User login
FDA okays first generic of ProAir HFA
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
Generic albuterol sulfate inhalation, from Perrigo Pharmaceutical, is indicated for the treatment or prevention of bronchospasm in people aged 4 years or older who have reversible obstructive airway disease, as well as for the prevention of exercise-induced bronchospasm.
“Approval of the first generic drug product for one of the most commonly used rescue inhalers in the US is part of our long-standing commitment to advance patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts, and to expand opportunities to bring generic copies of complex drugs to the market,” FDA Commissioner Stephen Hahn, MD, said in a news release.
Metered-dose inhalers are hard to duplicate because of the complexities of their formulation or mode of delivery. “As a result, too many complex drugs lack generic competition even after patents and exclusivities no longer block generic approval,” he explained.
“Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers,” Hahn added.
In the United States, more than 26 million people suffer from asthma; about 7 million of these people are children.
Perrigo said it will immediately launch a limited quantity of generic albuterol sulfate and, in collaboration with its development and manufacturing partner, Catalent Pharma Solutions, is ramping up production to meet future demand.
The company “anticipates that we will be in a position to provide a steady supply of this product by the fourth quarter of 2020,” Perrigo Executive Vice President and Rx Pharmaceuticals President Sharon Kochan said in a statement.
This article originally appeared on Medscape.com.
Pediatrics Board Review: Neonatal Seizures
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
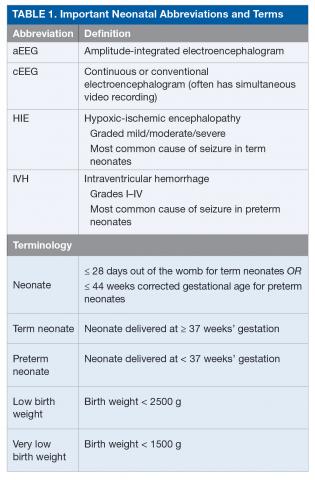
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
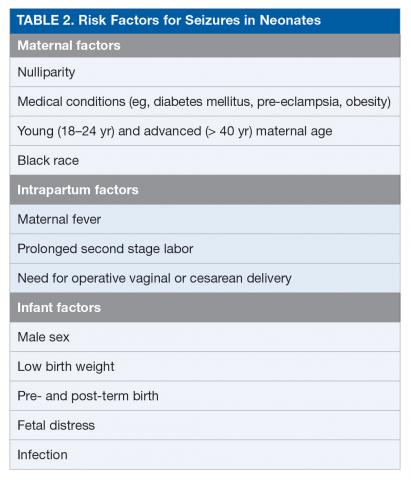
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
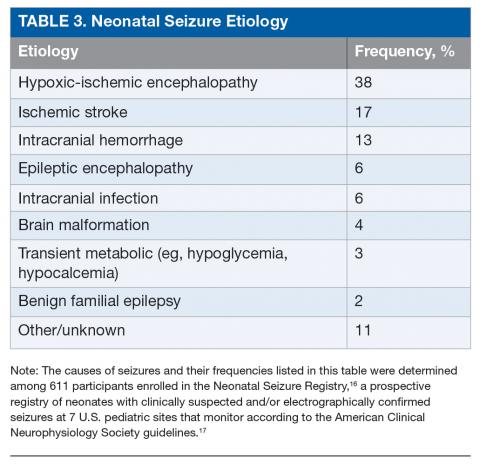
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
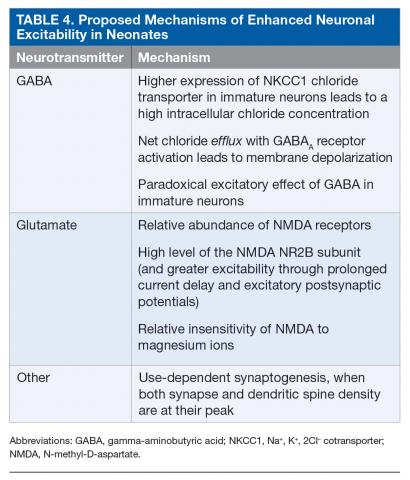
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
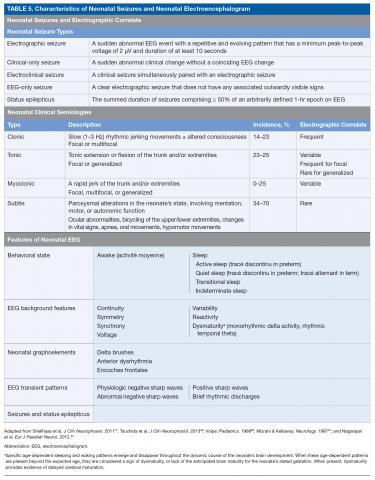
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
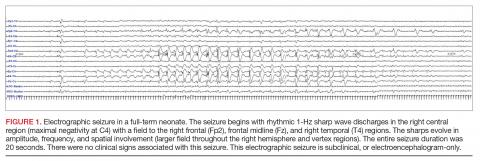
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
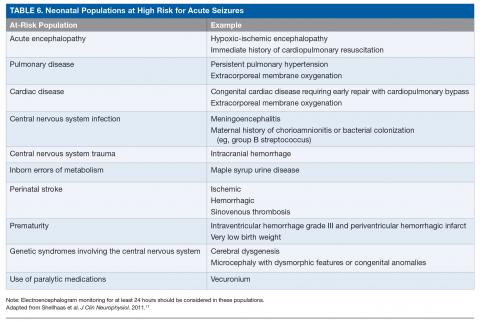
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
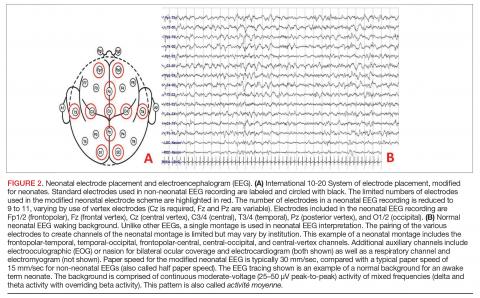
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
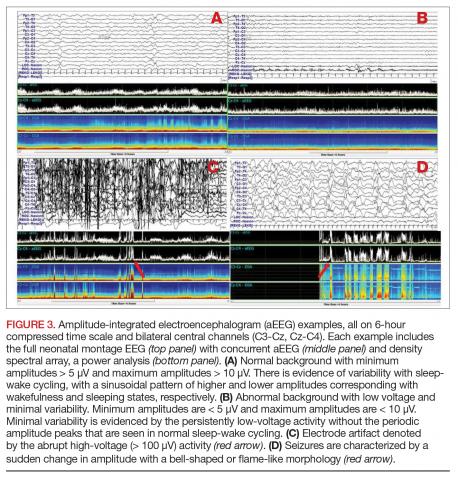
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.
Test your knowledge of this topic: Board Review Questions
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
1. Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–9.
2. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5.
3. Maartens IA, Wassenberg T, Buijs J, et al. Neurodevelopmental outcome in full-term newborns with refractory neonatal seizures. Acta Paediatr. 2012;101:e173–8.
4. Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9.
5. Pavlidis E, Spagnoli C, Pelosi A, et al. Neonatal status epilepticus: differences between preterm and term newborns. Eur J Paediatr Neurol. 2015;19:314–9.
6. Pisani F, Facini C, Pavlidis E, et al. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015;19:6–14.
7. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13.
8. Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22.
9. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91.
10. Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013;18:185–91.
11. Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol. 1999;150:763–9.
12. Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–3.
13. Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–28 e1.
14. Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol. 2009;7:13–7.
15. Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.
16. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89:893–9.
17. Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
18. Tekgul H, Gavreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80.
19. Yildiz EP, Tatli B, Ekici B, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatr Neurol. 2012;47:186–92.
20. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901.
21. Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5 e1.
22. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015;33:60–5.
23. Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82:1239–44.
24. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135:e1220–8.
25. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–9.
26. Harteman JC, Groenendaal F, Benders MJ, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6.
27. Simchen MJ, Goldstein G, Lubetsky A, et al. Factor v Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke. 2009;40:65–70.
28. deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23.
29. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video-EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30.
30. Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–8.
31. Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6.
32. Grinton BE, Heron SE, Pelekanos JT, et al. Familial neonatal seizures in 36 families: clinical and genetic features correlate with outcome. Epilepsia. 2015;56:1071–80.
33. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91.
34. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25
35. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and
implications for clinical applications. JAMA. 2005;293:2372–83.
36. Haukland LU, Kutzsche S, Hovden IA, Stiris T. Neonatal seizures with reversible EEG changes after antenatal venlafaxine exposure. Acta
Paediatr. 2013;102:e524–6.
37. Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33.
38. Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900.
39. Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84.
40. Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57.
41. Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset
epilepsy. J Neurosci. 1998;18:8356–8.
42. McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103.
43. Montgomery EM, Bardgett ME, Lall B, et al. Delayed neuronal loss after administration of intracerebrocentricular kainic acid to preweanling rats.
Brain Res Dev Brain Res. 1999;112:107–16.
44. Lynch M, Sayin U, Bownds J, et al. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci.
2000;12:2252–64.
45. Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. 46. Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–8.
47. Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic
brain injury. J Pediatr. 2009;155:318–23.
48. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology.
2016;86:2179–86.
49. Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for
the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
50. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8.
51. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44.
52. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25.
53. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101.
54. Guidelines on neonatal seizures. Geneva: World Health Organizatin; 2011.
55. Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
56. Connell J, Oozeer R, de Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–64.
57. Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78.
58. Scher MS, Alvin J, Gaus L, et al. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80.
59. Murray DM, Ryan CA, Boylan GB, et al. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic
monitoring. Pediatrics. 2006;118:41–6.
60. Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016;127:285–96.
61. Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia.
Neurology. 2011;76:556–62.
62. Hellström-Westas L, Liu X, Thoresen M, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia.
Pediatrics. 2010;126:e131–9.
63. Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24.
64. Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8.
65. Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57.
66. Shah DK, Zempel J, Barton T, et al. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6.
67. Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–42.
68. de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201–7.
69. Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;20:770–7.
70. Mackay M, Lavery S, Shah DK, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous
conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54.
71. Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–61.
72. Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–5.
73. Shah NA, Van Meurs KP, Davis AS., Amplitude-integrated electroencephalography: a survey of practices in the United States. Am J Perinatol.
2015;32:755–60.
74. Scher MS, Stein AD, Painter MJ, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med.
1999;341:485–9.
75. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46:111–5.
76. Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90.
77. Bassan H, Bental Y, Shany E, et al. Neonatal seizures: dilemmas in workup and management. Pediatr Neurol. 2008;38:415–21.
78. Sharpe CM, Capparelli EV, Mower A, et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked
changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9.
79. Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159:152–4.
80. Castro Conde JR, Hernandez-Borges AA, Domenech Martinez E, et al. Midazolam in neonatal seizures with no response to phenobarbital.
Neurology. 2005;64:876–9.
81. Hirsch LJ, Emerson RG, Claassen J, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.
Neurology. 2001;57:1036–42.
82. Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95.
83. Hellstrom-Westas L, Blennow G, Lindroth M, et al. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal
period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–101.
84. Gospe SM Jr. Neonatal vitamin-responsive epileptic encephalopathies. Chang Gung Med J. 2010;33:1–12.
85. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response.
Neurology. 2014;82:368–70.
86. Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49:320–5.
87. van Rooij LG, de Vries LS, Handryastuti S, et al. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-
integrated electroencephalography. Pediatrics. 2007;120:e354–63.
88. Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21.
89. Camfield C, Camfield P, Gordon K, et al. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–8.
SCC survival remains poor in epidermolysis bullosa
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
REPORTING FROM EB 2020
CDC expects eventual community spread of coronavirus in U.S.
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
How to beat bullying in the workplace
Cyberbullying can prove particularly insidious
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
Cyberbullying can prove particularly insidious
Cyberbullying can prove particularly insidious
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
Bullying happens to our patients and sometimes to the doctors in the medical community. As psychiatrists, we need to share information on how to spot it and deal with it in the workplace.
We can view bullying as the endpoint in a continuum with authority at one end and harassment at the other extreme. Discipline maintains order but those in charge can be misguided or mean spirited.
Bullying is bad and prevalent, but is it inevitable in the workplace? There are three categories: those who get bullied, those who bully, and those who witness bullying. Any one, two, or even all three can apply in a work environment. Some escape the problem, and for them, bullying remains theoretical, a phenomenon to understand.
How do we define bullying? You know it when you see it; bullying interferes with functioning. It includes harsh language, threats, snubbing, screaming, and undermining.
Case is illustrative
Helen, a medical consultant on a surgical unit, was reading a chart when another internist arrived for the same purpose. He introduced himself as a new full-time assistant to the head of medical consultations. Helen greeted him and said: “Since I started with this case, I will continue. There was probably an error in the referral process.” Bill looked concerned. “But he has uncontrolled diabetes.” Taken aback, Helen said: “I think I can handle it. I’ve been on the hospital staff for 25 years.”
Then the bullying began. On occasion, Bill and a resident consulted on patients Helen was treating already, as though her input were nonexistent. When Helen inquired about this, rather than attribute it to an error in communication within a large hospital, Bill diminished the value of her input. She asked, “How many medical consultants does a patient need?” She decided to confront Bill and tell him that he had no reason to treat her with disrespect. After that, Bill’s disparaging remarks intensified and he threatened her saying, “I’m not someone you want to go up against.” Bill sent her an email, “You are demeaning and harsh to the staff; if you want to retain your hospital credentials you must change your behavior.” In her response, Helen agreed to meet with Bill and she emailed, “It is not in my nature to mistreat anyone, staff or patient.” The meeting never happened.
