier declined to answer questions about a whistleblower report alleging improper training and inadequate protective measures for Department of Health & Human Services workers at the quarantine center at Travis Air Force Base, Calif.
Dr. Messonnier said that the CDC has been working closely with the Food and Drug Administration to address problems with the COVID-19 test kits that were unusable because of a large number of indeterminate results. The two agencies together have determined that of the three reactions that were initially deemed necessary for a definitive COVID-19 diagnosis, just two are sufficient, so new kits that omit the problematic chemical are being manufactured and distributed.
These new kits are rapidly being made available; the goal, said Dr. Messonnier, is to have to state and local public health departments equipped with test kits by about March 7.
As local tests become available, the most updated information will be coming from state and local public health departments, she stressed, adding that the CDC would continue to update case counts on Monday, Wednesday, and Friday of each week. Procedures are being developed for the management of patients presumed to have COVID-19, where local health departments see positive tests but the mandatory CDC confirmatory test hasn’t been completed.
While new cases emerge across Europe and Asia, China’s earlier COVID-19 explosion seems to be slowing. “It’s really good news that the case counts in China are decreasing,” both for the well-being of that country’s citizens, and as a sign of the disease’s potential global effects, said Dr. Messonnier. She added that epidemiologists and mathematical modelers are parsing case fatality rates as well.
She advised health care providers and public health officials to keep abreast of changes in CDC guidance by checking frequently at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
REPORTING FROM A CDC BRIEFING
Serum NfL levels may predict 10-year deep gray matter volumes
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Researchers have begun to study longitudinal changes in serum NfL levels as a way to monitor axonal damage in patients with MS and see how they relate to other measures of neuronal loss, such as brain atrophy, and clinical outcomes over the long-term. “Deep gray matter volumes have been shown to correlate with neurological outcomes in MS patients. In particular, thalamic volume has been shown to correlate with measures of cognitive processing speed, such as the Symbol Digit Modalities Test,” said senior author Tanuja Chitnis, MD, professor of neurology at Harvard Medical School in Boston.
She and her colleagues sought to determine whether annual serum NfL measures could predict 10-year deep gray matter atrophy measured by volumetric MRI in patients with MS. They examined patients who were enrolled in the Comprehensive Longitudinal Investigations in MS at Brigham and Women’s Hospital (CLIMB) study. Eligible participants were enrolled within 5 years of disease onset and had had annual blood samples drawn for as long as 10 years. In all, 122 patients met these criteria. The investigators measured serum NfL and compared it against deep gray matter volume in the thalamus, caudate, putamen, and globus pallidus from high-resolution 3-T MRI scans taken at year 10. Dr. Chitnis and colleagues assessed correlations between averaged annual NfL and 10-year MRI outcomes using univariate and multivariate linear regression models.
About 96% of participants were white, and about 2% were black. Approximately 73% of participants were female. Average age at the first symptom was 36 years, and average age at the first sample collection was 38 years.
The investigators found several negative associations between averaged NfL values and various MRI volumetric outcomes. Averaged annual serum NfL levels for the first 5 years were significantly and negatively associated with 10-year thalamic volumes in the unadjusted analysis. A 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000272 cm3 in thalamic volume. The association remained significant in an analysis adjusted for age, sex, and disease duration. In this analysis, a 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000259 cm3 in thalamic volume. Analyzing serum NfL levels beyond year 5 did not reveal a stronger association. Serum NfL levels during the first 5 years accounted for about 24% of the variance in 10-year thalamic volumes. Dr. Chitnis and colleagues found similar statistically significant associations between serum NfL levels and caudate, putamen, and globus pallidus volumes. “Therefore, early serum NfL levels contribute to the identification of patients who may require highly effective therapies,” she said.
“We will continue to validate these results. As well, we are exploring other early biomarkers that increase predictive power of long-term outcomes in MS, with the goal of identifying patients most appropriate for high-efficacy treatments.”
The study was supported by funds from the U.S. Department of Defense, Novartis, and the Swiss National Research Foundation. Dr. Chitnis has received personal compensation for consulting and advisory board membership from Biogen, Merck Serono, Novartis, and Sanofi.
SOURCE: Lokhande H et al. ACTRIMS Forum 2020, Abstract P018.
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Researchers have begun to study longitudinal changes in serum NfL levels as a way to monitor axonal damage in patients with MS and see how they relate to other measures of neuronal loss, such as brain atrophy, and clinical outcomes over the long-term. “Deep gray matter volumes have been shown to correlate with neurological outcomes in MS patients. In particular, thalamic volume has been shown to correlate with measures of cognitive processing speed, such as the Symbol Digit Modalities Test,” said senior author Tanuja Chitnis, MD, professor of neurology at Harvard Medical School in Boston.
She and her colleagues sought to determine whether annual serum NfL measures could predict 10-year deep gray matter atrophy measured by volumetric MRI in patients with MS. They examined patients who were enrolled in the Comprehensive Longitudinal Investigations in MS at Brigham and Women’s Hospital (CLIMB) study. Eligible participants were enrolled within 5 years of disease onset and had had annual blood samples drawn for as long as 10 years. In all, 122 patients met these criteria. The investigators measured serum NfL and compared it against deep gray matter volume in the thalamus, caudate, putamen, and globus pallidus from high-resolution 3-T MRI scans taken at year 10. Dr. Chitnis and colleagues assessed correlations between averaged annual NfL and 10-year MRI outcomes using univariate and multivariate linear regression models.
About 96% of participants were white, and about 2% were black. Approximately 73% of participants were female. Average age at the first symptom was 36 years, and average age at the first sample collection was 38 years.
The investigators found several negative associations between averaged NfL values and various MRI volumetric outcomes. Averaged annual serum NfL levels for the first 5 years were significantly and negatively associated with 10-year thalamic volumes in the unadjusted analysis. A 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000272 cm3 in thalamic volume. The association remained significant in an analysis adjusted for age, sex, and disease duration. In this analysis, a 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000259 cm3 in thalamic volume. Analyzing serum NfL levels beyond year 5 did not reveal a stronger association. Serum NfL levels during the first 5 years accounted for about 24% of the variance in 10-year thalamic volumes. Dr. Chitnis and colleagues found similar statistically significant associations between serum NfL levels and caudate, putamen, and globus pallidus volumes. “Therefore, early serum NfL levels contribute to the identification of patients who may require highly effective therapies,” she said.
