User login
Thyroid dysfunction is common in patients with diabetes
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
FROM CLINICAL ENDOCRINOLOGY
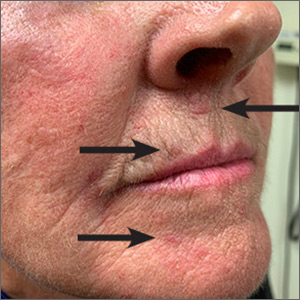
Red lesions on face
These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.
These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.
Pence named COVID-19 point person as CDC reports possible community spread
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Joint replacement: What’s new in 2020
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
MAUI, HAWAII – Outpatient total hip and knee replacement is “the latest craze” in orthopedic surgery, and it’s being driven by the might of Medicare, William Bugbee, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“In 2019, Medicare took total knee replacement off the inpatient-only list, meaning you could do it as an outpatient. And just in January 2020, they took total hips off that list. So I have to designate most of my hip and knee replacements as outpatients, even if I do it in the hospital and keep them for 1 night. And some of the private insurers have already gone to that, so they’ll deny coverage if I say I want a 1-day hospital stay, believe it or not,” according to Dr. Bugbee, chief of joint reconstruction in the department of orthopedics at the Scripps Clinic in La Jolla, Calif.
He provided a behind-the-scenes look at contemporary trends in joint replacement as well as tips on how rheumatologists can best help their patients get through the experience with excellent outcomes.
Joint replacement remains the best treatment for advanced arthritis of the hips and knees, he said. There is a high degree of confidence about the predictability and durability of the results. But joint replacement has become highly commoditized.
“We’re getting pummeled by Medicare to make this as cheap as possible,” the orthopedic surgeon explained. “An implant costs the hospital $3,000-$6,000. A care episode for a primary total joint replacement should cost a hospital $8,000-$15,000, which is about what Medicare pays for the [Diagnosis Related Group], so the margins are small. That’s why we’re being drilled on about how much we spend on every little thing. We hardly do any labs, x-rays, anything.”
As a result of recent advances in pre-, peri-, and postoperative management, outpatient joint replacement has become a safe and comparatively economical option for generally healthy patients.
“We’ve engineered a much better patient experience, so the assault and battery of 5, 10, 15 years ago isn’t so bad anymore,” Dr. Bugbee said.
Rheumatologists can expect to see a growing number of their patients undergoing total knee or hip replacement at outpatient surgery centers. That’s not a bad thing so long as the procedure is being done there because the outpatient center employs best practices in order to provide a highly efficient episode of care supported by excellent outcome data, he continued.
State-of-the-art perioperative management in 2020 includes accelerated-care pathways that allow ambulation within an hour or 2 after surgery along with same-day discharge, regional anesthesia with motor-sparing nerve blocks, and multimodal pain management with avoidance of intravenous narcotics except in opioid-tolerant patients. Tranexamic acid is now widely used in order to reduce operative blood loss.
“When I started practice 25 years ago, 50% of patients got a blood transfusion. I haven’t given a blood transfusion to a patient in probably 2 years. Tranexamic acid reduces blood loss by 500-700 cc with no discernible adverse effects. It’s truly remarkable,” he said.
Another important technical advance has been the routine use of oral dexamethasone. “Decadron is an antiemetic, it has anti-inflammatory effects, and it makes people happy. It’s a simple, cheap drug that has revolutionized care,” the surgeon continued.
Postoperative management has been streamlined. Dr. Bugbee is among many orthopedic surgeons who no longer routinely prescribe therapist-directed formal physical therapy for total hip arthroplasty patients, relying instead upon online tools and apps for self-administered physical therapy. Pedal exercise devices available online for $30 or so have been shown to be as effective as supervised physical therapy for knee rehabilitation.
What patients want to know about joint replacement
The question patients most often ask both their referring physician and the orthopedic surgeon is, “How long will my joint replacement last?” The best available data come from a couple of recent paired meta-analyses. The investigators reported 82% implant survivorship 25 years after primary total knee arthroplasty and 70% after unicondylar knee arthroplasty as well as a 25-year implant survivorship rate of 77% for total hip arthroplasty.
“I expected that hip arthroplasty survivorship rate to be much higher than 77%. The reason it’s not is probably because of the metal-on-metal bearing surface debacle of about 10 years ago. There’ve been lots of revisions because of that. We thought metal-on-metal implants were going to be all that, with microscopically low wear, but they turned out to be a nightmare because of metal ion release,” Dr. Bugbee observed.