Helen sought me out for psychiatric consultation and psychotherapy because she felt demoralized. Confused by Bill’s assault on her reputation, she needed a strategy and confirmation of her worth. We conceived a plan. Helen decided to get busy and get better. She redoubled her efforts to be cordial, and she remained effective with her patients. I suggested that she confide in a trusted senior attending at the hospital, which she did. She aired her insights to him. Excellence mattered and the threats disappeared. Bill had no power over Helen after all. She was a voluntary attending. She never succumbed to despair; rather she converted her response to the threats into useful energy.
When does authority become harassment?
A pecking order exists in every organization because, from the CEO to the janitor, it is necessary to maintain productivity. But when does this hierarchy become abusive? Discipline gets learned early. Those who are familiar with the comic strip “Calvin and Hobbes” by Bill Watterson may remember the 6-year-old boy asserting his intention to stay home from school – only to be forced to the bus stop by his mother. Call that authority, discipline, or even bullying, but it represents a child’s first encounter with obedience despite protest. When authority interferes rather than enhances effectiveness, question the methods used to attain order.
The vulnerable
If you do your job and you do it well, there should be no bullying. It is hard to know why a target gets chosen for harassment. However, some questions may need answering by the target. Does he or she avoid conflict even when there is bad behavior? Does past trauma immobilize him into passivity? Such issues necessitate self examination. Psychotherapy helps to uncover and clear up these issues.
Is bullying a fact of life?
In “Lord of the Flies,” William Golding portrays a fictional group of unsupervised boys abandoned on an island. An initial hierarchy descends into threats and eventual violence. Consider the animal world. In the wild, a wolf pack isolates a caribou from the herd to kill and devour. On a farm, llamas raised for yarn establish which llama is in charge. Those cases illustrate the hierarchy that exists because there is the need for food or reproductive prowess.
In the workplace, isolation of the target is common when authority extends to bullying. According to Robert Sapolsky, PhD, a neuroscientist and author, there are biological underpinnings for group conformity. This implies that colleagues who stand apart feel distress and get relief when they join the ganging up on a target. Those who watch harassment may hide from confronting it or even from pointing it out to protect themselves.
How do bullies think?
Challenge the bully at your own peril, because expecting a bully to change is futile. Recall Helen’s confrontation with Bill. It provoked him. His power to harass her came from his perceived position. Bullies regard the pleasant person as weak. Bullies can fall into two categories: Sadists who get pleasure from seeing others suffer, and opportunists. The latter focus only on their goals and disregard concerns expressed by others. Outside of the workplace, they may be reasonable. If workplace morale deteriorates along with productivity, the bully gets ousted. Otherwise, companies usually protect high performers at the expense of targets.
Is bullying different in medicine?
It can happen in a training program. The ingredients for bullying exist, including imbalance of power. Often, there are no witnesses, and there can be lack of accountability because of changing authorities. Just as technology can help make harassment possible, it also enables the target to spot and document inappropriate communications by saving emails and texts. Whenever a need for advancement exists along with changing authority, bullying gets tolerated. Who wants to be derailed by reporting? Those in training have the goal of completing a program. If they report bullying, they fear antagonism and retribution, a personal expense that can deter advancement.
Remedies
Let truth and fairness be your guide. That is easy to say and hard to do, but there are helpful personal and legal resources.
Personal capability
Get busy; get better. That became Helen’s method of choice. She focused on her role and productivity, not on her hurt. Helen shunned victimhood. With the help of psychotherapy and by confiding in a mentor, she prevailed. What works is recalling challenges that were mastered and the qualities that made for success. Acquire skills, build a good reputation, be assertive, not defensive.
The group is powerful, and that means it is important to build alliances above and below in the organizational hierarchy; cultivate friendship with trustworthy people. Occasionally, there is unwarranted ganging up on a manager, bullying from below. It is more likely to happen to a newly appointed supervisor. A way to avert that is to communicate with staff throughout the institution and remain accessible.
Legal options
What are legal options to confront bullying? Of note, workplace bullying is not necessarily illegal. According to one employment attorney, “There is no law that prohibits uncivil behavior on the job.” However, under Title VII of the federal law enacted in 1964, there are protected characteristics such as race, color, national origin, sex, age, and disability. The Equal Employment Opportunity Commission enforces Title VII. In cases of assault or stalking, harassment is illegal and criminal.
Some employees report to Human Resources or seek out their company’s employee assistance program. As useful as those options may be, they are part of the company and potentially partial to the administration. There can be incentives to protect those with power against a complainant. For assistance, it is preferable to enlist an outside attorney and a therapist in the community.
Advocacy exists. The Workplace Bullying Institute maintains a website, holds workshops, promotes literature, and offers information. The National Employment Lawyers Association can provide referrals or recommendations that come from other legal sources. Cases rarely reach court because of the expense of a trial; rather, the parties reach a financial settlement. When there is cause, an employment attorney can best pursue justice for the worker.
Conclusion
Get busy; get better is the solution to bullying. Avoid victimhood. That means prepare: Update the resume, seek opportunities, and identify allies. Bullies get beaten; as Abraham Lincoln said, “You can fool some of the people some of the time but you can’t fool all of the people all of the time.”
According to the Workplace Bullying Institute, 7 out of 10 bullied workers either resign or get fired. You should leave only when the leaving is better than the staying. Bullying brings out the worst in the workplace. Those who bully isolate the target. Coworkers often shun the target, fearing for their own position; they may even participate in the harassment. Psychiatrists need to remain sensitive to harassment in their own environment and for their patients. We have tools to address bullying in the workplace and a moral responsibility to combat it.
References
Workplace Bullying Institute (WBI)
National Employment Lawyers Association (NELA)
“Ozymandias” by Percy Bysshe Shelley
BY BEN HINDELL, PSY.D.
Cyberbullying is willful, repeated harm inflicted with the use of computers, cell phones, and other electronic devices. In some cases, a single message may be viewed by multiple people because the text and pictures are posted elsewhere.
Technology makes is possible to harass at any hour. The concept of willful harm is essential. Without interaction between sender and recipient, nuances are lost. Face to face might make a communication benign instead of malevolent or threatening. This has implications for the workplace, where colleagues increasingly communicate by email rather than discuss matters in person or by telephone.
Steps for survivors of cyberbullying
- Do not respond immediately to an inflammatory message, post, or email. Gather your thoughts and avoid responding in anger.
- Keep calm and rational, not emotional.
- Try to respond in person and work to avoid a conflict.
- Remember, your interpretation may differ from what was intended.
- Communicate openly and honestly and not defensively.
- Calmly indicate you were offended and you want the comments to stop.
- Move up the chain of command, if comments don’t cease.
- Save all messages and posts as evidence.
- Report the cyberbullying to your employer. Human resources may get involved.
- Detach from the cyberbully, if it continues. Block social media, cell phone messaging, and emails.
- Find support from friends, family, and a psychotherapist, if needed. As a last resort, it may become necessary to enlist an attorney.
- Take the high road; remain calm and professional at work. The bully may be seeking a reaction from the behavior. Prevent it.
All of the elements of workplace bullying apply to cyberbullying, but the latter can be more insidious. Psychiatrists and psychologists are able to support patients who deal with cyberbullying and help them cope successfully.