“We will continue to validate these results. As well, we are exploring other early biomarkers that increase predictive power of long-term outcomes in MS, with the goal of identifying patients most appropriate for high-efficacy treatments.”
The study was supported by funds from the U.S. Department of Defense, Novartis, and the Swiss National Research Foundation. Dr. Chitnis has received personal compensation for consulting and advisory board membership from Biogen, Merck Serono, Novartis, and Sanofi.
SOURCE: Lokhande H et al. ACTRIMS Forum 2020, Abstract P018.
WEST PALM BEACH, FLA. – , according to research presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Researchers have begun to study longitudinal changes in serum NfL levels as a way to monitor axonal damage in patients with MS and see how they relate to other measures of neuronal loss, such as brain atrophy, and clinical outcomes over the long-term. “Deep gray matter volumes have been shown to correlate with neurological outcomes in MS patients. In particular, thalamic volume has been shown to correlate with measures of cognitive processing speed, such as the Symbol Digit Modalities Test,” said senior author Tanuja Chitnis, MD, professor of neurology at Harvard Medical School in Boston.
She and her colleagues sought to determine whether annual serum NfL measures could predict 10-year deep gray matter atrophy measured by volumetric MRI in patients with MS. They examined patients who were enrolled in the Comprehensive Longitudinal Investigations in MS at Brigham and Women’s Hospital (CLIMB) study. Eligible participants were enrolled within 5 years of disease onset and had had annual blood samples drawn for as long as 10 years. In all, 122 patients met these criteria. The investigators measured serum NfL and compared it against deep gray matter volume in the thalamus, caudate, putamen, and globus pallidus from high-resolution 3-T MRI scans taken at year 10. Dr. Chitnis and colleagues assessed correlations between averaged annual NfL and 10-year MRI outcomes using univariate and multivariate linear regression models.
About 96% of participants were white, and about 2% were black. Approximately 73% of participants were female. Average age at the first symptom was 36 years, and average age at the first sample collection was 38 years.
The investigators found several negative associations between averaged NfL values and various MRI volumetric outcomes. Averaged annual serum NfL levels for the first 5 years were significantly and negatively associated with 10-year thalamic volumes in the unadjusted analysis. A 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000272 cm3 in thalamic volume. The association remained significant in an analysis adjusted for age, sex, and disease duration. In this analysis, a 1-pg/mL increase in the average sNfL value was associated with a decrease of 0.0000259 cm3 in thalamic volume. Analyzing serum NfL levels beyond year 5 did not reveal a stronger association. Serum NfL levels during the first 5 years accounted for about 24% of the variance in 10-year thalamic volumes. Dr. Chitnis and colleagues found similar statistically significant associations between serum NfL levels and caudate, putamen, and globus pallidus volumes. “Therefore, early serum NfL levels contribute to the identification of patients who may require highly effective therapies,” she said.
“We will continue to validate these results. As well, we are exploring other early biomarkers that increase predictive power of long-term outcomes in MS, with the goal of identifying patients most appropriate for high-efficacy treatments.”
The study was supported by funds from the U.S. Department of Defense, Novartis, and the Swiss National Research Foundation. Dr. Chitnis has received personal compensation for consulting and advisory board membership from Biogen, Merck Serono, Novartis, and Sanofi.
SOURCE: Lokhande H et al. ACTRIMS Forum 2020, Abstract P018.
REPORTING FROM ACTRIMS FORUM 2020
DDSEP® 9 Quick Quiz Question 2
Q2. Correct answer: D
Rationale
This patient has tropical sprue based on her travel to an endemic country, negative celiac serologies, labs revealing a macrocytic anemia and low albumin and characteristic histology (villous blunting, increased intraepithelial lymphocytes). Treatment is with tetracycline and folate. Diagnosis of tropical sprue is ultimately confirmed by a response to treatment. A gluten-free diet is not appropriate, as the patient does not have celiac disease, confirmed by normal celiac serologies. Ceftriaxone IV followed by Bactrim PO is the correct treatment for Whipple's disease. A diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) is beneficial treatment in some patients with IBS with abdominal bloating or pain. Rifaximin is the correct treatment for small intestine bacterial overgrowth or IBS-D.
References
1. Brown IS, Bettington A, Bettington M, Rosty C. Tropical sprue: revisiting an underrecognized disease. Am J Surg Pathol. 2014;38:666.
2. Shah VH, Rotterdam H, Kotler DP, et al. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717.
ginews@gastro.org
Q2. Correct answer: D
Rationale
This patient has tropical sprue based on her travel to an endemic country, negative celiac serologies, labs revealing a macrocytic anemia and low albumin and characteristic histology (villous blunting, increased intraepithelial lymphocytes). Treatment is with tetracycline and folate. Diagnosis of tropical sprue is ultimately confirmed by a response to treatment. A gluten-free diet is not appropriate, as the patient does not have celiac disease, confirmed by normal celiac serologies. Ceftriaxone IV followed by Bactrim PO is the correct treatment for Whipple's disease. A diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) is beneficial treatment in some patients with IBS with abdominal bloating or pain. Rifaximin is the correct treatment for small intestine bacterial overgrowth or IBS-D.
References
1. Brown IS, Bettington A, Bettington M, Rosty C. Tropical sprue: revisiting an underrecognized disease. Am J Surg Pathol. 2014;38:666.