The long-term joint survivorship data are based upon older implants. Encouraging albeit still preliminary data suggest contemporary implants may last significantly longer. The “clear winner,” he said, is a 36-mm ceramic head and a highly crosslinked polyethylene liner.
“That’s been a game changer, with a 10- to 20-fold decrease in wear compared to plastics for weight-bearing surfaces,” Dr. Bugbee said.
In terms of functional improvement, by various measures 85%-97% of patients are satisfied with the results of their total hip replacement, and 60% report returning to high-level recreational activities. Patient satisfaction scores are lower – 75%-90% – after total knee arthroplasty.
“The total knee replacement just doesn’t work like a regular joint,” the surgeon observed. “When I think of hip and knee replacements, I think of a hip as a Ferrari – it’s a high-performance joint replacement – and I think of the knee as a Ford – it’s serviceable, it does the job, and it’s okay but not fantastic.”
How referring physicians can optimize preoperative management and long-term follow-up
Orthopedic surgeons would appreciate help from rheumatologists and primary care physicians in preoperatively addressing the known modifiable risk factors for poor outcomes of joint replacement. These include obesity, smoking, depression, a hemoglobin A1c of 7% or more, and being on opioids. These risk factors are incompatible with outpatient hip or knee replacement.
“Let the surgeon know if you think outpatient joint replacement is a bad idea in your patient for medical reasons,” Dr. Bugbee urged.
Also, orthopedic surgeons can generally benefit from rheumatologist input regarding perioperative management of patients on standard disease-modifying antirheumatic drugs, biologics, or Janus kinase inhibitors as recommended in the guidelines published jointly by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“I can guarantee you that most orthopedic surgeons don’t know about these guidelines. The evidence base for these recommendations is not great, but these are the best guidelines we have,” Dr. Bugbee said.
After joint replacement surgery a patient should get an x-ray of the replacement every 5 years. And if a patient develops a painful hip after arthroplasty, it’s worthwhile to order blood chromium and cobalt levels.
“The implant weight-bearing surface matters. You can’t necessarily tell on x-ray what’s a metal-on-metal hip and what’s metal-on-plastic or ceramic. You already send people for a lot of labs. If you see a patient with a painful total hip replacement, just add a cobalt and chromium. If they’re elevated, talk to the orthopedist,” he advised.
The road ahead
Hip and knee replacement is an $18 billion market today. And it’s a major growth industry: According to a recent projection, there will be 1 million total hip replacements and 4 million total knee replacements annually 10 years from now, figures four times greater than projected for 2030 in an earlier 2005 estimate. The rapid growth is coming from the expanding elderly population combined with a virtual epidemic of posttraumatic arthritis in young people – but decidedly not from patients with joint failure attributable to rheumatoid arthritis.
“Congratulations! You’ve eradicated rheumatoid arthritis from my practice,” Dr. Bugbee declared. “Most of the rheumatoid arthritis patients who come to me come because they have osteoarthritis in their joint, not because of their rheumatoid arthritis.”
He reported serving as a consultant to Orthalign, Insight Medical, and Arthrex, and receiving royalties from Smith and Nephew and Depuy.
REPORTING FROM RWCS 2020
ACIP advocates pre-exposure Ebola vaccination for high-risk groups
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
Vaccination against the Ebola virus is recommended for first responders, health care personnel, and laboratory workers deemed at high risk of exposure, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The committee voted unanimously to recommended pre-exposure vaccination with the rVSVdeltaG-ZEBOV-GP vaccine for adults aged 18 years and older who are at potential risk of exposure to the Ebola species Zaire ebolavirus because they fall into any of the following three categories:
- They are responding to an outbreak of Ebola virus disease.
- They are working as health care personnel at a federally designated Ebola Treatment Center in the United States.
- The are working in laboratories or are other staff members at biosafety-level 4 facilities in the United States.
Mary Choi, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) presented data on the safety and effectiveness of the vaccine and the work group considerations in recommending vaccination in the three target populations.
In clinical trials, the most commonly reported adverse events associated with the vaccine were arthritis and arthralgia, Dr. Choi said, but the duration of those cases was limited to months and did not persist long term.
Pre-exposure vaccination for health care personnel, laboratory workers, and support staff would provide an additional layer of protection, she explained, in addition to existing safeguards such as personal protective equipment and engineering controls at the facility. The work group’s research showed that most of the target population believed that the desirable effects of that protection outweigh potentially undesirable effects, Dr. Choi noted.
Some committee members expressed concerns about vaccination of pregnant women. But the recommendations are presented as “population based, not shared decision making,” said Sharon E. Frey, MD, of Saint Louis University in St. Louis, Missouri.