Dr. Cohen is in private practice and is a clinical assistant professor of psychiatry at Weill Cornell Medical Center of New York-Presbyterian Hospital, and psychiatric consultant at the Hospital for Special Surgery, also in New York. She made changes to the patient’s story to protect confidentiality.
Dr. Hindell is a psychologist with the Mental Health Service of Colorado College, Colorado Springs. He also practices psychotherapy in Denver. Dr. Hindell is the son of Dr. Cohen.
ID Blog: SARS-CoV-2 – What’s in a name?
Coming up with a moniker for the new coronavirus shows the perils of naming names.
There is no Baby Book of Names or hurricane alphabet to readily name diseases and their causal entities. Throughout history and even in the modern era, a host of considerations have intruded on the decision as to what to call these blights upon humanity. Names have varied from inflammatory to misleading, from colloquial to scientific. And when it concerns a new epidemiological entity such as the latest coronavirus outbreak originating in China, health organizations, media, politicians, scientific taxonomy commissions, and the public at large all have a stake in the naming.
From “Wuhan virus” to “novel coronavirus-2019” to “COVID-19 virus,” the name of the new coronavirus that first appeared in China has been evolving to its now official designation: SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). But where did the final name come from, how does such a name become official, and who makes it so?
Virus taxonomy
The Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) named the new coronavirus SARS-CoV-2 based upon its genetic relationship to the original SARS-CoV that caused an outbreak of disease in 2002–2003.
According to the ICTV website, the first internationally organized attempts to introduce order into the bewildering variety of viruses took place at the International Congress of Microbiology held in Moscow in 1966 where a committee was created that later became the ICTV and was given the task of developing a single, universal taxonomic scheme for all the viruses infecting animals, plants, fungi, bacteria, and archaea. The ICTV was created as a committee of the virology division of the International Union of Microbiological Societies and is governed by statutes approved by the virology division. Virus classification and nomenclature are subject to rules set out in an International Code.
These designate that: “The universal virus classification system shall employ the hierarchical levels of realm, subrealm, kingdom, subkingdom, phylum, subphylum, class, subclass, order, suborder, family, subfamily, genus, subgenus and species.”
Many of the topmost areas of classification are based on whether the viruses are DNA or RNA, single or double stranded, and have a simple protein shell or a complex lipoprotein envelope. Other levels of classification include host species, type of replication, and type of diseases they cause, the later exemplified in the SARS designation for this virus.
There are 98 international study groups (SGs) covering all major virus orders, families, and genera that are part of the ICTV, and it was the one dedicated to the single-stranded RNA coronaviruses, the CSG, that came up with the SARS-CoV-2 name and first referenced it in their Feb 11 publication in the Cold Springs Harbor preprint journal bioRxiv.
“Based on phylogeny, taxonomy and established practice, the CSG formally recognizes this virus as a sister to severe acute respiratory syndrome coronaviruses (SARS-CoVs) of the species severe acute respiratory syndrome–related coronavirus and designates it as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” they wrote.
According to the National Center for Biotechnology Information Taxonomy Browser, with respect to the original SARS CoV virus, of which this is a relative, the full taxonomic designation is: Viruses, Riboviria, Nidovirales, Cornidovirineae, Coronaviridae, Orthocoronavirinae, Betacoronavirus, Sarbecovirus.
The problem with naming names
The World Health Organization currently is not using the official scientific name of the virus, but rather is merely labeling it with regard to the disease: COVID-19, which simply refers to coronavirus disease 2019.
They are following a modern standard by which disease names avoid inflammatory connotations with people and places. Too often in the past from syphilis as the “French pox,” the 1918 influenza as the “Spanish flu,” AIDS as the “gay plague,” Middle East Respiratory Syndrome (MERS), and the currently named “WuFlu,” which made an appearance early in the new outbreak and which is symbolic of a sudden wave of anti-Asian, and specifically Chinese, prejudice.
Chinatown districts even in the United States are being affected economically through unwarranted fear associated with the virus. And there have been equivalently virulent outbreaks of hate speech against Asian individuals in places untouched by the new virus.
However, although SARS-CoV-2 as a name avoids such problems, different considerations led the WHO to reject it in its discussions, determining that its use ties it to tightly to the much more deadly SARS-CoV-1 virus in the public mind, risking greater fear and panic, especially in Asia, where SARS-CoV-1 had the biggest impact.
Back in 1896, William Sykes, MD, writing in the first flush of the triumph of germ theory in modern medicine, attempted to give some guidance to how medical science should best come up with new names of diseases by merging the demands of common parlance with those of taxonomic legitimacy. His “On the Origin and History of Disease-Names,” published in the Lancet, had clearcut advice: “It is vain to attempt to replace a folk name or one widely adopted by the people by a new one deliberately coined by scholars, and this for the following reasons: first, whatever names may be accepted by medical men must be translated by them into the vernacular of their patients, and by a resulting reaction the vernacular name comes to be the commoner one with themselves; and, secondly, there is no continuity or unchangeableness in the terms invented by savants, which are amended, improved upon, and displaced by the next writer on the subject, or, even more absurdly still, by the very inventors themselves in a subsequent publication.”
This is the reason that virus taxonomy provides names based upon unchangeable scientific descriptors of the actual disease causing entity, as illustrated by the decisions of the ICTV. In addition, the genomic sequences being provided by the scientific community are all being organized under the SARS-CoV-2 name and thus are cementing that moniker as the only acceptable scientific one.
Whether the rest of the world universally adopts SARS-CoV-2 as a name is still in question. If the outbreak spreads significantly beyond its current limits, fear and confusion – and simply the need for a more familiar-sounding label – may lead the general public to adopt more colloquial designations than those that science attempts to impose, as Dr. Sykes suggested back in 1896. That remains to be seen.
mlesney@mdedge.com
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Coming up with a moniker for the new coronavirus shows the perils of naming names.
Coming up with a moniker for the new coronavirus shows the perils of naming names.
There is no Baby Book of Names or hurricane alphabet to readily name diseases and their causal entities. Throughout history and even in the modern era, a host of considerations have intruded on the decision as to what to call these blights upon humanity. Names have varied from inflammatory to misleading, from colloquial to scientific. And when it concerns a new epidemiological entity such as the latest coronavirus outbreak originating in China, health organizations, media, politicians, scientific taxonomy commissions, and the public at large all have a stake in the naming.
From “Wuhan virus” to “novel coronavirus-2019” to “COVID-19 virus,” the name of the new coronavirus that first appeared in China has been evolving to its now official designation: SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). But where did the final name come from, how does such a name become official, and who makes it so?
Virus taxonomy
The Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) named the new coronavirus SARS-CoV-2 based upon its genetic relationship to the original SARS-CoV that caused an outbreak of disease in 2002–2003.