2. Shah VH, Rotterdam H, Kotler DP, et al. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717.
ginews@gastro.org
Q2. Correct answer: D
Rationale
This patient has tropical sprue based on her travel to an endemic country, negative celiac serologies, labs revealing a macrocytic anemia and low albumin and characteristic histology (villous blunting, increased intraepithelial lymphocytes). Treatment is with tetracycline and folate. Diagnosis of tropical sprue is ultimately confirmed by a response to treatment. A gluten-free diet is not appropriate, as the patient does not have celiac disease, confirmed by normal celiac serologies. Ceftriaxone IV followed by Bactrim PO is the correct treatment for Whipple's disease. A diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) is beneficial treatment in some patients with IBS with abdominal bloating or pain. Rifaximin is the correct treatment for small intestine bacterial overgrowth or IBS-D.
References
1. Brown IS, Bettington A, Bettington M, Rosty C. Tropical sprue: revisiting an underrecognized disease. Am J Surg Pathol. 2014;38:666.
2. Shah VH, Rotterdam H, Kotler DP, et al. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717.
ginews@gastro.org
An 18-year-old woman presents for evaluation of chronic diarrhea, fatigue, and abdominal cramping. She was recently in Puerto Rico for 6 months visiting family and returned a few weeks ago. Her labs are significant for a hemoglobin of 11 g/L with an MCV of 109 fL. Her albumin is 3.6 g/dL. She had stool studies which ruled out infection, including parasites. TtG IgA and total IgA were within normal limits. EGD with multiple duodenal biopsies showed villous blunting with increased intraepithelial lymphocytes.
March 2020 - Question 1
Correct answer: C
Rationale
This patient has been exposed to HBV in the past and has cleared the virus. The HBVcore total Ab is indicative of prior exposure while the HBV surface Ab is detectable and gives immunity against reinfection under most routine clinical scenarios. Patients who have been exposed to HBV still have HBV ccc DNA within their hepatocytes that is dormant, but under extreme combined B and T cell immunosuppression, the patients are at risk for reverse seroconversion where they can lose HBV surface Ab and manifest HBV surface antigen and present as an acute HBV infection. Prophylaxis is required during therapy and for at least 12-18 months after therapy due to the long-lasting effects of anti-B cell monoclonal antibodies like rituximab. Reactivation of HCV in HCV Ab–positive, RNA-negative patients has not been reported.
Reference
1. Pauly MP, Tucker LY, Szpakowski JL, et al. Incidence of hepatitis B virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2018 Apr 24. doi: 10.1016/j. cgh.2018.04.033.
ginews@gastro.org
Correct answer: C
Rationale
This patient has been exposed to HBV in the past and has cleared the virus. The HBVcore total Ab is indicative of prior exposure while the HBV surface Ab is detectable and gives immunity against reinfection under most routine clinical scenarios. Patients who have been exposed to HBV still have HBV ccc DNA within their hepatocytes that is dormant, but under extreme combined B and T cell immunosuppression, the patients are at risk for reverse seroconversion where they can lose HBV surface Ab and manifest HBV surface antigen and present as an acute HBV infection. Prophylaxis is required during therapy and for at least 12-18 months after therapy due to the long-lasting effects of anti-B cell monoclonal antibodies like rituximab. Reactivation of HCV in HCV Ab–positive, RNA-negative patients has not been reported.
Reference
1. Pauly MP, Tucker LY, Szpakowski JL, et al. Incidence of hepatitis B virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2018 Apr 24. doi: 10.1016/j. cgh.2018.04.033.
ginews@gastro.org
Correct answer: C
Rationale
This patient has been exposed to HBV in the past and has cleared the virus. The HBVcore total Ab is indicative of prior exposure while the HBV surface Ab is detectable and gives immunity against reinfection under most routine clinical scenarios. Patients who have been exposed to HBV still have HBV ccc DNA within their hepatocytes that is dormant, but under extreme combined B and T cell immunosuppression, the patients are at risk for reverse seroconversion where they can lose HBV surface Ab and manifest HBV surface antigen and present as an acute HBV infection. Prophylaxis is required during therapy and for at least 12-18 months after therapy due to the long-lasting effects of anti-B cell monoclonal antibodies like rituximab. Reactivation of HCV in HCV Ab–positive, RNA-negative patients has not been reported.
Reference
1. Pauly MP, Tucker LY, Szpakowski JL, et al. Incidence of hepatitis B virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2018 Apr 24. doi: 10.1016/j. cgh.2018.04.033.
ginews@gastro.org
Q1. A 45-year-old man has recently been diagnosed with leukemia. The chemotherapeutic regimen will include rituximab and high-dose steroids. He is a former IV drug user but has been sober for 20 years. His lab work is as follows: ALT 25 U/L, HAV total antibody positive, HBs antibody positive, HBs antigen negative, HBc total positive, HCV antibody positive, HCV RNA undetected.
What is your diagnosis? - March 2020
Squamous metaplasia
Weight regain can accompany re-emergence of obesity-related comorbidities and, thus, early intervention is important. Although diet, exercise, and behavior modifications are fundamental, they can have limited efficacy. Thus, endoscopic management is important, with specific evaluation for gastrogastric fistulae, pouch dilation, and GJA dilation, all of which can be successfully intervened upon endoscopically. For GJA dilation in particular, APC has been used with promising results.1
In the normal GI tract, the esophagus is lined with squamous epithelium, and the stomach is lined with columnar epithelium. One of the most well-known and well-documented scenarios in which the typical mucosal lining is replaced by abnormal mucosa is Barrett’s esophagus (BE). BE is defined by the replacement of the normal distal squamous epithelial lining with columnar epithelium with a minimum length of 1 cm (tongues or circumferential) containing specialized intestinal metaplasia on histopathologic examination. It is well-documented that treatment of BE with thermal ablation and acid suppression therapy results in re-epithelialization of the esophagus with neosquamous mucosa.2 In contrast with this, in our patients, after we burned the gastric columnar mucosa with APC to treat their dilated GJA, the gastric pouch mucosa has been replaced with squamous epithelium, which we have termed “reverse BE.” To our knowledge, there are no reports of this condition in the literature, nor do we know the precise cause. There is a series of patients without a history of bariatric surgery who developed squamous metaplasia in the proximal gastric cardia.3 The authors hypothesized that this condition may be due to chronic mucosal injury owing to hiatal hernia, reflux, caustic ingestion, chronic gastritis, or pyloric stenosis. We suggest two potential mechanisms for this condition in our patients: 1) extending the ablation to the Z-line on the medial aspect of the pouch may allow for the distal extension of squamous mucosa during the healing process; and 2) acid suppression therapy with proton pump inhibitors after the procedure, in combination with a postoperative decrease in acid production, allows for a shift in the cell proliferation and differentiation in the pouch. Notably, although there are well-defined guidelines for surveillance of BE, owing to the risk of progression to esophageal adenocarcinoma,2 it is unclear what clinical significance this reverse BE may have in the future. It is important to continue to monitor these patients and clarify the natural history of this finding.