Several members noted that pregnant women should not be automatically included or excluded from vaccination if they fall into a high-risk population. And the committee agreed that additional guidance in the policy note will help assess risk and that organizations will determine the risk for their employees and whether to offer the vaccine.
The FDA approved the currently available U.S. vaccine for Ebola in 2019. Merck manufactures that vaccine.
The ACIP members had no relevant financial conflicts to disclose.
New CAR T-cell therapy eliminates MM and tumor propagating cells without fratricide in lab study
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
These cells proved to be active in vitro and in vivo against MM plasma cells, memory B cells, and MM-propagating cells, according to a report in Nature Communications.
This research is important because most MM patients eventually succumb to the disease and previously developed CAR T cells targeting B-cell maturation antigen (BCMA) on MM cells have shown high-response rates but limited durability.
Previous research showed that CD229/LY9 is a potential target for CAR T-cell therapy in MM because of its strong and homogeneous expression on the bulk of tumor cells, as well as chemotherapy-resistant myeloma progenitors; its absence from most normal cells; and dependence of MM cells on CD229 for their survival, according to Sabarinath V. Radhakrishnan, MD, of the University of Utah, Salt Lake City, and colleagues.
Using primary CD138+ tumor cells from three patients with plasma cell leukemia, a highly aggressive form of MM, which all showed high expression of CD229, the researchers found that CD229 CAR T cells exhibited high cytotoxic activity against these cells. In addition, when assessing two MM cell lines, U-266 and RPMI-8226, expressing different levels of CD229, they found that CD229 CAR T cells efficiently killed both cell lines in vitro.
“We do not observe fratricide during CD229 CAR T-cell production, as CD229 is downregulated in T cells during activation. In addition, while CD229 CAR T cells target normal CD229high T cells, they spare functional CD229neg/low T cells. These findings indicate that CD229 CAR T cells may be an effective treatment for patients with MM,” the authors concluded.
The study was funded by several nongovernmental organizations and the National Cancer Institute. Three of the authors are inventors on PCT application US2017/42840 “Antibodies and CAR T Cells for the Treatment of Multiple Myeloma” describing the therapeutic use of CD229 CAR T cells.
SOURCE: Radhakrishnan SV et al. Nat Commun. 2020 Feb 7;11(1):798. doi: 10.1038/s41467-020-14619-z.
FROM NATURE COMMUNICATIONS
HDL hypothesis: New trial expected to show why prior ones failed
NATIONAL HARBOR, MD. – If positive, a major ongoing phase 3 trial of CSL112, an agent designed to promote efflux of cholesterol from macrophages, is positioned to prove the HDL hypothesis, according to an outline of the rationale of the trial at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“Twenty papers now show better efflux means better outcomes independent of standard risk factors” and “we know this drug improves efflux,” explained C. Michael Gibson, MD, an interventional cardiologist at Beth Israel Deaconess Hospital, Boston.
The HDL hypothesis was derived from the Framingham Heart Study, which correlated high levels of HDL cholesterol with a reduced risk of adverse cardiovascular (CV) outcomes, according to Dr. Gibson. Just as elevated LDL proved to be a treatable risk factor for CV events, reduced HDL was the target of numerous trials to achieve the same types of benefits.
All have failed.
The problem has been in seeing HDL as a number without addressing its function, Dr. Gibson said. In essence, he believes “the HDL hypothesis not been really tested to date.”
CSL112 is a novel formulation of apolipoprotein A-1 (apoA-1) that has been purified from human plasma and reconstituted to form HDL. In the experimental and clinical setting, including the AEGIS I pilot study, weekly infusions of CSL112 have been associated with a degree of cholesterol efflux that predicts major CV risk reductions.
At the same time that the multinational event-driven AEGIS II trial will determine whether cholesterol efflux with CSL112 does translate into protection from CV events, it will also examine the HDL side of the lipid equation. Dr. Gibson said that it is specifically designed to circumvent the weaknesses of previous efforts to target HDL for reducing CV risk.
“The previous studies were conducted in the wrong patients with the wrong drugs given in the wrong doses at the wrong times,” said Dr. Gibson, who is also professor of medicine at Harvard Medical School, Boston.
One major difference from previous trials is that AEGIS II is enrolling patients with an acute coronary syndrome rather than stable atherosclerosis. Many of those being enrolled have had a recent event. Also, rather than raising HDL, the goal of CSL112 is to increase cholesterol efflux, which is now considered to be the key function of HDL. Furthermore, the time frame for the primary outcome, which is a composite of major adverse cardiac outcomes (MACE), is 90 days rather than several years.