According to the ICTV website, the first internationally organized attempts to introduce order into the bewildering variety of viruses took place at the International Congress of Microbiology held in Moscow in 1966 where a committee was created that later became the ICTV and was given the task of developing a single, universal taxonomic scheme for all the viruses infecting animals, plants, fungi, bacteria, and archaea. The ICTV was created as a committee of the virology division of the International Union of Microbiological Societies and is governed by statutes approved by the virology division. Virus classification and nomenclature are subject to rules set out in an International Code.
These designate that: “The universal virus classification system shall employ the hierarchical levels of realm, subrealm, kingdom, subkingdom, phylum, subphylum, class, subclass, order, suborder, family, subfamily, genus, subgenus and species.”
Many of the topmost areas of classification are based on whether the viruses are DNA or RNA, single or double stranded, and have a simple protein shell or a complex lipoprotein envelope. Other levels of classification include host species, type of replication, and type of diseases they cause, the later exemplified in the SARS designation for this virus.
There are 98 international study groups (SGs) covering all major virus orders, families, and genera that are part of the ICTV, and it was the one dedicated to the single-stranded RNA coronaviruses, the CSG, that came up with the SARS-CoV-2 name and first referenced it in their Feb 11 publication in the Cold Springs Harbor preprint journal bioRxiv.
“Based on phylogeny, taxonomy and established practice, the CSG formally recognizes this virus as a sister to severe acute respiratory syndrome coronaviruses (SARS-CoVs) of the species severe acute respiratory syndrome–related coronavirus and designates it as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” they wrote.
According to the National Center for Biotechnology Information Taxonomy Browser, with respect to the original SARS CoV virus, of which this is a relative, the full taxonomic designation is: Viruses, Riboviria, Nidovirales, Cornidovirineae, Coronaviridae, Orthocoronavirinae, Betacoronavirus, Sarbecovirus.
The problem with naming names
The World Health Organization currently is not using the official scientific name of the virus, but rather is merely labeling it with regard to the disease: COVID-19, which simply refers to coronavirus disease 2019.
They are following a modern standard by which disease names avoid inflammatory connotations with people and places. Too often in the past from syphilis as the “French pox,” the 1918 influenza as the “Spanish flu,” AIDS as the “gay plague,” Middle East Respiratory Syndrome (MERS), and the currently named “WuFlu,” which made an appearance early in the new outbreak and which is symbolic of a sudden wave of anti-Asian, and specifically Chinese, prejudice.
Chinatown districts even in the United States are being affected economically through unwarranted fear associated with the virus. And there have been equivalently virulent outbreaks of hate speech against Asian individuals in places untouched by the new virus.
However, although SARS-CoV-2 as a name avoids such problems, different considerations led the WHO to reject it in its discussions, determining that its use ties it to tightly to the much more deadly SARS-CoV-1 virus in the public mind, risking greater fear and panic, especially in Asia, where SARS-CoV-1 had the biggest impact.
Back in 1896, William Sykes, MD, writing in the first flush of the triumph of germ theory in modern medicine, attempted to give some guidance to how medical science should best come up with new names of diseases by merging the demands of common parlance with those of taxonomic legitimacy. His “On the Origin and History of Disease-Names,” published in the Lancet, had clearcut advice: “It is vain to attempt to replace a folk name or one widely adopted by the people by a new one deliberately coined by scholars, and this for the following reasons: first, whatever names may be accepted by medical men must be translated by them into the vernacular of their patients, and by a resulting reaction the vernacular name comes to be the commoner one with themselves; and, secondly, there is no continuity or unchangeableness in the terms invented by savants, which are amended, improved upon, and displaced by the next writer on the subject, or, even more absurdly still, by the very inventors themselves in a subsequent publication.”
This is the reason that virus taxonomy provides names based upon unchangeable scientific descriptors of the actual disease causing entity, as illustrated by the decisions of the ICTV. In addition, the genomic sequences being provided by the scientific community are all being organized under the SARS-CoV-2 name and thus are cementing that moniker as the only acceptable scientific one.
Whether the rest of the world universally adopts SARS-CoV-2 as a name is still in question. If the outbreak spreads significantly beyond its current limits, fear and confusion – and simply the need for a more familiar-sounding label – may lead the general public to adopt more colloquial designations than those that science attempts to impose, as Dr. Sykes suggested back in 1896. That remains to be seen.
mlesney@mdedge.com
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
There is no Baby Book of Names or hurricane alphabet to readily name diseases and their causal entities. Throughout history and even in the modern era, a host of considerations have intruded on the decision as to what to call these blights upon humanity. Names have varied from inflammatory to misleading, from colloquial to scientific. And when it concerns a new epidemiological entity such as the latest coronavirus outbreak originating in China, health organizations, media, politicians, scientific taxonomy commissions, and the public at large all have a stake in the naming.
From “Wuhan virus” to “novel coronavirus-2019” to “COVID-19 virus,” the name of the new coronavirus that first appeared in China has been evolving to its now official designation: SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). But where did the final name come from, how does such a name become official, and who makes it so?
Virus taxonomy
The Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) named the new coronavirus SARS-CoV-2 based upon its genetic relationship to the original SARS-CoV that caused an outbreak of disease in 2002–2003.
According to the ICTV website, the first internationally organized attempts to introduce order into the bewildering variety of viruses took place at the International Congress of Microbiology held in Moscow in 1966 where a committee was created that later became the ICTV and was given the task of developing a single, universal taxonomic scheme for all the viruses infecting animals, plants, fungi, bacteria, and archaea. The ICTV was created as a committee of the virology division of the International Union of Microbiological Societies and is governed by statutes approved by the virology division. Virus classification and nomenclature are subject to rules set out in an International Code.
These designate that: “The universal virus classification system shall employ the hierarchical levels of realm, subrealm, kingdom, subkingdom, phylum, subphylum, class, subclass, order, suborder, family, subfamily, genus, subgenus and species.”
Many of the topmost areas of classification are based on whether the viruses are DNA or RNA, single or double stranded, and have a simple protein shell or a complex lipoprotein envelope. Other levels of classification include host species, type of replication, and type of diseases they cause, the later exemplified in the SARS designation for this virus.
There are 98 international study groups (SGs) covering all major virus orders, families, and genera that are part of the ICTV, and it was the one dedicated to the single-stranded RNA coronaviruses, the CSG, that came up with the SARS-CoV-2 name and first referenced it in their Feb 11 publication in the Cold Springs Harbor preprint journal bioRxiv.
“Based on phylogeny, taxonomy and established practice, the CSG formally recognizes this virus as a sister to severe acute respiratory syndrome coronaviruses (SARS-CoVs) of the species severe acute respiratory syndrome–related coronavirus and designates it as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” they wrote.
According to the National Center for Biotechnology Information Taxonomy Browser, with respect to the original SARS CoV virus, of which this is a relative, the full taxonomic designation is: Viruses, Riboviria, Nidovirales, Cornidovirineae, Coronaviridae, Orthocoronavirinae, Betacoronavirus, Sarbecovirus.
The problem with naming names
The World Health Organization currently is not using the official scientific name of the virus, but rather is merely labeling it with regard to the disease: COVID-19, which simply refers to coronavirus disease 2019.