References
1. Brunaldi VO, Jirapinyo P, de Moura DTH et al. Endoscopic treatment of weight regain following Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2018;28:266-76.
2. Weusten B, Bisschops R, Coron E et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-8.
3. Fass R, Sampliner RE. Extension of squamous epithelium into the proximal stomach: a newly recognized mucosal abnormality. Endoscopy. 2000;32:27-32.
Christopher C. Thompson is a consultant for Boston Scientific and Medtronic, a consultant for and has institutional grants from USGI Medical, Olympus, and Apollo Endosurgery.
ginews@gastro.org
Squamous metaplasia
Weight regain can accompany re-emergence of obesity-related comorbidities and, thus, early intervention is important. Although diet, exercise, and behavior modifications are fundamental, they can have limited efficacy. Thus, endoscopic management is important, with specific evaluation for gastrogastric fistulae, pouch dilation, and GJA dilation, all of which can be successfully intervened upon endoscopically. For GJA dilation in particular, APC has been used with promising results.1
In the normal GI tract, the esophagus is lined with squamous epithelium, and the stomach is lined with columnar epithelium. One of the most well-known and well-documented scenarios in which the typical mucosal lining is replaced by abnormal mucosa is Barrett’s esophagus (BE). BE is defined by the replacement of the normal distal squamous epithelial lining with columnar epithelium with a minimum length of 1 cm (tongues or circumferential) containing specialized intestinal metaplasia on histopathologic examination. It is well-documented that treatment of BE with thermal ablation and acid suppression therapy results in re-epithelialization of the esophagus with neosquamous mucosa.2 In contrast with this, in our patients, after we burned the gastric columnar mucosa with APC to treat their dilated GJA, the gastric pouch mucosa has been replaced with squamous epithelium, which we have termed “reverse BE.” To our knowledge, there are no reports of this condition in the literature, nor do we know the precise cause. There is a series of patients without a history of bariatric surgery who developed squamous metaplasia in the proximal gastric cardia.3 The authors hypothesized that this condition may be due to chronic mucosal injury owing to hiatal hernia, reflux, caustic ingestion, chronic gastritis, or pyloric stenosis. We suggest two potential mechanisms for this condition in our patients: 1) extending the ablation to the Z-line on the medial aspect of the pouch may allow for the distal extension of squamous mucosa during the healing process; and 2) acid suppression therapy with proton pump inhibitors after the procedure, in combination with a postoperative decrease in acid production, allows for a shift in the cell proliferation and differentiation in the pouch. Notably, although there are well-defined guidelines for surveillance of BE, owing to the risk of progression to esophageal adenocarcinoma,2 it is unclear what clinical significance this reverse BE may have in the future. It is important to continue to monitor these patients and clarify the natural history of this finding.
References
1. Brunaldi VO, Jirapinyo P, de Moura DTH et al. Endoscopic treatment of weight regain following Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2018;28:266-76.
2. Weusten B, Bisschops R, Coron E et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-8.
3. Fass R, Sampliner RE. Extension of squamous epithelium into the proximal stomach: a newly recognized mucosal abnormality. Endoscopy. 2000;32:27-32.
Christopher C. Thompson is a consultant for Boston Scientific and Medtronic, a consultant for and has institutional grants from USGI Medical, Olympus, and Apollo Endosurgery.
ginews@gastro.org
Squamous metaplasia
Weight regain can accompany re-emergence of obesity-related comorbidities and, thus, early intervention is important. Although diet, exercise, and behavior modifications are fundamental, they can have limited efficacy. Thus, endoscopic management is important, with specific evaluation for gastrogastric fistulae, pouch dilation, and GJA dilation, all of which can be successfully intervened upon endoscopically. For GJA dilation in particular, APC has been used with promising results.1
In the normal GI tract, the esophagus is lined with squamous epithelium, and the stomach is lined with columnar epithelium. One of the most well-known and well-documented scenarios in which the typical mucosal lining is replaced by abnormal mucosa is Barrett’s esophagus (BE). BE is defined by the replacement of the normal distal squamous epithelial lining with columnar epithelium with a minimum length of 1 cm (tongues or circumferential) containing specialized intestinal metaplasia on histopathologic examination. It is well-documented that treatment of BE with thermal ablation and acid suppression therapy results in re-epithelialization of the esophagus with neosquamous mucosa.2 In contrast with this, in our patients, after we burned the gastric columnar mucosa with APC to treat their dilated GJA, the gastric pouch mucosa has been replaced with squamous epithelium, which we have termed “reverse BE.” To our knowledge, there are no reports of this condition in the literature, nor do we know the precise cause. There is a series of patients without a history of bariatric surgery who developed squamous metaplasia in the proximal gastric cardia.3 The authors hypothesized that this condition may be due to chronic mucosal injury owing to hiatal hernia, reflux, caustic ingestion, chronic gastritis, or pyloric stenosis. We suggest two potential mechanisms for this condition in our patients: 1) extending the ablation to the Z-line on the medial aspect of the pouch may allow for the distal extension of squamous mucosa during the healing process; and 2) acid suppression therapy with proton pump inhibitors after the procedure, in combination with a postoperative decrease in acid production, allows for a shift in the cell proliferation and differentiation in the pouch. Notably, although there are well-defined guidelines for surveillance of BE, owing to the risk of progression to esophageal adenocarcinoma,2 it is unclear what clinical significance this reverse BE may have in the future. It is important to continue to monitor these patients and clarify the natural history of this finding.