In patients with ACS, “it is the early period of vulnerability where efflux of cholesterol really appears to have the greatest influence on outcomes,” Dr. Gibson explained.
The failure of previous efforts to treat HDL now appears to be based on an incomplete understanding of the goals, according to Dr. Gibson. The doomed cholesteryl ester transfer protein (CETP) drugs, for example, effectively increased HDL levels, but generated a form of HDL that “was not all that functional.”
He noted that niacin raises HDL but has off-target effects. Apo-A1 Milano, a mutant variation of apo-A1, is now understood to reduce the endogenous form, which Dr. Gibson said might explain its counterproductive effect on CV protection.
Using a garbage truck analogy to explain the growing appreciation of factors involved in cholesterol accumulation in the macrophage, Dr. Gibson characterized ABCA1, a transporter protein sitting on the surface of the macrophage, as the loader. He described LCAT (lecithin-cholesterol acyltransferase), an enzyme that converts cholesterol into cholesteryl ester, as the compactor. He sees CRL112 as an empty garbage truck sent into the macrophage to reverse the process.
“We are moving beyond thinking of HDL as a number to try to better appreciate its function,” Dr. Gibson said.
The AEGIS II trial was opened in March of 2018. It has a planned enrollment of 17,400 patients, with an estimated completion date of October 2021.
Dr. Gibson reports financial relationships with Bayer, Janssen, Johnson & Johnson, and CSL Behring, the sponsor of the AEGIS II trial.
NATIONAL HARBOR, MD. – If positive, a major ongoing phase 3 trial of CSL112, an agent designed to promote efflux of cholesterol from macrophages, is positioned to prove the HDL hypothesis, according to an outline of the rationale of the trial at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“Twenty papers now show better efflux means better outcomes independent of standard risk factors” and “we know this drug improves efflux,” explained C. Michael Gibson, MD, an interventional cardiologist at Beth Israel Deaconess Hospital, Boston.
The HDL hypothesis was derived from the Framingham Heart Study, which correlated high levels of HDL cholesterol with a reduced risk of adverse cardiovascular (CV) outcomes, according to Dr. Gibson. Just as elevated LDL proved to be a treatable risk factor for CV events, reduced HDL was the target of numerous trials to achieve the same types of benefits.
All have failed.
The problem has been in seeing HDL as a number without addressing its function, Dr. Gibson said. In essence, he believes “the HDL hypothesis not been really tested to date.”
CSL112 is a novel formulation of apolipoprotein A-1 (apoA-1) that has been purified from human plasma and reconstituted to form HDL. In the experimental and clinical setting, including the AEGIS I pilot study, weekly infusions of CSL112 have been associated with a degree of cholesterol efflux that predicts major CV risk reductions.
At the same time that the multinational event-driven AEGIS II trial will determine whether cholesterol efflux with CSL112 does translate into protection from CV events, it will also examine the HDL side of the lipid equation. Dr. Gibson said that it is specifically designed to circumvent the weaknesses of previous efforts to target HDL for reducing CV risk.
“The previous studies were conducted in the wrong patients with the wrong drugs given in the wrong doses at the wrong times,” said Dr. Gibson, who is also professor of medicine at Harvard Medical School, Boston.
One major difference from previous trials is that AEGIS II is enrolling patients with an acute coronary syndrome rather than stable atherosclerosis. Many of those being enrolled have had a recent event. Also, rather than raising HDL, the goal of CSL112 is to increase cholesterol efflux, which is now considered to be the key function of HDL. Furthermore, the time frame for the primary outcome, which is a composite of major adverse cardiac outcomes (MACE), is 90 days rather than several years.
In patients with ACS, “it is the early period of vulnerability where efflux of cholesterol really appears to have the greatest influence on outcomes,” Dr. Gibson explained.
The failure of previous efforts to treat HDL now appears to be based on an incomplete understanding of the goals, according to Dr. Gibson. The doomed cholesteryl ester transfer protein (CETP) drugs, for example, effectively increased HDL levels, but generated a form of HDL that “was not all that functional.”
He noted that niacin raises HDL but has off-target effects. Apo-A1 Milano, a mutant variation of apo-A1, is now understood to reduce the endogenous form, which Dr. Gibson said might explain its counterproductive effect on CV protection.