They are following a modern standard by which disease names avoid inflammatory connotations with people and places. Too often in the past from syphilis as the “French pox,” the 1918 influenza as the “Spanish flu,” AIDS as the “gay plague,” Middle East Respiratory Syndrome (MERS), and the currently named “WuFlu,” which made an appearance early in the new outbreak and which is symbolic of a sudden wave of anti-Asian, and specifically Chinese, prejudice.
Chinatown districts even in the United States are being affected economically through unwarranted fear associated with the virus. And there have been equivalently virulent outbreaks of hate speech against Asian individuals in places untouched by the new virus.
However, although SARS-CoV-2 as a name avoids such problems, different considerations led the WHO to reject it in its discussions, determining that its use ties it to tightly to the much more deadly SARS-CoV-1 virus in the public mind, risking greater fear and panic, especially in Asia, where SARS-CoV-1 had the biggest impact.
Back in 1896, William Sykes, MD, writing in the first flush of the triumph of germ theory in modern medicine, attempted to give some guidance to how medical science should best come up with new names of diseases by merging the demands of common parlance with those of taxonomic legitimacy. His “On the Origin and History of Disease-Names,” published in the Lancet, had clearcut advice: “It is vain to attempt to replace a folk name or one widely adopted by the people by a new one deliberately coined by scholars, and this for the following reasons: first, whatever names may be accepted by medical men must be translated by them into the vernacular of their patients, and by a resulting reaction the vernacular name comes to be the commoner one with themselves; and, secondly, there is no continuity or unchangeableness in the terms invented by savants, which are amended, improved upon, and displaced by the next writer on the subject, or, even more absurdly still, by the very inventors themselves in a subsequent publication.”
This is the reason that virus taxonomy provides names based upon unchangeable scientific descriptors of the actual disease causing entity, as illustrated by the decisions of the ICTV. In addition, the genomic sequences being provided by the scientific community are all being organized under the SARS-CoV-2 name and thus are cementing that moniker as the only acceptable scientific one.
Whether the rest of the world universally adopts SARS-CoV-2 as a name is still in question. If the outbreak spreads significantly beyond its current limits, fear and confusion – and simply the need for a more familiar-sounding label – may lead the general public to adopt more colloquial designations than those that science attempts to impose, as Dr. Sykes suggested back in 1896. That remains to be seen.
mlesney@mdedge.com
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
China’s health authorities release large coronavirus case series
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
The Chinese Center for Disease Control and Prevention has released the largest case series to date for novel coronavirus 2019 (COVID-19), and a summary of key findings appears in JAMA.
- The virus, which spread from a single city to a whole country in only 30 days, has so far has caused over 72,314 cases as of Feb. 11, 2020, and 1,023 fatalities (2.3%) overall.
- The age distribution shows that most of the cases (87%) occurred in patients aged 30-79 years, while 10% were in patients 29 years and younger and 3% at 80 years and older.
- Following the SARS outbreak in 2002-2003, the Chinese government adjusted its epidemic response protocol. For example, according to the summary, while there were 300 cases and 5 deaths with SARS before the Chinese government reported it to the World Health Organization, there were only 27 cases and no deaths with COVID-19 before it was reported to that agency.
- A major goal, the authors wrote, is to buy enough time for scientific research, hopefully before the disease has become too widespread.
The summary argues that, while some measures the Chinese government has taken could be seen as extreme, the overall benefits and lives saved outweigh the potential infringement on civil liberties. It also suggests that countries need to work together in situations like this because disease pathogens do not respect geopolitical borders.
SOURCE: Wu Z, McGoogan JM. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
FROM JAMA
Lipidologists welcome bempedoic acid as new lipid-lowering option
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Bempedoic acid, the first agent in a new class of drugs that reduce LDL cholesterol, received Food and Drug Administration approval on Feb. 21 for treating selected hypercholesterolemic patients and is a welcome addition to the medicine cabinet, say lipid experts.
However, it is a tertiary option at least until results from a 14,000 patient clinical-outcome trial of bempedoic acid come out, likely in 2022, they agreed.
“I’m excited to have a new tool in the toolkit for treating high-risk patients, but I will always reach first for the drugs proven to reduce clinical outcomes,” said Erin D. Michos, MD, director of Women’s Cardiovascular Health and associate director of Preventive Cardiology at Johns Hopkins Medicine in Baltimore. That sentiment, shared by other experts, should for the time being relegate bempedoic acid (Nexletol) to a backup role behind statins, ezetimibe, and the PCSK9 inhibitor antibodies that are all now on the U.S. market and all buttressed with evidence of their ability to cut cardiovascular disease death and other CVD outcomes from large outcome studies.
The existing evidence base for bempedoic acid rests primarily two multicenter, randomized, placebo-controlled clinical trials of bempedoic acid in patients with LDL levels above 70 mg/dL while on maximally tolerated lipid-lowering therapy. In CLEAR Harmony, results showed that treatment with bempedoic acid cut LDL-cholesterol levels by an average of 18% more compared with placebo (N Engl J Med 2019;380:1022-32). In CLEAR Wisdom, bempedoic acid reduced LDL cholesterol levels by 17% (JAMA. 2019;322[18]:1780-8).
While those two trials proved the drug’s ability to lower levels of LDL cholesterol, they lacked the power to address whether this effect cut the incidence of CVD events, a question that the CLEAR Outcomes trial aims to answer.
“I believe in the lipid hypothesis, but the main thing we need to see is whether bempedoic acid leads to a meaningful reduction in CVD events. The window for bempedoic acid will remain narrow until we see the outcomes results,” Dr. Michos said in an interview.
Bempedoic acid is a prodrug that’s activated in liver and targets the same cholesterol synthesis pathway as statins by inhibition of ATP-citrate lyase, an enzyme that’s upstream of HMG-CoA reductase, thereby enhancing LDL cholesterol clearance via up-regulation of LDL receptors
.
“,” said Jennifer G. Robinson, MD, professor of epidemiology and medicine and director of the Prevention Intervention Center of the University of Iowa in Iowa City. That would be just a portion of the newly labeled target population. The FDA’s approved label for bempedoic acid cites the drug as an “adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL-C.”
The current lack of outcomes evidence for bempedoic acid was not an issue for Robert H. Eckel, MD, an endocrinologist and lipid management specialist at the University of Colorado at Denver in Aurora. Having results from CLEAR Outcomes “may be helpful, but LDL cholesterol lowering in the range where the FDA has indicated using bempedoic acid seems all we need for now,” he said in an interview. Viewing bempedoic acid as potentially useful for both HeFH and ASCVD patients, Dr. Eckel particularly cited the possibility of using the new drug in combination with ezetimibe, another oral, once-daily agent with a moderate but additive effect for cutting LDL cholesterol.
Combined treatment with bempedoic acid and ezetimibe “may be successful in avoiding [using] a PCSK9 inhibitor in some patients, and in particular patients with HeFH or those who are statin intolerant.” But like his colleagues, Dr. Eckel agreed that, for the moment, ezetimibe has an edge over bempedoic acid because of its more extensive evidence base. “If the combination of bempedoic acid and ezetimibe is not needed, the decision [of which one of these to use] needs to depend on the outcome trial results for ezetimibe,” he said. Other factors clinicians could apply if faced with choosing between these two agents include the significant reduction in high-sensitivity C-reactive protein that bempedoic acid produces; the downside that bempedoic acid can cause in some patients an early and persistent rise in serum uric acid levels that can trigger gout flares in patients with a history of gout or at risk for gout; and cost, he said.
Cost is the room-dwelling elephant that colors many decisions about which lipid-lowering drug to use for patients, with options running the price gamut from the generic and uniformly affordable statins and ezetimibe, to the notoriously pricey PCSK9 inhibitors that remain for many patients either prohibitively expensive or hard to get covered by some insurers. Bempedoic acid seems on track to fall somewhere between these two poles, although staff members from Esperion, the company that developed and will market bempedoic acid as Nexletol starting on March 30, declared in a conference call on Feb. 24 that “cost will not be an issue,” for indicated patients prescribed the drug. Company representatives cited a program of coupons, discounts, and rebates they have planned that they anticipate will allow patients who meet the labeled indications to have an out-of-pocket cost for bempedoic acid of “as low as” $10 for a 90-pill supply. They also noted their goal of getting bempedoic acid onto the lowest tier of the Medicare formulary.
How these steps actually play out in the fun house of U.S. prescription drug pricing and preauthorizations remains to be seen. “Out-of-pocket costs are not the real drivers” of drug access, noted Dr. Robinson. “Insurers will likely start with restricted access and prior authorization requirements, just as they did with ezetimibe when it was on patent and prior to having the results from a CVD outcomes trial.” For the time being, bempedoic acid can generally be seen as “expensive ezetimibe,” summed up Dr. Robinson.
Despite that somewhat dismissive characterization, experts are intrigued by the possibility of combining two moderately potent, oral, and safe lipid-lowering drugs in selected patients as a potential alternative to the still financially challenging PCSK9 inhibitors. Combining bempedoic acid and ezetimibe “has a lot of appeal,” said Dr. Michos. “Even though preauthorization has gotten better, it’s still a challenge to get a PCSK9 inhibitor approved.”
Much of her enthusiasm stems from a study reported last year that randomized 301 patients to treatment with bempedoic acid, ezetimibe, or both. The results showed that combined treatment has a similar safety profile to treatment with either drug alone, and produced a cut in LDL cholesterol that was roughly additive for the reductions produced by each drug by itself: Ezetimibe alone cut LDL by about 23%, bempedoic acid alone by about 17%, and the two dosed together once daily resulted in an average 36% drop (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864671). The results showed that, “in patients requiring intensive LDL cholesterol lowering, who cannot afford PCSK9 inhibitors, or have statin intolerance, bempedoic acid and ezetimibe are stronger together and can serve as an alternative approach for lipid management in ASCVD prevention,” wrote Dr. Michos and a coauthor in a commentary that appeared with the study results (Eur J Prev Cardiol. 2019 Jul 29. doi: 10.1177/2047487319864672).
The concept of combined bempedoic acid and ezetimibe treatment is so appealing that the bempedoic acid manufacturer, Esperion, has already developed a single-pill formulation of the two drugs that received FDA marketing approval on February 26. A company statement said that marketing of this combined formulation, Nexlizet, will start in July 2020.
Although interest in bempedoic acid seems running high for patients included in the new FDA indication, Dr. Michos and others see possibly greater potential for what would now be off-label use for primary prevention in high-risk patients without HeFH, patients who generally don’t qualify for insurance coverage of a PCSK9 inhibitor.
“Use in primary prevention in [non-HeFH] patients with insufficient lowering of LDL cholesterol wouldn’t surprise me,” but a big concern will be out-of-pocket cost when off-label use precludes insurance coverage or discount eligibility, noted Dr. Eckel. An Esperion spokesperson said that the undiscounted, wholesale acquisition cost for bempedoic acid is expected to be roughly $10/pill, or about $300 for a 30-day supply, positioning it more or less midway between generic statins and ezetimibe and the list price for a PCSk9 inhibitor of roughly $500/month.
“I’m most excited about bempedoic acid in the off-label space, for patients who can’t get approved for a PCSK9 inhibitor, for treating patients with subclinical ASCVD, or really high-risk patients with multiple risk factors including diabetes,” especially when these patients are intolerant of a high-intensity statin regimen, said Dr. Michos. “I have a clinic full of patients” who can’t take their full, indicated dosage of a high-intensity statin, and when those patients also can’t get on treatment with a PCSK9 inhibitor then bempedoic acid will be an important part of their alternative regimen, she explained.
Dr. Michos had no disclosures. Dr. Robinson has received research funding from Esperion and from several other companies, and she has been a consultant to Amgen, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi. Dr. Eckel has received honoraria from Kowa, Merck, Novo Nordisk, and Sanofi/Regeneron.
This article was updated 2/27/20.
Localized Acanthosis Nigricans at the Site of Repetitive Insulin Injections
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.
A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13
In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.
A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13
In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.
A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13
In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
Practice Points
- Benign acanthosis nigricans (AN) is most often related to insulin resistance and presents as asymptomatic, hyperpigmented, velvety plaques on the neck, axillae, groin, and other body folds.
- Benign AN related to insulin resistance occurs when insulin binds to insulinlike growth factor 1 on keratinocytes and stimulates proliferations.
- Although insulin injections have been associated with several cutaneous side effects, including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation, localized AN is an uncommonly reported cutaneous adverse effect.
Neurologists report low job satisfaction

The report found that only 18% of neurologists were very happy at work, and 41% overall identified themselves as burned out. Among reasons for burnout, 61% reported mounting bureaucratic tasks as their top reason, with 40% listing spending too many hours at work.
Coping strategies varied, with isolation from others topping the list at 46%, followed by talking with close friends and family and exercise tied at 40%.
Less than half (46%) claimed there was no impact on patients, but most (65%) don’t intend to seek professional help for depression and/or burnout and haven’t done so in the past. Similarly, 48% reported it’s unlikely they’d participate in a workplace program – in fact, only 33% said they would.
A slideshow laying out the findings in the report is available on Medscape.
A closer look at the numbers
Over 15,000 physicians across 29 specialties completed the 10-minute survey in the summer of 2019; 62% were men and most of the group were Baby Boomers (ages 55-73), then Generation X (ages 40-54), and lastly Millennials (ages 25-39). Of the specialties surveyed, neurologists scored lowest in the happiness-at-work category, with only 18% saying they were happy. Neurologists also scored lowest in happiness outside of work (44%). Half the neurologists surveyed said they were burned out, which was slightly more than the surveyed group of physicians in general. The biggest contributors to burnout were bureaucratic tasks, too many hours at work, and lack of control. Most coped by isolating themselves, talking with family members or friends, exercising and sleep. About 65% did not seek help for burnout or depression. The main reasons were being too busy, preferring to deal with it themselves, or feeling that the problem was not significant enough to warrant intervention.