References
1. Brunaldi VO, Jirapinyo P, de Moura DTH et al. Endoscopic treatment of weight regain following Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2018;28:266-76.
2. Weusten B, Bisschops R, Coron E et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-8.
3. Fass R, Sampliner RE. Extension of squamous epithelium into the proximal stomach: a newly recognized mucosal abnormality. Endoscopy. 2000;32:27-32.
Christopher C. Thompson is a consultant for Boston Scientific and Medtronic, a consultant for and has institutional grants from USGI Medical, Olympus, and Apollo Endosurgery.
ginews@gastro.org
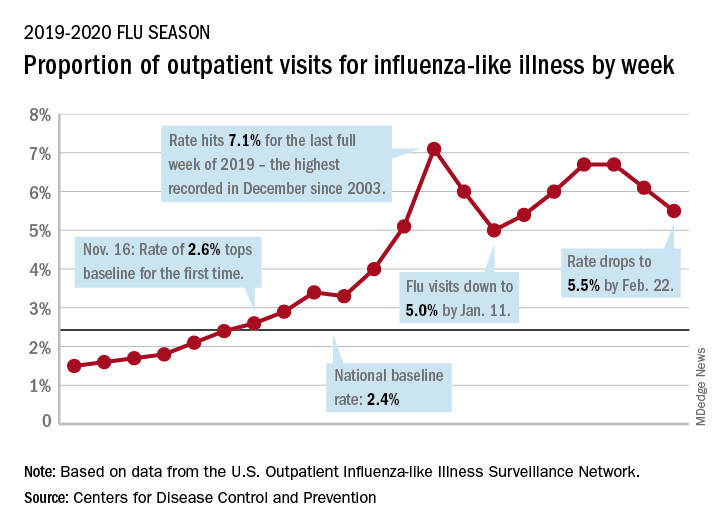
We describe three unique presentations of patients with a prior history of Roux-en-Y gastric bypass who were referred for endoscopic treatment of weight regain. All of the patients had failed prior attempts at lifestyle modifications and pharmacologic weight loss treatment. On physical examination, their body mass indices ranged from 27 to 30 kg/m2, but examination and complete laboratory evaluation, including thyroid-stimulating hormone, were otherwise normal. During the first upper gastrointestinal esophagogastroduodenoscopies, the pouch and the gastrojejunal anastomosis (GJA) were characterized by healthy appearing mucosa. In all three cases, the pouch size measured between 3 and 5 cm and the GJA was dilated, with diameters of more than 20 mm (Figure A). Laser resurfacing of the stoma by argon plasma coagulation (APC) at 0.8 L/min and 70 watts was successfully performed in a 1-cm concentric ring fashion around the gastric side of the GJA (Figure B). Three months after the initial APC, the patients returned for reevaluation. The pouch mucosa again seemed to be normal, but there was persistent, albeit improved, dilation of the GJA. Repeat APC sessions were performed without any adverse events.
On their next follow-up esophagogastroduodenoscopies, all patients were noted to have an abnormal color of approximately 25% of their gastric pouch epithelium, which seemed to be more similar to the esophageal epithelium (Figure C). The GJAs remained dilated, measuring more than 14 mm, and their third APC sessions were performed (Figure D). The patients did well after the procedures, with significant weight loss. A 1-year follow-up endoscopy showed a 10-mm GJA, but the gastric pouch was now approximately 80%-100% covered with this abnormal epithelium (Figure E). A forceps biopsy was performed for histologic evaluation (Figure F).
What is the histopathologic diagnosis of this finding?
DoD and VA Release Updated List of Agent Orange Locations
The VA has released an updated list of locations outside of Vietnam where tactical herbicides have been used, tested, or stored by the US military. The list, which includes the “rainbow” herbicides (Agents Orange, Pink, Green, Purple, Blue, and White), comes from the DoD, after a “thorough review” of research, reports, and government publications in response to a November 2018 US Government Accountability Office (GAO) report.
The GAO made 6 recommendations, including that the DoD develop a process for updating the list, and that the DoD and the VA develop a process for coordinating the communication of the information. The DoD concurred with 4 recommendations.
The VA, responding to the GAO report, said it was “concerned that the report conflates the terms commercial herbicides with tactical herbicides, which are distinct from one another.” Certain testing and storage locations (eg, Kelly Air Force Base), it noted, are added to the list based on the presence of commercial herbicides or “mere components” of Agent Orange or other rainbow agents.
The distinction is important for veterans applying for disability benefits. The impetus for creating the list of testing and storage sites, the VA says, was to carry out the administration of providing disability benefits in accordance with the applicable Agent Orange statute and regulations. Exposure to tactical herbicides (herbicides intended for military operations in Vietnam) is required for the VA to grant benefits on a presumptive basis for Agent Orange conditions outside of Vietnam. Thus, the VA concludes in its response, unless the commercial herbicides were the “same composition, forms, and mixtures” as the estimated 20 million gallons of rainbow agents specifically produced for operations in Vietnam, the “discussion is misleading.”
The VA also did not concur with the recommendation that it take the lead on developing “clear and transparent criteria” for what constitutes a location to be included on the list.
The DoD and VA did agree with the recommendation that the DoD should be the lead agency for producing and updating the list, while the VA will be the lead agency in providing information to veterans. The list will be updated as verifiable information becomes available, said Defense Secretary Mark Esper.
The full list of locations is available at https://www.publichealth.va.gov/docs/agentorange/dod_herbicides_outside_vietnam.pdf.
The GAO report is available at https://www.gao.gov/assets/gao-19-24.pdf.
The VA has released an updated list of locations outside of Vietnam where tactical herbicides have been used, tested, or stored by the US military. The list, which includes the “rainbow” herbicides (Agents Orange, Pink, Green, Purple, Blue, and White), comes from the DoD, after a “thorough review” of research, reports, and government publications in response to a November 2018 US Government Accountability Office (GAO) report.
The GAO made 6 recommendations, including that the DoD develop a process for updating the list, and that the DoD and the VA develop a process for coordinating the communication of the information. The DoD concurred with 4 recommendations.