Using a garbage truck analogy to explain the growing appreciation of factors involved in cholesterol accumulation in the macrophage, Dr. Gibson characterized ABCA1, a transporter protein sitting on the surface of the macrophage, as the loader. He described LCAT (lecithin-cholesterol acyltransferase), an enzyme that converts cholesterol into cholesteryl ester, as the compactor. He sees CRL112 as an empty garbage truck sent into the macrophage to reverse the process.
“We are moving beyond thinking of HDL as a number to try to better appreciate its function,” Dr. Gibson said.
The AEGIS II trial was opened in March of 2018. It has a planned enrollment of 17,400 patients, with an estimated completion date of October 2021.
Dr. Gibson reports financial relationships with Bayer, Janssen, Johnson & Johnson, and CSL Behring, the sponsor of the AEGIS II trial.
NATIONAL HARBOR, MD. – If positive, a major ongoing phase 3 trial of CSL112, an agent designed to promote efflux of cholesterol from macrophages, is positioned to prove the HDL hypothesis, according to an outline of the rationale of the trial at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
“Twenty papers now show better efflux means better outcomes independent of standard risk factors” and “we know this drug improves efflux,” explained C. Michael Gibson, MD, an interventional cardiologist at Beth Israel Deaconess Hospital, Boston.
The HDL hypothesis was derived from the Framingham Heart Study, which correlated high levels of HDL cholesterol with a reduced risk of adverse cardiovascular (CV) outcomes, according to Dr. Gibson. Just as elevated LDL proved to be a treatable risk factor for CV events, reduced HDL was the target of numerous trials to achieve the same types of benefits.
All have failed.
The problem has been in seeing HDL as a number without addressing its function, Dr. Gibson said. In essence, he believes “the HDL hypothesis not been really tested to date.”
CSL112 is a novel formulation of apolipoprotein A-1 (apoA-1) that has been purified from human plasma and reconstituted to form HDL. In the experimental and clinical setting, including the AEGIS I pilot study, weekly infusions of CSL112 have been associated with a degree of cholesterol efflux that predicts major CV risk reductions.
At the same time that the multinational event-driven AEGIS II trial will determine whether cholesterol efflux with CSL112 does translate into protection from CV events, it will also examine the HDL side of the lipid equation. Dr. Gibson said that it is specifically designed to circumvent the weaknesses of previous efforts to target HDL for reducing CV risk.
“The previous studies were conducted in the wrong patients with the wrong drugs given in the wrong doses at the wrong times,” said Dr. Gibson, who is also professor of medicine at Harvard Medical School, Boston.
One major difference from previous trials is that AEGIS II is enrolling patients with an acute coronary syndrome rather than stable atherosclerosis. Many of those being enrolled have had a recent event. Also, rather than raising HDL, the goal of CSL112 is to increase cholesterol efflux, which is now considered to be the key function of HDL. Furthermore, the time frame for the primary outcome, which is a composite of major adverse cardiac outcomes (MACE), is 90 days rather than several years.
In patients with ACS, “it is the early period of vulnerability where efflux of cholesterol really appears to have the greatest influence on outcomes,” Dr. Gibson explained.
The failure of previous efforts to treat HDL now appears to be based on an incomplete understanding of the goals, according to Dr. Gibson. The doomed cholesteryl ester transfer protein (CETP) drugs, for example, effectively increased HDL levels, but generated a form of HDL that “was not all that functional.”
He noted that niacin raises HDL but has off-target effects. Apo-A1 Milano, a mutant variation of apo-A1, is now understood to reduce the endogenous form, which Dr. Gibson said might explain its counterproductive effect on CV protection.
Using a garbage truck analogy to explain the growing appreciation of factors involved in cholesterol accumulation in the macrophage, Dr. Gibson characterized ABCA1, a transporter protein sitting on the surface of the macrophage, as the loader. He described LCAT (lecithin-cholesterol acyltransferase), an enzyme that converts cholesterol into cholesteryl ester, as the compactor. He sees CRL112 as an empty garbage truck sent into the macrophage to reverse the process.
“We are moving beyond thinking of HDL as a number to try to better appreciate its function,” Dr. Gibson said.
The AEGIS II trial was opened in March of 2018. It has a planned enrollment of 17,400 patients, with an estimated completion date of October 2021.
Dr. Gibson reports financial relationships with Bayer, Janssen, Johnson & Johnson, and CSL Behring, the sponsor of the AEGIS II trial.
EXPERT ANALYSIS FROM CRT 2020
Morning and evening skin care: What to tell patients
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Early results with ‘optimized’ gene therapy in hemophilia A
First-in-human results suggest a gene therapy, BAY 2599023, can increase factor VIII levels in patients with hemophilia A, but it isn’t clear if BAY 2599023 can eliminate the need for prophylaxis.