A majority of the neurologists surveyed (70%-80%) are married and 85% say they have a good marriage. Almost half of neurologists take 3-4 weeks of vacation and a third take 1-2 weeks. Neurologists surveyed drive mostly Hondas and Toyotas; 4% drive Teslas and 3% drive Porsches. One third of neurologists exercise two to three times per week and 10% exercise daily. Only 20% have a drink more than four times per week.
Looking for solutions
“It is a bit distressing to see how many neurologists are unhappy at work and unhappy even outside of work,” said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. Many neurologists claim to be burned out and a small percent report depression. Most do not seek help, do not take care of themselves well enough, and do not vacation or exercise enough, added Dr. Rapoport, who also is a past president of the International Headache Society and is editor in chief of Neurology Reviews.
Dr. Rapoport believes that some studies about this situation should be done by the American Academy of Neurology and other subspecialty organizations (for example, the American Headache Society), and results should be published in the neurology and subspecialty journals. Further work in this area should include suggestions for rectifying the situation and encouraging neurologists to seek help and improve their lifestyle. “I think that one of the ways that headache specialists have avoided burnout and depression is by focusing on one subspecialty area and engaging in different types of activities, such as seeing patients in the office and hospital, giving lectures, traveling to meetings, writing papers, and balancing their professional and personal lives. It appears that we need help as a profession, and we had better help ourselves to get it.”

The report found that only 18% of neurologists were very happy at work, and 41% overall identified themselves as burned out. Among reasons for burnout, 61% reported mounting bureaucratic tasks as their top reason, with 40% listing spending too many hours at work.
Coping strategies varied, with isolation from others topping the list at 46%, followed by talking with close friends and family and exercise tied at 40%.
Less than half (46%) claimed there was no impact on patients, but most (65%) don’t intend to seek professional help for depression and/or burnout and haven’t done so in the past. Similarly, 48% reported it’s unlikely they’d participate in a workplace program – in fact, only 33% said they would.
A slideshow laying out the findings in the report is available on Medscape.
A closer look at the numbers
Over 15,000 physicians across 29 specialties completed the 10-minute survey in the summer of 2019; 62% were men and most of the group were Baby Boomers (ages 55-73), then Generation X (ages 40-54), and lastly Millennials (ages 25-39). Of the specialties surveyed, neurologists scored lowest in the happiness-at-work category, with only 18% saying they were happy. Neurologists also scored lowest in happiness outside of work (44%). Half the neurologists surveyed said they were burned out, which was slightly more than the surveyed group of physicians in general. The biggest contributors to burnout were bureaucratic tasks, too many hours at work, and lack of control. Most coped by isolating themselves, talking with family members or friends, exercising and sleep. About 65% did not seek help for burnout or depression. The main reasons were being too busy, preferring to deal with it themselves, or feeling that the problem was not significant enough to warrant intervention.
A majority of the neurologists surveyed (70%-80%) are married and 85% say they have a good marriage. Almost half of neurologists take 3-4 weeks of vacation and a third take 1-2 weeks. Neurologists surveyed drive mostly Hondas and Toyotas; 4% drive Teslas and 3% drive Porsches. One third of neurologists exercise two to three times per week and 10% exercise daily. Only 20% have a drink more than four times per week.
Looking for solutions
“It is a bit distressing to see how many neurologists are unhappy at work and unhappy even outside of work,” said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. Many neurologists claim to be burned out and a small percent report depression. Most do not seek help, do not take care of themselves well enough, and do not vacation or exercise enough, added Dr. Rapoport, who also is a past president of the International Headache Society and is editor in chief of Neurology Reviews.
Dr. Rapoport believes that some studies about this situation should be done by the American Academy of Neurology and other subspecialty organizations (for example, the American Headache Society), and results should be published in the neurology and subspecialty journals. Further work in this area should include suggestions for rectifying the situation and encouraging neurologists to seek help and improve their lifestyle. “I think that one of the ways that headache specialists have avoided burnout and depression is by focusing on one subspecialty area and engaging in different types of activities, such as seeing patients in the office and hospital, giving lectures, traveling to meetings, writing papers, and balancing their professional and personal lives. It appears that we need help as a profession, and we had better help ourselves to get it.”

The report found that only 18% of neurologists were very happy at work, and 41% overall identified themselves as burned out. Among reasons for burnout, 61% reported mounting bureaucratic tasks as their top reason, with 40% listing spending too many hours at work.
Coping strategies varied, with isolation from others topping the list at 46%, followed by talking with close friends and family and exercise tied at 40%.
Less than half (46%) claimed there was no impact on patients, but most (65%) don’t intend to seek professional help for depression and/or burnout and haven’t done so in the past. Similarly, 48% reported it’s unlikely they’d participate in a workplace program – in fact, only 33% said they would.
A slideshow laying out the findings in the report is available on Medscape.
A closer look at the numbers
Over 15,000 physicians across 29 specialties completed the 10-minute survey in the summer of 2019; 62% were men and most of the group were Baby Boomers (ages 55-73), then Generation X (ages 40-54), and lastly Millennials (ages 25-39). Of the specialties surveyed, neurologists scored lowest in the happiness-at-work category, with only 18% saying they were happy. Neurologists also scored lowest in happiness outside of work (44%). Half the neurologists surveyed said they were burned out, which was slightly more than the surveyed group of physicians in general. The biggest contributors to burnout were bureaucratic tasks, too many hours at work, and lack of control. Most coped by isolating themselves, talking with family members or friends, exercising and sleep. About 65% did not seek help for burnout or depression. The main reasons were being too busy, preferring to deal with it themselves, or feeling that the problem was not significant enough to warrant intervention.
A majority of the neurologists surveyed (70%-80%) are married and 85% say they have a good marriage. Almost half of neurologists take 3-4 weeks of vacation and a third take 1-2 weeks. Neurologists surveyed drive mostly Hondas and Toyotas; 4% drive Teslas and 3% drive Porsches. One third of neurologists exercise two to three times per week and 10% exercise daily. Only 20% have a drink more than four times per week.
Looking for solutions
“It is a bit distressing to see how many neurologists are unhappy at work and unhappy even outside of work,” said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. Many neurologists claim to be burned out and a small percent report depression. Most do not seek help, do not take care of themselves well enough, and do not vacation or exercise enough, added Dr. Rapoport, who also is a past president of the International Headache Society and is editor in chief of Neurology Reviews.
Dr. Rapoport believes that some studies about this situation should be done by the American Academy of Neurology and other subspecialty organizations (for example, the American Headache Society), and results should be published in the neurology and subspecialty journals. Further work in this area should include suggestions for rectifying the situation and encouraging neurologists to seek help and improve their lifestyle. “I think that one of the ways that headache specialists have avoided burnout and depression is by focusing on one subspecialty area and engaging in different types of activities, such as seeing patients in the office and hospital, giving lectures, traveling to meetings, writing papers, and balancing their professional and personal lives. It appears that we need help as a profession, and we had better help ourselves to get it.”