The VA, responding to the GAO report, said it was “concerned that the report conflates the terms commercial herbicides with tactical herbicides, which are distinct from one another.” Certain testing and storage locations (eg, Kelly Air Force Base), it noted, are added to the list based on the presence of commercial herbicides or “mere components” of Agent Orange or other rainbow agents.
The distinction is important for veterans applying for disability benefits. The impetus for creating the list of testing and storage sites, the VA says, was to carry out the administration of providing disability benefits in accordance with the applicable Agent Orange statute and regulations. Exposure to tactical herbicides (herbicides intended for military operations in Vietnam) is required for the VA to grant benefits on a presumptive basis for Agent Orange conditions outside of Vietnam. Thus, the VA concludes in its response, unless the commercial herbicides were the “same composition, forms, and mixtures” as the estimated 20 million gallons of rainbow agents specifically produced for operations in Vietnam, the “discussion is misleading.”
The VA also did not concur with the recommendation that it take the lead on developing “clear and transparent criteria” for what constitutes a location to be included on the list.
The DoD and VA did agree with the recommendation that the DoD should be the lead agency for producing and updating the list, while the VA will be the lead agency in providing information to veterans. The list will be updated as verifiable information becomes available, said Defense Secretary Mark Esper.
The full list of locations is available at https://www.publichealth.va.gov/docs/agentorange/dod_herbicides_outside_vietnam.pdf.
The GAO report is available at https://www.gao.gov/assets/gao-19-24.pdf.
The VA has released an updated list of locations outside of Vietnam where tactical herbicides have been used, tested, or stored by the US military. The list, which includes the “rainbow” herbicides (Agents Orange, Pink, Green, Purple, Blue, and White), comes from the DoD, after a “thorough review” of research, reports, and government publications in response to a November 2018 US Government Accountability Office (GAO) report.
The GAO made 6 recommendations, including that the DoD develop a process for updating the list, and that the DoD and the VA develop a process for coordinating the communication of the information. The DoD concurred with 4 recommendations.
The VA, responding to the GAO report, said it was “concerned that the report conflates the terms commercial herbicides with tactical herbicides, which are distinct from one another.” Certain testing and storage locations (eg, Kelly Air Force Base), it noted, are added to the list based on the presence of commercial herbicides or “mere components” of Agent Orange or other rainbow agents.
The distinction is important for veterans applying for disability benefits. The impetus for creating the list of testing and storage sites, the VA says, was to carry out the administration of providing disability benefits in accordance with the applicable Agent Orange statute and regulations. Exposure to tactical herbicides (herbicides intended for military operations in Vietnam) is required for the VA to grant benefits on a presumptive basis for Agent Orange conditions outside of Vietnam. Thus, the VA concludes in its response, unless the commercial herbicides were the “same composition, forms, and mixtures” as the estimated 20 million gallons of rainbow agents specifically produced for operations in Vietnam, the “discussion is misleading.”
The VA also did not concur with the recommendation that it take the lead on developing “clear and transparent criteria” for what constitutes a location to be included on the list.
The DoD and VA did agree with the recommendation that the DoD should be the lead agency for producing and updating the list, while the VA will be the lead agency in providing information to veterans. The list will be updated as verifiable information becomes available, said Defense Secretary Mark Esper.
The full list of locations is available at https://www.publichealth.va.gov/docs/agentorange/dod_herbicides_outside_vietnam.pdf.
The GAO report is available at https://www.gao.gov/assets/gao-19-24.pdf.
Team approach greatly reduces inappropriate PPI use
A multidisciplinary, patient-centered approach can dramatically reduce inappropriate use of proton pump inhibitors (PPI), based on the success of a quality improvement study conducted at an academic, primary care clinic in New York.
In 1 year, the program reduced inappropriate PPI use from 80% to 30%, reported lead author Naren Nallapeta, MD, an internal medicine resident at the State University of New York at Buffalo, and colleagues.
According to the investigators, inappropriate use of PPIs is a common and growing concern. Recent patent expirations have led to wider availability of generic and over-the-counter options; and the consequences of unnecessary usage can be serious.
“PPI intake has been found to have a significant association with community-acquired pneumonia, Clostridium difficile associated diarrhea, impaired B12 absorption, hypomagnesemia, hip fractures, acute and chronic kidney disease, and spontaneous bacterial peritonitis in patients with cirrhotic ascites,” the investigators wrote in the Journal of Clinical Gastroenterology.
In 2017, the American Gastroenterological Association released guidelines that include indications for PPIs. But these guidelines often go unheeded, the investigators noted. Multiple studies have documented rates of inappropriate PPI use ranging from 54.1% to 82%. Previous studies have yielded mixed results: Simple physician education alone was found insufficient to reduce inappropriate prescriptions in a primary care setting, whereas studies involving pharmacy personnel have had positive results in various treatment centers.
In a survey of their own clinic, the Erie County Medical Center, an internal medicine service located in a tertiary care safety net hospital at the University at Buffalo, the investigators found that 80% of PPI prescriptions were inappropriate. This prompted a goal to reduce inappropriate use to less than 60% within 1 year.
To achieve this goal, the investigators started a quality improvement project based on the Plan-Do-Study-Act Cycle (PDSA) Model of health care improvement. The quality improvement team included internal medicine attending physicians and physician residents, gastroenterologists, patients, nurses, administrative and information technology staff, and a social worker.
After identifying root causes, the team deployed a variety of strategies to cut down on inappropriate PPI use. First, a new prompt in the electronic medical record reminded physicians to discuss PPI use with patients. Physicians were also given additional training concerning appropriate indications for PPIs, as well as a pocket guide and brochures that could be used for patient education. These efforts were supplemented by an enhanced nursing workflow, as well as continuous reinforcement with positive feedback for health care workers involved.
One year later, results exceeded expectations. Based on data from 180 patients, inappropriate use decreased from 80% to 30%, with a mean discontinuation rate of approximately 50%, an average that was maintained for 6 months beyond the end of the study. The annual, direct cost savings of the program totaled $13,992.