Of four patients who received BAY 2599023, three achieved clinically meaningful factor VIII levels, and all were able to stop prophylaxis at some point. However, one patient had to resume prophylaxis, and all four have experienced bleeds since receiving BAY 2599023.
This is a dose-escalating trial (NCT03588299), so researchers are still attempting to determine the optimal dose of BAY 2599023. Upcoming results in two patients treated at the highest dose should provide more insight, according to Steven W. Pipe, MD, of the University of Michigan in Ann Arbor.
Dr. Pipe presented results with BAY 2599023 at the annual congress of the European Association for Haemophilia and Allied Disorders.
He noted that between 30% and 70% of hemophilia patients may have preexisting neutralizing antibodies to specific adeno-associated virus (AAV) serotypes. Developing gene therapies with varying serotypes could reduce this problem and increase eligibility for gene therapy. Bayer had this in mind when developing BAY 2599023.
BAY 2599023 is a non-replicating AAV vector, based on the AAV serotype hu37, which contains a single-stranded DNA genome encoding a B-domain deleted factor VIII, under the control of a liver-specific promotor/enhancer combination optimized for transgenic expression, Dr. Pipe explained.
He presented results with BAY 2599023 in four patients with severe hemophilia A. At baseline, patients were receiving prophylaxis and did not have factor VIII inhibitors or detectable immunity to the AAVhu37 capsid.
First cohort
Two patients received BAY 2599023 at a dose of 0.5 × 1013 GC/kg. One patient achieved a factor VIII level above 5% at this dose and was able to stop prophylaxis temporarily. The patient experienced two bleeds on study and had to resume prophylaxis because of a target joint.
“He came into the study with a particularly challenging target joint in his ankle, and he had known from experience on prophylaxis that, if his factor level got too low, he would have breakthrough bleeds,” Dr. Pipe explained. “So we agreed that if he didn’t achieve a sustained level above 5%, he would go back on prophylaxis. He was able to stay off prophylaxis for 6 weeks, but we had to put him back on because of breakthrough bleeding.”
Dr. Pipe and colleagues have been following this patient for about a year, and the patient still has factor VIII levels of about 2% to 3% after a washout of prophylaxis.
The other patient who received a dose of 0.5 × 1013 GC/kg came off prophylaxis early on and has sustained factor VIII levels of 20%. This patient had four bleeds early in the trial but has since done very well, Dr. Pipe said. The patient had 99 bleeds in the year prior to receiving BAY 2599023.
Second cohort
Two subsequent patients received BAY 2599023 at a dose of 1 × 1013 GC/kg. Both patients have stopped prophylaxis.
One patient achieved factor VIII levels in the 5%-10% range and stopped prophylaxis at day 49. The patient has received on-demand treatment for four bleeds. The other patient achieved “robust” factor VIII expression levels “well above 20%,” according to Dr. Pipe. This patient stopped prophylaxis at day 10 and experienced bleeds early on but has remained bleed-free and treatment-free for 7 months.
Dr. Pipe noted that this patient experienced a spike in transaminases “very early on” after receiving BAY 2599023.
“So he was put on prednisone, but his liver enzymes almost immediately corrected to within the normal range,” Dr. Pipe said. “He has finished the cascading dose of prednisone, his liver enzymes have remained stable, his factor VIII expression has remained stable, and he continues to do very well.”
Next steps
Based on results in the first two cohorts, researchers are proceeding with a higher dose of BAY 2599023.
“The overall goal of this study is to identify the optimal dose that can be taken forward to the planned phase 3 study,” Dr. Pipe said. “What they [Bayer] would like to bring to the community is a product that offers a chance for maximum eligibility based on low screen out because of preexisting immunity and maximize the number of patients who are in the normal range.”
“For patients to embrace gene therapy, it really has to get them at least the possibility to land within the normal range and, hopefully, be not just liberated from prophylaxis but maybe liberated from the need for factor VIII at all,” Dr. Pipe added. “I think if these AAV platform therapies can’t deliver that on a relatively consistent basis, it’s going to be challenging to get patients to embrace this technology.”
The phase 1/2 trial is sponsored by Bayer in collaboration with Ultragenyx Pharmaceutical Inc. Dr. Pipe disclosed relationships with Bayer and other companies.
SOURCE: Pipe SW et al. EAHAD 2020. Abstract P190.
First-in-human results suggest a gene therapy, BAY 2599023, can increase factor VIII levels in patients with hemophilia A, but it isn’t clear if BAY 2599023 can eliminate the need for prophylaxis.