When indicated, patients on long-term PPIs were referred to the gastroenterology service for esophagogastroduodenoscopy. About half of the referred patients (49.8%) completed this procedure, which exceeded the baseline completion rate of less than 30%.
According to principal author Smita Bakhai, MD, of the department of internal medicine at the University at Buffalo, and a physician with UBMB Internal Medicine, the strategies used in this study are broadly applicable.
“The multidisciplinary approach, including patient engagement, would work well in any setting, even with limited resources and without the use of pharmacy personnel,” Dr. Bakhai said in an interview. “We used a patient-centered approach and physicians used shared decision making with patients to taper and discontinue PPIs or switch them to an H2-blocking agent when [patients] did not need to be on chronic PPIs.”
Dr. Bakhai went on to highlight the importance of working with multiple stakeholders.
“This project is unique because it was a resident-led project,” she said. “As academicians, we should always engage the fellows and the residents in any quality improvement work that we do, because they are the doctors of the future.”
Beyond care providers and patients, Dr. Bakhai emphasized the need to involve administrative leadership who can guarantee long-term resources and cultivate the right culture.
“Without the resources, you can’t sustain the project,” Dr. Bakhai said. “You have to have allocated resources, and a culture of safety and quality in the environment that you are doing the project. It has to be a supportive environment. We had all of those things, and that’s why we succeeded.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Nallapeta N et al. J Clin Gastroenterol. 2020 Feb 14. doi: 10.1097/MCG.0000000000001317.
A multidisciplinary, patient-centered approach can dramatically reduce inappropriate use of proton pump inhibitors (PPI), based on the success of a quality improvement study conducted at an academic, primary care clinic in New York.
In 1 year, the program reduced inappropriate PPI use from 80% to 30%, reported lead author Naren Nallapeta, MD, an internal medicine resident at the State University of New York at Buffalo, and colleagues.
According to the investigators, inappropriate use of PPIs is a common and growing concern. Recent patent expirations have led to wider availability of generic and over-the-counter options; and the consequences of unnecessary usage can be serious.
“PPI intake has been found to have a significant association with community-acquired pneumonia, Clostridium difficile associated diarrhea, impaired B12 absorption, hypomagnesemia, hip fractures, acute and chronic kidney disease, and spontaneous bacterial peritonitis in patients with cirrhotic ascites,” the investigators wrote in the Journal of Clinical Gastroenterology.
In 2017, the American Gastroenterological Association released guidelines that include indications for PPIs. But these guidelines often go unheeded, the investigators noted. Multiple studies have documented rates of inappropriate PPI use ranging from 54.1% to 82%. Previous studies have yielded mixed results: Simple physician education alone was found insufficient to reduce inappropriate prescriptions in a primary care setting, whereas studies involving pharmacy personnel have had positive results in various treatment centers.
In a survey of their own clinic, the Erie County Medical Center, an internal medicine service located in a tertiary care safety net hospital at the University at Buffalo, the investigators found that 80% of PPI prescriptions were inappropriate. This prompted a goal to reduce inappropriate use to less than 60% within 1 year.
To achieve this goal, the investigators started a quality improvement project based on the Plan-Do-Study-Act Cycle (PDSA) Model of health care improvement. The quality improvement team included internal medicine attending physicians and physician residents, gastroenterologists, patients, nurses, administrative and information technology staff, and a social worker.
After identifying root causes, the team deployed a variety of strategies to cut down on inappropriate PPI use. First, a new prompt in the electronic medical record reminded physicians to discuss PPI use with patients. Physicians were also given additional training concerning appropriate indications for PPIs, as well as a pocket guide and brochures that could be used for patient education. These efforts were supplemented by an enhanced nursing workflow, as well as continuous reinforcement with positive feedback for health care workers involved.
One year later, results exceeded expectations. Based on data from 180 patients, inappropriate use decreased from 80% to 30%, with a mean discontinuation rate of approximately 50%, an average that was maintained for 6 months beyond the end of the study. The annual, direct cost savings of the program totaled $13,992.
When indicated, patients on long-term PPIs were referred to the gastroenterology service for esophagogastroduodenoscopy. About half of the referred patients (49.8%) completed this procedure, which exceeded the baseline completion rate of less than 30%.
According to principal author Smita Bakhai, MD, of the department of internal medicine at the University at Buffalo, and a physician with UBMB Internal Medicine, the strategies used in this study are broadly applicable.
“The multidisciplinary approach, including patient engagement, would work well in any setting, even with limited resources and without the use of pharmacy personnel,” Dr. Bakhai said in an interview. “We used a patient-centered approach and physicians used shared decision making with patients to taper and discontinue PPIs or switch them to an H2-blocking agent when [patients] did not need to be on chronic PPIs.”
Dr. Bakhai went on to highlight the importance of working with multiple stakeholders.
“This project is unique because it was a resident-led project,” she said. “As academicians, we should always engage the fellows and the residents in any quality improvement work that we do, because they are the doctors of the future.”
Beyond care providers and patients, Dr. Bakhai emphasized the need to involve administrative leadership who can guarantee long-term resources and cultivate the right culture.
“Without the resources, you can’t sustain the project,” Dr. Bakhai said. “You have to have allocated resources, and a culture of safety and quality in the environment that you are doing the project. It has to be a supportive environment. We had all of those things, and that’s why we succeeded.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Nallapeta N et al. J Clin Gastroenterol. 2020 Feb 14. doi: 10.1097/MCG.0000000000001317.
A multidisciplinary, patient-centered approach can dramatically reduce inappropriate use of proton pump inhibitors (PPI), based on the success of a quality improvement study conducted at an academic, primary care clinic in New York.
In 1 year, the program reduced inappropriate PPI use from 80% to 30%, reported lead author Naren Nallapeta, MD, an internal medicine resident at the State University of New York at Buffalo, and colleagues.
According to the investigators, inappropriate use of PPIs is a common and growing concern. Recent patent expirations have led to wider availability of generic and over-the-counter options; and the consequences of unnecessary usage can be serious.