Of four patients who received BAY 2599023, three achieved clinically meaningful factor VIII levels, and all were able to stop prophylaxis at some point. However, one patient had to resume prophylaxis, and all four have experienced bleeds since receiving BAY 2599023.
This is a dose-escalating trial (NCT03588299), so researchers are still attempting to determine the optimal dose of BAY 2599023. Upcoming results in two patients treated at the highest dose should provide more insight, according to Steven W. Pipe, MD, of the University of Michigan in Ann Arbor.
Dr. Pipe presented results with BAY 2599023 at the annual congress of the European Association for Haemophilia and Allied Disorders.
He noted that between 30% and 70% of hemophilia patients may have preexisting neutralizing antibodies to specific adeno-associated virus (AAV) serotypes. Developing gene therapies with varying serotypes could reduce this problem and increase eligibility for gene therapy. Bayer had this in mind when developing BAY 2599023.
BAY 2599023 is a non-replicating AAV vector, based on the AAV serotype hu37, which contains a single-stranded DNA genome encoding a B-domain deleted factor VIII, under the control of a liver-specific promotor/enhancer combination optimized for transgenic expression, Dr. Pipe explained.
He presented results with BAY 2599023 in four patients with severe hemophilia A. At baseline, patients were receiving prophylaxis and did not have factor VIII inhibitors or detectable immunity to the AAVhu37 capsid.
First cohort
Two patients received BAY 2599023 at a dose of 0.5 × 1013 GC/kg. One patient achieved a factor VIII level above 5% at this dose and was able to stop prophylaxis temporarily. The patient experienced two bleeds on study and had to resume prophylaxis because of a target joint.
“He came into the study with a particularly challenging target joint in his ankle, and he had known from experience on prophylaxis that, if his factor level got too low, he would have breakthrough bleeds,” Dr. Pipe explained. “So we agreed that if he didn’t achieve a sustained level above 5%, he would go back on prophylaxis. He was able to stay off prophylaxis for 6 weeks, but we had to put him back on because of breakthrough bleeding.”
Dr. Pipe and colleagues have been following this patient for about a year, and the patient still has factor VIII levels of about 2% to 3% after a washout of prophylaxis.
The other patient who received a dose of 0.5 × 1013 GC/kg came off prophylaxis early on and has sustained factor VIII levels of 20%. This patient had four bleeds early in the trial but has since done very well, Dr. Pipe said. The patient had 99 bleeds in the year prior to receiving BAY 2599023.
Second cohort
Two subsequent patients received BAY 2599023 at a dose of 1 × 1013 GC/kg. Both patients have stopped prophylaxis.
One patient achieved factor VIII levels in the 5%-10% range and stopped prophylaxis at day 49. The patient has received on-demand treatment for four bleeds. The other patient achieved “robust” factor VIII expression levels “well above 20%,” according to Dr. Pipe. This patient stopped prophylaxis at day 10 and experienced bleeds early on but has remained bleed-free and treatment-free for 7 months.
Dr. Pipe noted that this patient experienced a spike in transaminases “very early on” after receiving BAY 2599023.
“So he was put on prednisone, but his liver enzymes almost immediately corrected to within the normal range,” Dr. Pipe said. “He has finished the cascading dose of prednisone, his liver enzymes have remained stable, his factor VIII expression has remained stable, and he continues to do very well.”
Next steps
Based on results in the first two cohorts, researchers are proceeding with a higher dose of BAY 2599023.
“The overall goal of this study is to identify the optimal dose that can be taken forward to the planned phase 3 study,” Dr. Pipe said. “What they [Bayer] would like to bring to the community is a product that offers a chance for maximum eligibility based on low screen out because of preexisting immunity and maximize the number of patients who are in the normal range.”
“For patients to embrace gene therapy, it really has to get them at least the possibility to land within the normal range and, hopefully, be not just liberated from prophylaxis but maybe liberated from the need for factor VIII at all,” Dr. Pipe added. “I think if these AAV platform therapies can’t deliver that on a relatively consistent basis, it’s going to be challenging to get patients to embrace this technology.”
The phase 1/2 trial is sponsored by Bayer in collaboration with Ultragenyx Pharmaceutical Inc. Dr. Pipe disclosed relationships with Bayer and other companies.
SOURCE: Pipe SW et al. EAHAD 2020. Abstract P190.
First-in-human results suggest a gene therapy, BAY 2599023, can increase factor VIII levels in patients with hemophilia A, but it isn’t clear if BAY 2599023 can eliminate the need for prophylaxis.
Of four patients who received BAY 2599023, three achieved clinically meaningful factor VIII levels, and all were able to stop prophylaxis at some point. However, one patient had to resume prophylaxis, and all four have experienced bleeds since receiving BAY 2599023.
This is a dose-escalating trial (NCT03588299), so researchers are still attempting to determine the optimal dose of BAY 2599023. Upcoming results in two patients treated at the highest dose should provide more insight, according to Steven W. Pipe, MD, of the University of Michigan in Ann Arbor.
Dr. Pipe presented results with BAY 2599023 at the annual congress of the European Association for Haemophilia and Allied Disorders.
He noted that between 30% and 70% of hemophilia patients may have preexisting neutralizing antibodies to specific adeno-associated virus (AAV) serotypes. Developing gene therapies with varying serotypes could reduce this problem and increase eligibility for gene therapy. Bayer had this in mind when developing BAY 2599023.
BAY 2599023 is a non-replicating AAV vector, based on the AAV serotype hu37, which contains a single-stranded DNA genome encoding a B-domain deleted factor VIII, under the control of a liver-specific promotor/enhancer combination optimized for transgenic expression, Dr. Pipe explained.
He presented results with BAY 2599023 in four patients with severe hemophilia A. At baseline, patients were receiving prophylaxis and did not have factor VIII inhibitors or detectable immunity to the AAVhu37 capsid.
First cohort
Two patients received BAY 2599023 at a dose of 0.5 × 1013 GC/kg. One patient achieved a factor VIII level above 5% at this dose and was able to stop prophylaxis temporarily. The patient experienced two bleeds on study and had to resume prophylaxis because of a target joint.
“He came into the study with a particularly challenging target joint in his ankle, and he had known from experience on prophylaxis that, if his factor level got too low, he would have breakthrough bleeds,” Dr. Pipe explained. “So we agreed that if he didn’t achieve a sustained level above 5%, he would go back on prophylaxis. He was able to stay off prophylaxis for 6 weeks, but we had to put him back on because of breakthrough bleeding.”
Dr. Pipe and colleagues have been following this patient for about a year, and the patient still has factor VIII levels of about 2% to 3% after a washout of prophylaxis.
The other patient who received a dose of 0.5 × 1013 GC/kg came off prophylaxis early on and has sustained factor VIII levels of 20%. This patient had four bleeds early in the trial but has since done very well, Dr. Pipe said. The patient had 99 bleeds in the year prior to receiving BAY 2599023.
Second cohort
Two subsequent patients received BAY 2599023 at a dose of 1 × 1013 GC/kg. Both patients have stopped prophylaxis.
One patient achieved factor VIII levels in the 5%-10% range and stopped prophylaxis at day 49. The patient has received on-demand treatment for four bleeds. The other patient achieved “robust” factor VIII expression levels “well above 20%,” according to Dr. Pipe. This patient stopped prophylaxis at day 10 and experienced bleeds early on but has remained bleed-free and treatment-free for 7 months.
Dr. Pipe noted that this patient experienced a spike in transaminases “very early on” after receiving BAY 2599023.
“So he was put on prednisone, but his liver enzymes almost immediately corrected to within the normal range,” Dr. Pipe said. “He has finished the cascading dose of prednisone, his liver enzymes have remained stable, his factor VIII expression has remained stable, and he continues to do very well.”
Next steps
Based on results in the first two cohorts, researchers are proceeding with a higher dose of BAY 2599023.
“The overall goal of this study is to identify the optimal dose that can be taken forward to the planned phase 3 study,” Dr. Pipe said. “What they [Bayer] would like to bring to the community is a product that offers a chance for maximum eligibility based on low screen out because of preexisting immunity and maximize the number of patients who are in the normal range.”
“For patients to embrace gene therapy, it really has to get them at least the possibility to land within the normal range and, hopefully, be not just liberated from prophylaxis but maybe liberated from the need for factor VIII at all,” Dr. Pipe added. “I think if these AAV platform therapies can’t deliver that on a relatively consistent basis, it’s going to be challenging to get patients to embrace this technology.”
The phase 1/2 trial is sponsored by Bayer in collaboration with Ultragenyx Pharmaceutical Inc. Dr. Pipe disclosed relationships with Bayer and other companies.
SOURCE: Pipe SW et al. EAHAD 2020. Abstract P190.
REPORTING FROM EAHAD 2020
More evidence backs LDL below 70 to reduce recurrent stroke
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
REPORTING FROM ISC 2020