“PPI intake has been found to have a significant association with community-acquired pneumonia, Clostridium difficile associated diarrhea, impaired B12 absorption, hypomagnesemia, hip fractures, acute and chronic kidney disease, and spontaneous bacterial peritonitis in patients with cirrhotic ascites,” the investigators wrote in the Journal of Clinical Gastroenterology.
In 2017, the American Gastroenterological Association released guidelines that include indications for PPIs. But these guidelines often go unheeded, the investigators noted. Multiple studies have documented rates of inappropriate PPI use ranging from 54.1% to 82%. Previous studies have yielded mixed results: Simple physician education alone was found insufficient to reduce inappropriate prescriptions in a primary care setting, whereas studies involving pharmacy personnel have had positive results in various treatment centers.
In a survey of their own clinic, the Erie County Medical Center, an internal medicine service located in a tertiary care safety net hospital at the University at Buffalo, the investigators found that 80% of PPI prescriptions were inappropriate. This prompted a goal to reduce inappropriate use to less than 60% within 1 year.
To achieve this goal, the investigators started a quality improvement project based on the Plan-Do-Study-Act Cycle (PDSA) Model of health care improvement. The quality improvement team included internal medicine attending physicians and physician residents, gastroenterologists, patients, nurses, administrative and information technology staff, and a social worker.
After identifying root causes, the team deployed a variety of strategies to cut down on inappropriate PPI use. First, a new prompt in the electronic medical record reminded physicians to discuss PPI use with patients. Physicians were also given additional training concerning appropriate indications for PPIs, as well as a pocket guide and brochures that could be used for patient education. These efforts were supplemented by an enhanced nursing workflow, as well as continuous reinforcement with positive feedback for health care workers involved.
One year later, results exceeded expectations. Based on data from 180 patients, inappropriate use decreased from 80% to 30%, with a mean discontinuation rate of approximately 50%, an average that was maintained for 6 months beyond the end of the study. The annual, direct cost savings of the program totaled $13,992.
When indicated, patients on long-term PPIs were referred to the gastroenterology service for esophagogastroduodenoscopy. About half of the referred patients (49.8%) completed this procedure, which exceeded the baseline completion rate of less than 30%.
According to principal author Smita Bakhai, MD, of the department of internal medicine at the University at Buffalo, and a physician with UBMB Internal Medicine, the strategies used in this study are broadly applicable.
“The multidisciplinary approach, including patient engagement, would work well in any setting, even with limited resources and without the use of pharmacy personnel,” Dr. Bakhai said in an interview. “We used a patient-centered approach and physicians used shared decision making with patients to taper and discontinue PPIs or switch them to an H2-blocking agent when [patients] did not need to be on chronic PPIs.”
Dr. Bakhai went on to highlight the importance of working with multiple stakeholders.
“This project is unique because it was a resident-led project,” she said. “As academicians, we should always engage the fellows and the residents in any quality improvement work that we do, because they are the doctors of the future.”
Beyond care providers and patients, Dr. Bakhai emphasized the need to involve administrative leadership who can guarantee long-term resources and cultivate the right culture.
“Without the resources, you can’t sustain the project,” Dr. Bakhai said. “You have to have allocated resources, and a culture of safety and quality in the environment that you are doing the project. It has to be a supportive environment. We had all of those things, and that’s why we succeeded.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Nallapeta N et al. J Clin Gastroenterol. 2020 Feb 14. doi: 10.1097/MCG.0000000000001317.
FROM JOURNAL OF CLINICAL GASTROENTEROLGOY
Children bearing the brunt of declining flu activity
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
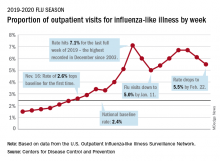
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
Oncology dominates clinical trial landscape
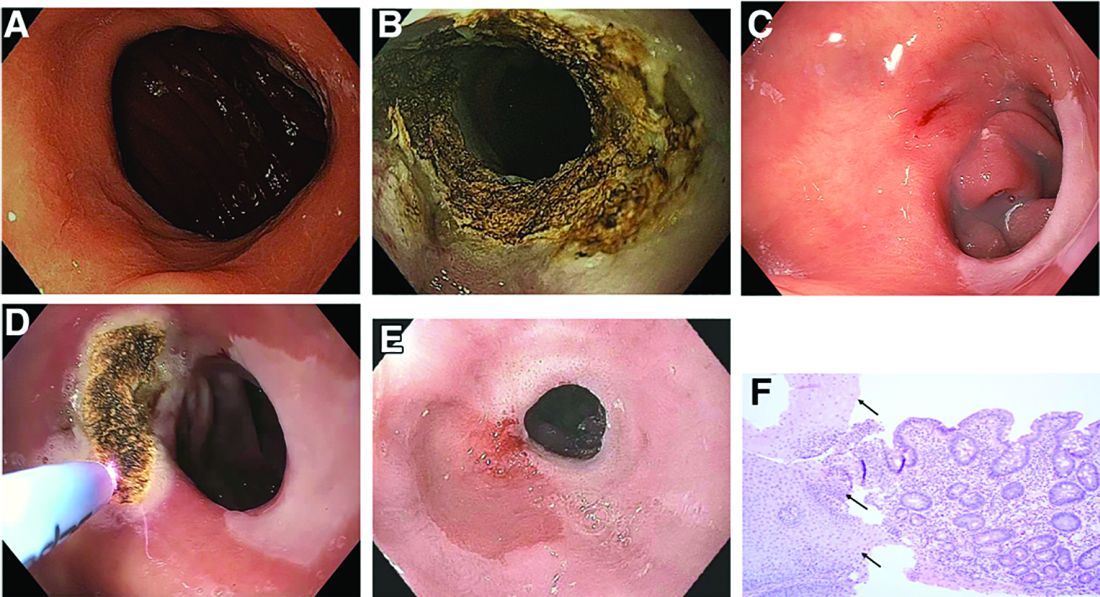
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.
“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Keep your eye on tapinarof, a topical antipsoriatic therapy
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR