User login
Cutaneous Collagenous Vasculopathy
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
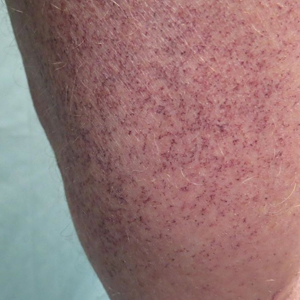
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
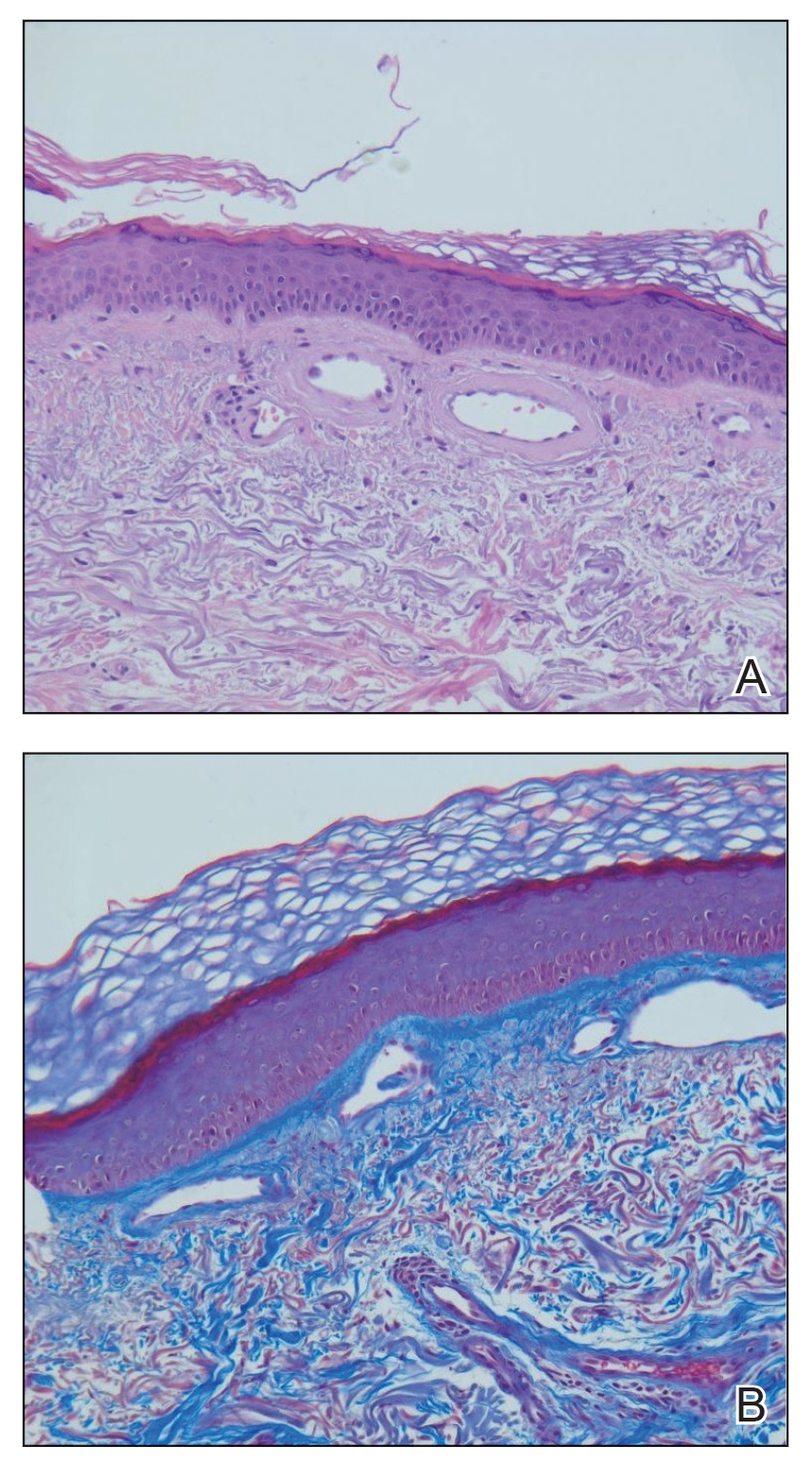
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
Practice Points
- Cutaneous collagenous vasculopathy (CCV) should be in the differential diagnosis of widespread telangiectases.
- Biopsy is needed to differentiate between CCV and generalized essential telangiectasia because of their similar clinical features.
- There may be underlying comorbidities associated with CCV, but the exact cause of the condition has yet to be found.
Persistent Chlorotrichosis With Chronic Sun Exposure
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
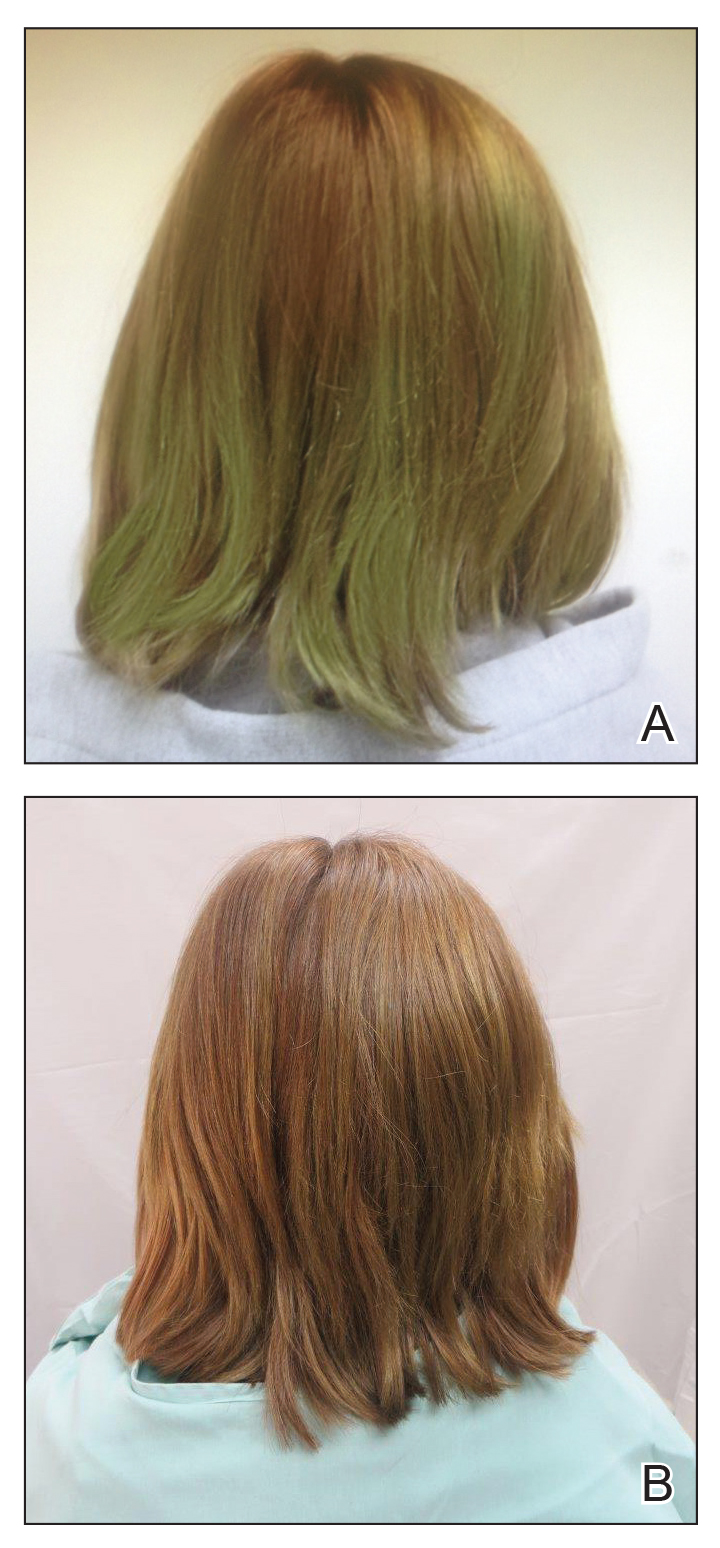
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
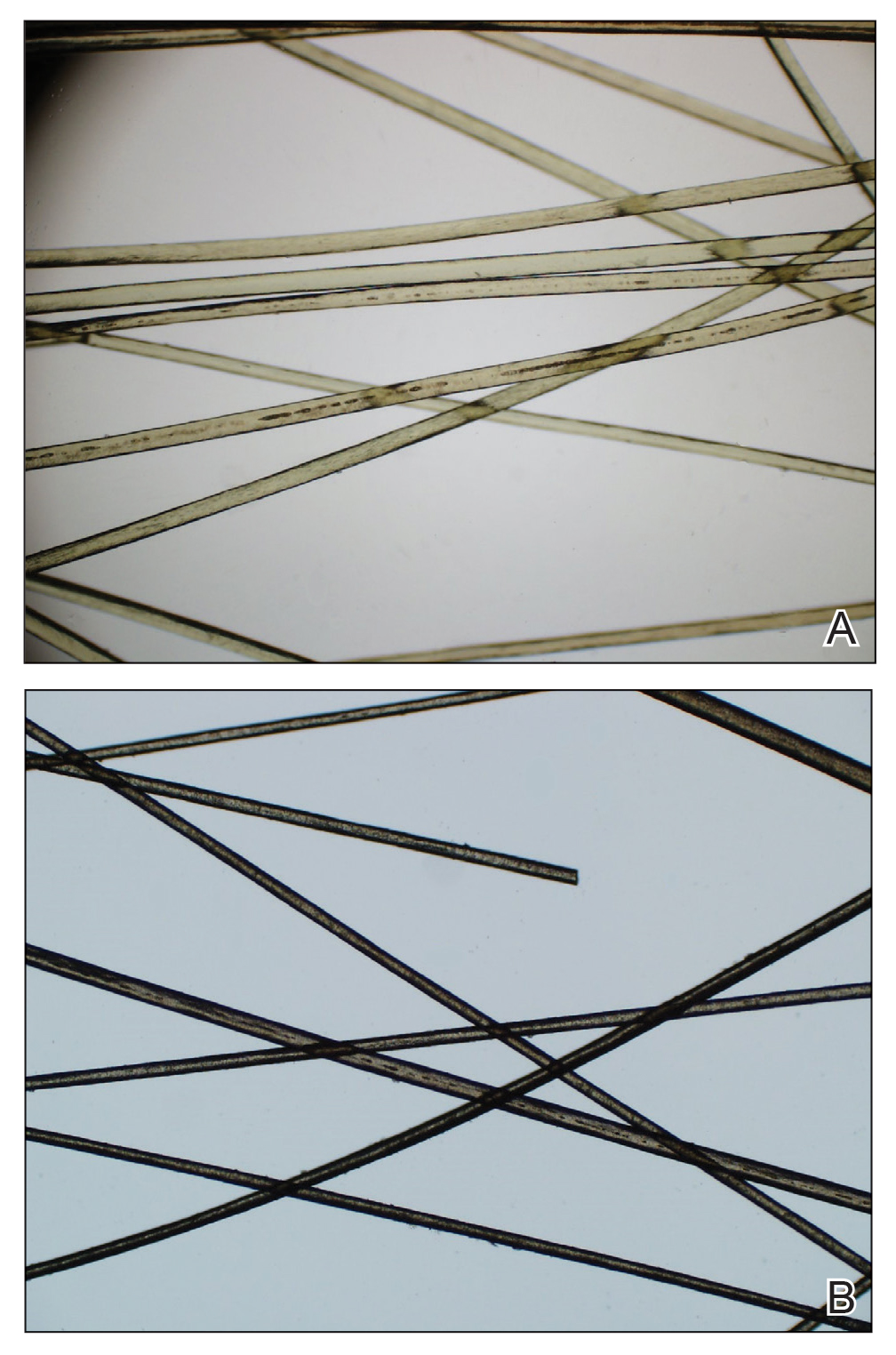
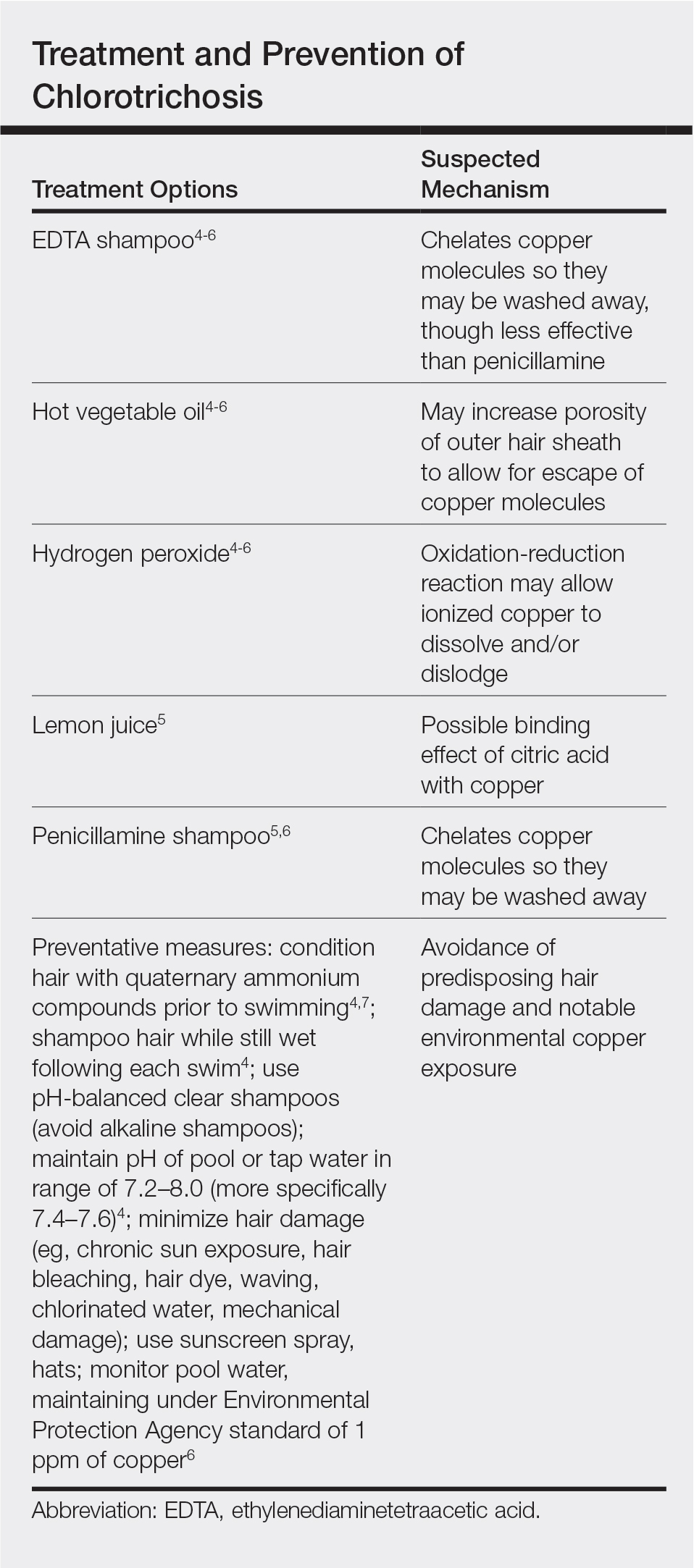
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
Practice Points
- Chlorotrichosis is the deposition of copper onto hair, which causes a green discoloration and most commonly occurs in blonde patients with excessive exposure to chlorinated water.
- Hair cuticle damage from hair care practices, such as use of heat or chemicals, can predispose patients to the development of chlorotrichosis.
- Although a number of treatments have been proposed, the use of penicillamine shampoo seems to be particularly effective and works via chelation of the adherent copper molecules.
Comparison shows tighter treat-to-target approach provides better outcomes in RA
Implementing a more stringent treat‐to‐target strategy could provide better outcomes for patients with early RA, according to a recent comparative study.
The findings confirm the feasibility of adopting a treat‐to‐target approach to ensure optimal outcomes are achieved for patients with early-stage disease.
“The objective of the present study was to compare achievement of remission during 2 years of follow-up in two early RA cohorts implementing different treat‐to‐target strategies,” wrote Vibeke Norvang, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, and colleagues. The findings were published in Arthritis & Rheumatology.
The researchers performed a pooled analysis of data from the randomized ARCTIC trial and the Norwegian Very Early Arthritis Clinic (NOR-VEAC) observational study. The combined cohort included a total of 429 disease-modifying antirheumatic drug (DMARD)–naive early RA patients, 189 and 330 from each study, respectively.
The American College of Rheumatology/European League Against Rheumatism Boolean remission criteria differed between the two cohorts, with more stringent criteria in ARCTIC than in NOR-VEAC. Remission was defined as scores of less than 1.6 and 2.6 on the Disease Activity Scores in 44 joints and 28 joints, respectively.
At 12- and 24-month follow-up, the researchers found that the odds of achieving remission were greater in ARCTIC than in NOR-VEAC (odds ratios, 1.97; 95% confidence interval, 1.21-3.20 vs. OR, 1.82; 95% CI, 1.05-3.16).
“We found that more than half of patients in each cohort had reached the study-specific remission targets at 6 months, and this increased to more than 60% in each cohort at 12 and 24 months,” they reported.
With respect to drug therapy, all study patients started with methotrexate monotherapy at a mean dose of 16.0 mg and 15.5 mg in ARCTIC and NOR-VEAC, respectively. In addition, similar rates of escalation to a biologic DMARD were observed in both studies (25.6% vs. 25.4%) at 24 months.
The researchers acknowledged that a key limitation of the study was comparing outcomes in two cohorts with different study designs; hence, the risk of bias in estimates of effect cannot be excluded.
“Targeting a more stringent remission and implementing more frequent visits provide further potential for favorable outcomes of a treat‐to‐target strategy,” they concluded.
The study was supported by legacy funds provided to the department of rheumatology at Diakonhjemmet Hospital. Three authors reported financial relationships with AbbVie, Amgen, Corrona, Genentech, Janssen, Mylan, Pfizer, and other companies.
SOURCE: Norvang V et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41232.
Implementing a more stringent treat‐to‐target strategy could provide better outcomes for patients with early RA, according to a recent comparative study.
The findings confirm the feasibility of adopting a treat‐to‐target approach to ensure optimal outcomes are achieved for patients with early-stage disease.
“The objective of the present study was to compare achievement of remission during 2 years of follow-up in two early RA cohorts implementing different treat‐to‐target strategies,” wrote Vibeke Norvang, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, and colleagues. The findings were published in Arthritis & Rheumatology.
The researchers performed a pooled analysis of data from the randomized ARCTIC trial and the Norwegian Very Early Arthritis Clinic (NOR-VEAC) observational study. The combined cohort included a total of 429 disease-modifying antirheumatic drug (DMARD)–naive early RA patients, 189 and 330 from each study, respectively.
The American College of Rheumatology/European League Against Rheumatism Boolean remission criteria differed between the two cohorts, with more stringent criteria in ARCTIC than in NOR-VEAC. Remission was defined as scores of less than 1.6 and 2.6 on the Disease Activity Scores in 44 joints and 28 joints, respectively.
At 12- and 24-month follow-up, the researchers found that the odds of achieving remission were greater in ARCTIC than in NOR-VEAC (odds ratios, 1.97; 95% confidence interval, 1.21-3.20 vs. OR, 1.82; 95% CI, 1.05-3.16).
“We found that more than half of patients in each cohort had reached the study-specific remission targets at 6 months, and this increased to more than 60% in each cohort at 12 and 24 months,” they reported.
With respect to drug therapy, all study patients started with methotrexate monotherapy at a mean dose of 16.0 mg and 15.5 mg in ARCTIC and NOR-VEAC, respectively. In addition, similar rates of escalation to a biologic DMARD were observed in both studies (25.6% vs. 25.4%) at 24 months.
The researchers acknowledged that a key limitation of the study was comparing outcomes in two cohorts with different study designs; hence, the risk of bias in estimates of effect cannot be excluded.
“Targeting a more stringent remission and implementing more frequent visits provide further potential for favorable outcomes of a treat‐to‐target strategy,” they concluded.
The study was supported by legacy funds provided to the department of rheumatology at Diakonhjemmet Hospital. Three authors reported financial relationships with AbbVie, Amgen, Corrona, Genentech, Janssen, Mylan, Pfizer, and other companies.
SOURCE: Norvang V et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41232.
Implementing a more stringent treat‐to‐target strategy could provide better outcomes for patients with early RA, according to a recent comparative study.
The findings confirm the feasibility of adopting a treat‐to‐target approach to ensure optimal outcomes are achieved for patients with early-stage disease.
“The objective of the present study was to compare achievement of remission during 2 years of follow-up in two early RA cohorts implementing different treat‐to‐target strategies,” wrote Vibeke Norvang, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, and colleagues. The findings were published in Arthritis & Rheumatology.
The researchers performed a pooled analysis of data from the randomized ARCTIC trial and the Norwegian Very Early Arthritis Clinic (NOR-VEAC) observational study. The combined cohort included a total of 429 disease-modifying antirheumatic drug (DMARD)–naive early RA patients, 189 and 330 from each study, respectively.
The American College of Rheumatology/European League Against Rheumatism Boolean remission criteria differed between the two cohorts, with more stringent criteria in ARCTIC than in NOR-VEAC. Remission was defined as scores of less than 1.6 and 2.6 on the Disease Activity Scores in 44 joints and 28 joints, respectively.
At 12- and 24-month follow-up, the researchers found that the odds of achieving remission were greater in ARCTIC than in NOR-VEAC (odds ratios, 1.97; 95% confidence interval, 1.21-3.20 vs. OR, 1.82; 95% CI, 1.05-3.16).
“We found that more than half of patients in each cohort had reached the study-specific remission targets at 6 months, and this increased to more than 60% in each cohort at 12 and 24 months,” they reported.
With respect to drug therapy, all study patients started with methotrexate monotherapy at a mean dose of 16.0 mg and 15.5 mg in ARCTIC and NOR-VEAC, respectively. In addition, similar rates of escalation to a biologic DMARD were observed in both studies (25.6% vs. 25.4%) at 24 months.
The researchers acknowledged that a key limitation of the study was comparing outcomes in two cohorts with different study designs; hence, the risk of bias in estimates of effect cannot be excluded.
“Targeting a more stringent remission and implementing more frequent visits provide further potential for favorable outcomes of a treat‐to‐target strategy,” they concluded.
The study was supported by legacy funds provided to the department of rheumatology at Diakonhjemmet Hospital. Three authors reported financial relationships with AbbVie, Amgen, Corrona, Genentech, Janssen, Mylan, Pfizer, and other companies.
SOURCE: Norvang V et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41232.
FROM ARTHRITIS & RHEUMATOLOGY
Diagnosing insomnia takes time
Give new patients 1 hour, expert advises
LAS VEGAS – Clinicians should spend 1 hour with patients who present with a chief complaint of insomnia, rather than rushing to a treatment after a 10- to 15-minute office visit, according to John W. Winkelman, MD, PhD.
“Why? Because sleep problems are usually multifactorial, involving psychiatric illness, sleep disorders, medical illness, medication, and poor sleep hygiene/stress,” he said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “There are usually many contributing problems, and sleep quality is only as strong as the weakest link. Maybe you don’t have an hour [to meet with new patients], but you need to give adequate time, otherwise you’re not going to do justice to the problem.”
“Ask, ‘what is it that bothers you most about your insomnia? Is it the time awake at night, your total sleep time, or how you feel during the day?’ Because we’re going to use different approaches based on that chief complaint of the insomnia,” said Dr. Winkelman, chief of the Massachusetts General Sleep Disorders Clinical Research Program in the department of psychiatry at Harvard Medical School, Boston. “Cognitive-behavioral therapy for insomnia [CBT-I], for instance, is very good at reducing time awake at night. It won’t increase total sleep time, but it reduces time awake at night dramatically.”
According to the DSM-5, insomnia disorder is marked by dissatisfaction with sleep quality or quantity associated with at least one of the following: difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening. “Just getting up to pee five times a night is not insomnia,” he said. “Just taking an hour and a half to fall asleep at the beginning of the night is not insomnia. There has to be distress or dysfunction related to the sleep disturbance, for a minimum of three times per week for 3 months.”
Most sleep problems are transient, but 25%-30% last more than 1 year. The differential diagnosis for chronic insomnia includes primary psychiatric disorders, medications, substances, restless legs syndrome, sleep schedule disorders, and obstructive sleep apnea.
“In general, we do not order sleep studies in people with insomnia unless we suspect sleep apnea; it’s just a waste of time,” said Dr. Winkelman, who is also a professor of psychiatry at Harvard Medical School. Indications for polysomnography include loud snoring plus one of the following: daytime sleepiness, witnessed apneas, or refractory hypertension. Other indications include abnormal behaviors or movements during sleep, unexplained excessive daytime sleepiness, and refractory sleep complaints, especially repetitive brief awakenings.
Many common cognitive and behavioral issues can produce or worsen insomnia, including inconsistent bedtimes and wake times. “That irregular schedule wreaks havoc with sleep,” he said. “It messes up the circadian rhythm. Also, homeostatic drive needs to build up: We need to be awake 16 or more hours in order to be sleepy. If people are sleeping until noon on Sundays and then trying to go to bed at their usual time, 10 or 11 at night, they’ve only been awake 10 or 11 hours. That’s why they’re going to have problems falling asleep. Also, a lot of people doze off after dinner in front of the TV. That doesn’t help.”
Spending excessive time in bed can also trigger or worsen insomnia. Dr. Winkelman recommends that people restrict their access to bed to the number of hours it is reasonable to sleep. “I see a lot of people in their 70s and 80s spending 10 hours in bed,” he said. “It doesn’t sound that crazy, but there is no way they’re going to get 10 hours of sleep. It’s physically impossible, so they spend 2 or 3 hours awake at night.” Clock-watching is another no-no. “In the middle of the night you wake up, look at the clock, and say to yourself: ‘Oh my god, I’ve been awake for 3 hours. I have 4 hours left. I need 7 hours. That means I need to go to sleep now!’ ”
An estimated 30%-40% of people with chronic insomnia have a psychiatric disorder. That means “you have to be thorough in your evaluation and act as if you’re doing a structured interview,” Dr. Winkelman said. “Ask about obsessive-compulsive disorder, generalized anxiety disorder, PTSD, et cetera, so that you understand the complete myriad of psychiatric illnesses, because psychiatric illnesses run in gangs. Comorbidity is generally the rule.”
The first-line treatment for chronic insomnia disorder is CBT-I, a multicomponent approach that includes time-in-bed restriction, stimulus control, cognitive therapy, relaxation therapy, and sleep hygiene. According to Dr. Winkelman, the cornerstone of CBT-I is time-in-bed restriction. “Many people with insomnia are spending 8.5 hours in bed to get 6.5 hours of sleep,” he said. “What you do is restrict access to bed to 6.5 hours; you initially sleep deprive them. Over the first few weeks, they hate you. After a few weeks when they start sleeping well, you start gradually increasing time in bed, but they rarely get back to the 8.5 hours in bed they were spending beforehand.”
Online CBT-I programs such as Sleepio can also be effective for improving sleep latency and wake after sleep onset, but not for total sleep time (JAMA Psychiatry. 2017;74[1]:68-75). “Not everybody responds to CBT; 50% don’t respond at a couple of months,” he said. “These are the people you need to think about medication for.”
Medications commonly used for chronic insomnia include benzodiazepine receptor agonists (BzRAs) – temazepam, eszopiclone, triazolam, zolpidem, and zaleplon are Food and Drug Administration approved – melatonin agonists, orexin antagonists, sedating antidepressants, anticonvulsants, and dopaminergic antagonists. “Each of the agents in these categories has somewhat similar mechanisms of action, and similar efficacy and contraindications,” Dr. Winkelman said. “The best way to divide the benzodiazepine receptor agonists is based on half-life. How long do you want drug on receptor in somebody with insomnia? Probably not much longer than 8 hours. Nevertheless, some psychiatrists love clonazepam, which has a 40-hour half-life. The circumstances under which clonazepam should be used for insomnia are small, such as in people with a daytime anxiety disorder.”
Consider trying triazolam, zolpidem, and zaleplon for patients who have problems falling asleep, he said, while oxazepam and eszopiclone are sensible options for people who have difficulty falling and staying asleep. Clinical response to BzRAs is common, yet only about half of people who have insomnia remit with one of these agents.
Dr. Winkelman said that patients and physicians often ask him whether BzRAs and other agents used as sleep aids are addictive. Abuse is identified when recurrent use causes clinically and functionally significant impairment, such as health problems; disability; and failure to meet major responsibilities at work, home, or school. “These are concerns with BzRAs. Misuse and abuse generally occur in younger people. Once you get to 35 years old, misuse rates get very low. In older people, rates of side effects go up.
“Tolerance, physiological and psychological dependence, and nonmedical diversion are also of concern,” he said. However, for the majority of people, BzRA hypnotics are effective and safe.
As for other agents, meta-analyses have demonstrated that melatonin 1-3 mg can help people fall asleep when it’s not being endogenously released. “That’s during the day,” he said. “That might be most relevant for jet lag and for people doing shift work.” Two orexin antagonists on the market for insomnia include suvorexant and lemborexant 10-20 mg. Advantages of these include little abuse liability and few side effects. “In one head-to-head polysomnography study in the elderly, lemborexant was superior to zolpidem 6.25 mg CR on both objective and subjective ability to fall asleep and stay asleep,” Dr. Winkelman said. (JAMA Netw Open. 2019;2[12]:e1918254).
Antidepressants are another treatment option, including mirtazapine 15-30 mg, trazodone 25-100 mg, and amitriptyline and doxepin (10-50 mg). Advantages include little abuse liability, while potential drawbacks include daytime sedation, weight gain, and anticholinergic side effects. Meanwhile, atypical antipsychotics such as quetiapine 25-100 mg have long been known to be helpful for sleep. “Advantages are that they’re anxiolytic, they’re mood stabilizing, and there is little abuse liability,” Dr. Winkelman said. “Drawbacks are that they’re probably less effective than BzRAs, they cause daytime sedation, weight gain, risks of extrapyramidal symptoms and glucose and lipid abnormalities.”
Dr. Winkelman said that he uses “a fair amount” of the anticonvulsant gabapentin as a second- or third-line hypnotic agent. “I usually start with 300 mg [at bedtime],” he added. “Drawbacks are that it’s probably less effective than BzRAs; it affects cognition; and can cause daytime sedation, dizziness, and weight gain. There are also concerns about abuse.”
Dr. Winkelman reported that he has received grant/research support from Merck, the RLS Foundation, and Luitpold Pharmaceuticals. He is also a consultant for Advance Medical, Avadel Pharmaceuticals, and UpToDate and is a member of the speakers’ bureau for Luitpold.
Give new patients 1 hour, expert advises
Give new patients 1 hour, expert advises
LAS VEGAS – Clinicians should spend 1 hour with patients who present with a chief complaint of insomnia, rather than rushing to a treatment after a 10- to 15-minute office visit, according to John W. Winkelman, MD, PhD.
“Why? Because sleep problems are usually multifactorial, involving psychiatric illness, sleep disorders, medical illness, medication, and poor sleep hygiene/stress,” he said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “There are usually many contributing problems, and sleep quality is only as strong as the weakest link. Maybe you don’t have an hour [to meet with new patients], but you need to give adequate time, otherwise you’re not going to do justice to the problem.”
“Ask, ‘what is it that bothers you most about your insomnia? Is it the time awake at night, your total sleep time, or how you feel during the day?’ Because we’re going to use different approaches based on that chief complaint of the insomnia,” said Dr. Winkelman, chief of the Massachusetts General Sleep Disorders Clinical Research Program in the department of psychiatry at Harvard Medical School, Boston. “Cognitive-behavioral therapy for insomnia [CBT-I], for instance, is very good at reducing time awake at night. It won’t increase total sleep time, but it reduces time awake at night dramatically.”
According to the DSM-5, insomnia disorder is marked by dissatisfaction with sleep quality or quantity associated with at least one of the following: difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening. “Just getting up to pee five times a night is not insomnia,” he said. “Just taking an hour and a half to fall asleep at the beginning of the night is not insomnia. There has to be distress or dysfunction related to the sleep disturbance, for a minimum of three times per week for 3 months.”
Most sleep problems are transient, but 25%-30% last more than 1 year. The differential diagnosis for chronic insomnia includes primary psychiatric disorders, medications, substances, restless legs syndrome, sleep schedule disorders, and obstructive sleep apnea.
“In general, we do not order sleep studies in people with insomnia unless we suspect sleep apnea; it’s just a waste of time,” said Dr. Winkelman, who is also a professor of psychiatry at Harvard Medical School. Indications for polysomnography include loud snoring plus one of the following: daytime sleepiness, witnessed apneas, or refractory hypertension. Other indications include abnormal behaviors or movements during sleep, unexplained excessive daytime sleepiness, and refractory sleep complaints, especially repetitive brief awakenings.
Many common cognitive and behavioral issues can produce or worsen insomnia, including inconsistent bedtimes and wake times. “That irregular schedule wreaks havoc with sleep,” he said. “It messes up the circadian rhythm. Also, homeostatic drive needs to build up: We need to be awake 16 or more hours in order to be sleepy. If people are sleeping until noon on Sundays and then trying to go to bed at their usual time, 10 or 11 at night, they’ve only been awake 10 or 11 hours. That’s why they’re going to have problems falling asleep. Also, a lot of people doze off after dinner in front of the TV. That doesn’t help.”
Spending excessive time in bed can also trigger or worsen insomnia. Dr. Winkelman recommends that people restrict their access to bed to the number of hours it is reasonable to sleep. “I see a lot of people in their 70s and 80s spending 10 hours in bed,” he said. “It doesn’t sound that crazy, but there is no way they’re going to get 10 hours of sleep. It’s physically impossible, so they spend 2 or 3 hours awake at night.” Clock-watching is another no-no. “In the middle of the night you wake up, look at the clock, and say to yourself: ‘Oh my god, I’ve been awake for 3 hours. I have 4 hours left. I need 7 hours. That means I need to go to sleep now!’ ”
An estimated 30%-40% of people with chronic insomnia have a psychiatric disorder. That means “you have to be thorough in your evaluation and act as if you’re doing a structured interview,” Dr. Winkelman said. “Ask about obsessive-compulsive disorder, generalized anxiety disorder, PTSD, et cetera, so that you understand the complete myriad of psychiatric illnesses, because psychiatric illnesses run in gangs. Comorbidity is generally the rule.”
The first-line treatment for chronic insomnia disorder is CBT-I, a multicomponent approach that includes time-in-bed restriction, stimulus control, cognitive therapy, relaxation therapy, and sleep hygiene. According to Dr. Winkelman, the cornerstone of CBT-I is time-in-bed restriction. “Many people with insomnia are spending 8.5 hours in bed to get 6.5 hours of sleep,” he said. “What you do is restrict access to bed to 6.5 hours; you initially sleep deprive them. Over the first few weeks, they hate you. After a few weeks when they start sleeping well, you start gradually increasing time in bed, but they rarely get back to the 8.5 hours in bed they were spending beforehand.”
Online CBT-I programs such as Sleepio can also be effective for improving sleep latency and wake after sleep onset, but not for total sleep time (JAMA Psychiatry. 2017;74[1]:68-75). “Not everybody responds to CBT; 50% don’t respond at a couple of months,” he said. “These are the people you need to think about medication for.”
Medications commonly used for chronic insomnia include benzodiazepine receptor agonists (BzRAs) – temazepam, eszopiclone, triazolam, zolpidem, and zaleplon are Food and Drug Administration approved – melatonin agonists, orexin antagonists, sedating antidepressants, anticonvulsants, and dopaminergic antagonists. “Each of the agents in these categories has somewhat similar mechanisms of action, and similar efficacy and contraindications,” Dr. Winkelman said. “The best way to divide the benzodiazepine receptor agonists is based on half-life. How long do you want drug on receptor in somebody with insomnia? Probably not much longer than 8 hours. Nevertheless, some psychiatrists love clonazepam, which has a 40-hour half-life. The circumstances under which clonazepam should be used for insomnia are small, such as in people with a daytime anxiety disorder.”
Consider trying triazolam, zolpidem, and zaleplon for patients who have problems falling asleep, he said, while oxazepam and eszopiclone are sensible options for people who have difficulty falling and staying asleep. Clinical response to BzRAs is common, yet only about half of people who have insomnia remit with one of these agents.
Dr. Winkelman said that patients and physicians often ask him whether BzRAs and other agents used as sleep aids are addictive. Abuse is identified when recurrent use causes clinically and functionally significant impairment, such as health problems; disability; and failure to meet major responsibilities at work, home, or school. “These are concerns with BzRAs. Misuse and abuse generally occur in younger people. Once you get to 35 years old, misuse rates get very low. In older people, rates of side effects go up.
“Tolerance, physiological and psychological dependence, and nonmedical diversion are also of concern,” he said. However, for the majority of people, BzRA hypnotics are effective and safe.
As for other agents, meta-analyses have demonstrated that melatonin 1-3 mg can help people fall asleep when it’s not being endogenously released. “That’s during the day,” he said. “That might be most relevant for jet lag and for people doing shift work.” Two orexin antagonists on the market for insomnia include suvorexant and lemborexant 10-20 mg. Advantages of these include little abuse liability and few side effects. “In one head-to-head polysomnography study in the elderly, lemborexant was superior to zolpidem 6.25 mg CR on both objective and subjective ability to fall asleep and stay asleep,” Dr. Winkelman said. (JAMA Netw Open. 2019;2[12]:e1918254).
Antidepressants are another treatment option, including mirtazapine 15-30 mg, trazodone 25-100 mg, and amitriptyline and doxepin (10-50 mg). Advantages include little abuse liability, while potential drawbacks include daytime sedation, weight gain, and anticholinergic side effects. Meanwhile, atypical antipsychotics such as quetiapine 25-100 mg have long been known to be helpful for sleep. “Advantages are that they’re anxiolytic, they’re mood stabilizing, and there is little abuse liability,” Dr. Winkelman said. “Drawbacks are that they’re probably less effective than BzRAs, they cause daytime sedation, weight gain, risks of extrapyramidal symptoms and glucose and lipid abnormalities.”
Dr. Winkelman said that he uses “a fair amount” of the anticonvulsant gabapentin as a second- or third-line hypnotic agent. “I usually start with 300 mg [at bedtime],” he added. “Drawbacks are that it’s probably less effective than BzRAs; it affects cognition; and can cause daytime sedation, dizziness, and weight gain. There are also concerns about abuse.”
Dr. Winkelman reported that he has received grant/research support from Merck, the RLS Foundation, and Luitpold Pharmaceuticals. He is also a consultant for Advance Medical, Avadel Pharmaceuticals, and UpToDate and is a member of the speakers’ bureau for Luitpold.
LAS VEGAS – Clinicians should spend 1 hour with patients who present with a chief complaint of insomnia, rather than rushing to a treatment after a 10- to 15-minute office visit, according to John W. Winkelman, MD, PhD.
“Why? Because sleep problems are usually multifactorial, involving psychiatric illness, sleep disorders, medical illness, medication, and poor sleep hygiene/stress,” he said at an annual psychopharmacology update held by the Nevada Psychiatric Association. “There are usually many contributing problems, and sleep quality is only as strong as the weakest link. Maybe you don’t have an hour [to meet with new patients], but you need to give adequate time, otherwise you’re not going to do justice to the problem.”
“Ask, ‘what is it that bothers you most about your insomnia? Is it the time awake at night, your total sleep time, or how you feel during the day?’ Because we’re going to use different approaches based on that chief complaint of the insomnia,” said Dr. Winkelman, chief of the Massachusetts General Sleep Disorders Clinical Research Program in the department of psychiatry at Harvard Medical School, Boston. “Cognitive-behavioral therapy for insomnia [CBT-I], for instance, is very good at reducing time awake at night. It won’t increase total sleep time, but it reduces time awake at night dramatically.”
According to the DSM-5, insomnia disorder is marked by dissatisfaction with sleep quality or quantity associated with at least one of the following: difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening. “Just getting up to pee five times a night is not insomnia,” he said. “Just taking an hour and a half to fall asleep at the beginning of the night is not insomnia. There has to be distress or dysfunction related to the sleep disturbance, for a minimum of three times per week for 3 months.”
Most sleep problems are transient, but 25%-30% last more than 1 year. The differential diagnosis for chronic insomnia includes primary psychiatric disorders, medications, substances, restless legs syndrome, sleep schedule disorders, and obstructive sleep apnea.
“In general, we do not order sleep studies in people with insomnia unless we suspect sleep apnea; it’s just a waste of time,” said Dr. Winkelman, who is also a professor of psychiatry at Harvard Medical School. Indications for polysomnography include loud snoring plus one of the following: daytime sleepiness, witnessed apneas, or refractory hypertension. Other indications include abnormal behaviors or movements during sleep, unexplained excessive daytime sleepiness, and refractory sleep complaints, especially repetitive brief awakenings.
Many common cognitive and behavioral issues can produce or worsen insomnia, including inconsistent bedtimes and wake times. “That irregular schedule wreaks havoc with sleep,” he said. “It messes up the circadian rhythm. Also, homeostatic drive needs to build up: We need to be awake 16 or more hours in order to be sleepy. If people are sleeping until noon on Sundays and then trying to go to bed at their usual time, 10 or 11 at night, they’ve only been awake 10 or 11 hours. That’s why they’re going to have problems falling asleep. Also, a lot of people doze off after dinner in front of the TV. That doesn’t help.”
Spending excessive time in bed can also trigger or worsen insomnia. Dr. Winkelman recommends that people restrict their access to bed to the number of hours it is reasonable to sleep. “I see a lot of people in their 70s and 80s spending 10 hours in bed,” he said. “It doesn’t sound that crazy, but there is no way they’re going to get 10 hours of sleep. It’s physically impossible, so they spend 2 or 3 hours awake at night.” Clock-watching is another no-no. “In the middle of the night you wake up, look at the clock, and say to yourself: ‘Oh my god, I’ve been awake for 3 hours. I have 4 hours left. I need 7 hours. That means I need to go to sleep now!’ ”
An estimated 30%-40% of people with chronic insomnia have a psychiatric disorder. That means “you have to be thorough in your evaluation and act as if you’re doing a structured interview,” Dr. Winkelman said. “Ask about obsessive-compulsive disorder, generalized anxiety disorder, PTSD, et cetera, so that you understand the complete myriad of psychiatric illnesses, because psychiatric illnesses run in gangs. Comorbidity is generally the rule.”
The first-line treatment for chronic insomnia disorder is CBT-I, a multicomponent approach that includes time-in-bed restriction, stimulus control, cognitive therapy, relaxation therapy, and sleep hygiene. According to Dr. Winkelman, the cornerstone of CBT-I is time-in-bed restriction. “Many people with insomnia are spending 8.5 hours in bed to get 6.5 hours of sleep,” he said. “What you do is restrict access to bed to 6.5 hours; you initially sleep deprive them. Over the first few weeks, they hate you. After a few weeks when they start sleeping well, you start gradually increasing time in bed, but they rarely get back to the 8.5 hours in bed they were spending beforehand.”
Online CBT-I programs such as Sleepio can also be effective for improving sleep latency and wake after sleep onset, but not for total sleep time (JAMA Psychiatry. 2017;74[1]:68-75). “Not everybody responds to CBT; 50% don’t respond at a couple of months,” he said. “These are the people you need to think about medication for.”
Medications commonly used for chronic insomnia include benzodiazepine receptor agonists (BzRAs) – temazepam, eszopiclone, triazolam, zolpidem, and zaleplon are Food and Drug Administration approved – melatonin agonists, orexin antagonists, sedating antidepressants, anticonvulsants, and dopaminergic antagonists. “Each of the agents in these categories has somewhat similar mechanisms of action, and similar efficacy and contraindications,” Dr. Winkelman said. “The best way to divide the benzodiazepine receptor agonists is based on half-life. How long do you want drug on receptor in somebody with insomnia? Probably not much longer than 8 hours. Nevertheless, some psychiatrists love clonazepam, which has a 40-hour half-life. The circumstances under which clonazepam should be used for insomnia are small, such as in people with a daytime anxiety disorder.”
Consider trying triazolam, zolpidem, and zaleplon for patients who have problems falling asleep, he said, while oxazepam and eszopiclone are sensible options for people who have difficulty falling and staying asleep. Clinical response to BzRAs is common, yet only about half of people who have insomnia remit with one of these agents.
Dr. Winkelman said that patients and physicians often ask him whether BzRAs and other agents used as sleep aids are addictive. Abuse is identified when recurrent use causes clinically and functionally significant impairment, such as health problems; disability; and failure to meet major responsibilities at work, home, or school. “These are concerns with BzRAs. Misuse and abuse generally occur in younger people. Once you get to 35 years old, misuse rates get very low. In older people, rates of side effects go up.
“Tolerance, physiological and psychological dependence, and nonmedical diversion are also of concern,” he said. However, for the majority of people, BzRA hypnotics are effective and safe.
As for other agents, meta-analyses have demonstrated that melatonin 1-3 mg can help people fall asleep when it’s not being endogenously released. “That’s during the day,” he said. “That might be most relevant for jet lag and for people doing shift work.” Two orexin antagonists on the market for insomnia include suvorexant and lemborexant 10-20 mg. Advantages of these include little abuse liability and few side effects. “In one head-to-head polysomnography study in the elderly, lemborexant was superior to zolpidem 6.25 mg CR on both objective and subjective ability to fall asleep and stay asleep,” Dr. Winkelman said. (JAMA Netw Open. 2019;2[12]:e1918254).
Antidepressants are another treatment option, including mirtazapine 15-30 mg, trazodone 25-100 mg, and amitriptyline and doxepin (10-50 mg). Advantages include little abuse liability, while potential drawbacks include daytime sedation, weight gain, and anticholinergic side effects. Meanwhile, atypical antipsychotics such as quetiapine 25-100 mg have long been known to be helpful for sleep. “Advantages are that they’re anxiolytic, they’re mood stabilizing, and there is little abuse liability,” Dr. Winkelman said. “Drawbacks are that they’re probably less effective than BzRAs, they cause daytime sedation, weight gain, risks of extrapyramidal symptoms and glucose and lipid abnormalities.”
Dr. Winkelman said that he uses “a fair amount” of the anticonvulsant gabapentin as a second- or third-line hypnotic agent. “I usually start with 300 mg [at bedtime],” he added. “Drawbacks are that it’s probably less effective than BzRAs; it affects cognition; and can cause daytime sedation, dizziness, and weight gain. There are also concerns about abuse.”
Dr. Winkelman reported that he has received grant/research support from Merck, the RLS Foundation, and Luitpold Pharmaceuticals. He is also a consultant for Advance Medical, Avadel Pharmaceuticals, and UpToDate and is a member of the speakers’ bureau for Luitpold.
EXPERT ANALYSIS FROM NPA 2020
BASILAR: Endovascular treatment improves outcomes in BAO stroke
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
REPORTING FROM ISC 2020
Docs spurn state attempts to criminalize treatment of transgender kids
Many US endocrinologists are crying foul as a growing number of state lawmakers are attempting to enact legislation that would prohibit, and in some cases criminalize, medical treatment for minors with gender dysphoria.
As of press time, 13 states had introduced such bills, and legislators in two additional states said they were drafting bills. So far, one — in South Dakota — was defeated in a Senate committee, and another, in Florida, was essentially tabled without being enacted.
They all have a common goal of preventing minors from receiving puberty blockers, cross-sex hormones, or gender-affirmation surgery.
“These things are being proposed based on a lot of misinformation,” said Stephen Rosenthal, MD, professor of pediatrics at the University of California, San Francisco (UCSF), and a past president of the Pediatric Endocrine Society.
Lawmakers “are not looking at the scientific evidence that supports current clinical practice guidelines,” Rosenthal, who treats transgender children, told Medscape Medical News.
And “People just aren’t really understanding the harm that regulating this kind of medicine would do,” stressed Cassandra Brady, MD, assistant professor of pediatric endocrinology at Vanderbilt University School of Medicine, Memphis, Tennessee.
The bills come at a time when gender identity clinics for minors around the world have seen a significant uptick in cases. And, as widely reported by Medscape Medical News, some clinicians have begun to question whether treatment decisions are outpacing science.
Queries about use of puberty blockers and cross-sex hormones have embroiled the United Kingdom’s only publicly funded Gender Identity Development Service (GIDS) in controversy, for example, with five clinicians resigning last year over concerns about overuse of the treatments.
And earlier this month, the UK National Health Service (NHS) announced an independent review of services including the use of puberty blockers and cross-sex hormones in youth with gender dysphoria.
Meanwhile, the topic has ignited debate in Sweden, where a report from the Board of Health and Welfare confirmed a 1,500% rise between 2008 and 2018 in gender dysphoria diagnoses among 13- to 17-year-olds born as girls, as detailed by The Guardian.
Indeed, there is some indication of a so-called “rapid-onset gender dysphoria” in born females who say they wish to become males and some clinicians have said this represents a “social” phenomenon.
But guidelines from US clinical organizations – including the American Academy of Pediatrics issued in 2018, the Endocrine Society as reported by Medscape Medical News in 2017, and the US Professional Association for Transgender Health (USPATH) – all support the use of medical treatment in adolescents with gender dysphoria who have received mental health evaluations from appropriately trained professionals.
More data needed but evidence to intervene is compelling
Joshua Safer, MD, FACP, FACE, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York City, says the data “even if it’s rudimentary, are convincing that there is a biological component to gender identity.”
Attempts to manipulate gender identity in people who are born intersex, for example, have uniformly failed, he noted.
Yet it’s still not known what causes gender identity – whether it might be a result of a cluster of genes or a bundle in the brain, or some other biological process – said Safer, who treats transgender adults, but not children, and is also a coauthor of the aforementioned Endocrine Society Clinical Practice Guideline on Endocrine Treatment of Gender Dysphoric/Gender Incongruent Persons.
This is an area for future research, he noted.
Nevertheless, “The data for interventions for transgender people ... is compelling,” he added, noting evidence for improved mental health morbidity among those gender-questioning people who have medical interventions.
“Those data are modest at this point and we need better data, but they do all move in the same direction,” he asserted.
Meanwhile, a large group of around 1,800 parents of transgender and nonbinary children have called on legislators to withdraw the proposals in an open letter organized by the Human Rights Campaign.
“We know better than anyone what our children need in order to thrive: access to best practice, evidence-based gender-affirming healthcare,” the parents write.
“These healthcare decisions must be made on a case-by-case basis, in careful consultation with a medical team, and with the goal of reducing the physical and emotional distress experienced by many transgender children,” they continue.
“They should not be made by politicians who think they know better than medical professionals,” they add.
The American Academy of Child and Adolescent Psychiatry has also condemned state efforts “to block access to these recognized interventions,” it said in a statement.
Proponents of laws speak of harms
Most of the state proposals portray medical interventions as harmful to minors.
Missouri’s proposed legislation labels surgical or hormonal treatment for a child under age 18 “abuse or neglect”; a physician or anyone who assists or provides for the child would be charged with a felony.
One of the first bills was introduced in South Dakota in January. House bill 1057 would have charged clinicians providing gender-affirming care in anyone under age 16 with a misdemeanor punishable by up to a year in prison and a $2,000 fine.
The bill was defeated in the Senate after the South Dakota State Medical Association and several other physicians, families, and adolescents testified against the proposal, according to the Argus Leader.
The Endocrine Society applauded the failure and noted in a statement that it “supports physicians’ ability to provide the best evidence-based treatment to their patients,” and that “these decisions should be made by the family and physician, and not dictated by policymakers.”
Jack Turban, MD, a resident physician in child and adolescent psychiatry at Massachusetts General Hospital, Boston, who conducted a pivotal study of some 26,000 transgender adults showing that early administration of puberty blockers led to lower odds of lifetime suicidal ideation, also expressed dismay over the bills in an opinion piece for the New York Times.
“The potential benefits of providing gender-affirmative care typically outweigh the minor risks associated with treatment,” wrote Turban.
“State legislators need to educate themselves about these young people and their medical care before introducing legislation that will hurt them,” he added.
Few states seem to have approached clinicians for feedback
In Tennessee, lawmakers have approached some clinicians at Vanderbilt and have appreciated the feedback they’ve received so far, said Brady.
But that may be an exception. It seems that few medical organizations have been consulted in the crafting of bills in the other states: Colorado, Florida, Idaho, Illinois, Kentucky, Mississippi, Missouri, New Hampshire, Oklahoma, South Carolina, South Dakota, and West Virginia. Lawmakers in Ohio and Utah also are drafting proposals.
Physicians could be charged with a felony in Florida, Idaho, Kentucky, Missouri, and reportedly, in the Ohio proposal under development.
The bills have been introduced at the behest of some conservative groups that doubt the existence of gender dysphoria or who have questions about treatment: the Eagle Forum, the Alliance Defending Freedom, and the Kelsey Coalition.
In a recent tweet clarifying its position on state efforts, the Kelsey Coalition said it “supports all bills that protect children, even those that may provide criminal penalties, because we believe these medical interventions should never be performed on children.”
“However, we do not support state bills that are not victim-led or used for political gain,” they added.
Existing knowledge imperfect but treatment indicated for some
The bills have also garnered support from some endocrinologists who have raised concerns about puberty blockers and other medical treatments for gender dysphoria.
One is Michael K. Laidlaw, MD, a Rocklin, California–based endocrinologist who has not treated transgender people but frequently writes about the subject, most recently calling the use of puberty blockers “a public health emergency.”
Laidlaw joined several other clinicians who do not treat transgender people in testifying in favor of the South Dakota bill.
Last year, as previously reported by Medscape Medical News, Laidlaw, along with others, criticized the Endocrine Society’s 2017 Clinical Practice Guideline on Treating Dysphoric/Gender-Incongruent Persons in a letter to the Journal of Clinical Endocrinology & Metabolism.
They stated that there is no lab, imaging, or other objective test to diagnose someone as transgender and that “the consequences of this gender-affirmative therapy are not trivial and include potential sterility, sexual dysfunction, thromboembolic and cardiovascular disease, and malignancy.”
Laidlaw told Medscape Medical News at the time that “If we’re talking about [transgender] adults [who have gone through puberty of their biological sex] and who can make a decision, if they have been truly notified of the risks and benefits [of cross-sex hormones] and have also had psychological evaluation, and they decide, ‘This is still the right course for me,’ then I don’t have any objection.”
But considering the use of cross-sex hormones in children and adolescents is “quite a different story,” he contended.
In May 2019, Rosenthal, Safer and colleagues responded to Laidlaw’s letter in the same journal, stating that for the right person, puberty blockers and cross-sex hormones are appropriate, and that medications can improve mental health outcomes.
“We agree that research to validate the safety and efficacy of all forms of treatment is desirable,” they wrote, noting some of that research is underway.
“However, we believe physicians would fall short in their duty of care if they withheld hormonal treatment of gender dysphoria/incongruence in pubertal youth, when indicated, given the existing state of knowledge, imperfect though it is.”
Research to validate safety and efficacy of transgender TX underway
Rosenthal’s center at UCSF is one of four in the United States that has been carrying out a National Institutes of Health-funded long-term observational study of the impact of early medical intervention on transgender adolescents.
It will take time to get those results, but in the meantime, clinicians should act on what is known now, said Rosenthal.
“We already have very compelling data to suggest that the benefits [of treatment] outweigh the potential harms,” he said.
Rosenthal told Medscape Medical News that Laidlaw has advanced the notion that clinicians who prescribe puberty blockers are forcing those individuals into a transgender outcome.
“We don’t push anybody down any path,” he said. “The guidelines make these treatments available in a very specific subset of people who are evaluated by skilled mental health professionals,” said Rosenthal.
Both he and Safer acknowledge that puberty blockers do have the potential for some harm. For instance, a frank discussion needs to happen about the likely lack of future fertility, said Rosenthal.
“Everything we do in medicine has a theoretical risk of harm,” noted Safer.
However, he said, to deny a puberty blocker to an individual approaching puberty who is distraught about growing breasts — but then to possibly have to surgically remove them later — is in itself doing harm.
“Puberty blockers are exactly the epitome of ‘do no harm’ in this case,” argued Safer.
The medications are reversible, he said, adding that they also give an individual and the family time to think through whether the adolescent is transgender, and, if yes, what they want to do in terms of taking cross-sex hormones in the future or getting other interventions.
Safer acknowledged that this doesn’t mean there aren’t still some concerns, however.
For instance, once puberty blockers — which have the potential to interfere with bone development — are started, “How much harm are you willing to risk? Maybe a couple of years is okay, but maybe 6 years is not,” he said.
“So, we do discuss how quickly...you have to get to your next decision point, whether it be to actually introduce hormones or not to introduce hormones,” he explained.
State proposals will have chilling effect on gender-questioning kids
Clinicians say that even if the proposals do not become law, just the fact of their existence could have a chilling effect on gender-questioning children, their families, and doctors considering whether to provide treatment.
“They’re already in a hard position,” Brady said of her patients.
“They’re coming here to seek something for a life that they’ve already not wanted to live and then we have people who are trying to put a real big block on that – I see that obviously affecting their mental health,” she observed.
“I can’t imagine how their lives would be without this care,” Brady said.
With the bills being out there, “two things can happen – one is, it can be very depressing and limiting, but it can also embolden people,” Rosenthal told Medscape Medical News.
“The people behind these things are the same people that have tried to stop our research at the National Institutes of Health (NIH),” he explained.
“These people are going to do everything they can, whether it’s to go state by state to try and exhaust us, or go to the NIH and try to get them to pull the plug on our research,” said Rosenthal.
Safer believes it’s ill-considered to try to legislate any aspect of medicine.
“The pitfalls of trying to legislate these things are myriad,” he said.
“Across all of medicine, interventions are very customized. Can you imagine a state legislature trying to legislate the optimal approach in medicines that can and cannot be given to people with diabetes? How crazy that would be,” he noted.
Rosenthal has served on an advisory panel for Endo Pharmaceuticals and is a grantee of the NIH. Safer has also served on an advisory panel for Endo Pharmaceuticals. Brady has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Many US endocrinologists are crying foul as a growing number of state lawmakers are attempting to enact legislation that would prohibit, and in some cases criminalize, medical treatment for minors with gender dysphoria.
As of press time, 13 states had introduced such bills, and legislators in two additional states said they were drafting bills. So far, one — in South Dakota — was defeated in a Senate committee, and another, in Florida, was essentially tabled without being enacted.
They all have a common goal of preventing minors from receiving puberty blockers, cross-sex hormones, or gender-affirmation surgery.
“These things are being proposed based on a lot of misinformation,” said Stephen Rosenthal, MD, professor of pediatrics at the University of California, San Francisco (UCSF), and a past president of the Pediatric Endocrine Society.
Lawmakers “are not looking at the scientific evidence that supports current clinical practice guidelines,” Rosenthal, who treats transgender children, told Medscape Medical News.
And “People just aren’t really understanding the harm that regulating this kind of medicine would do,” stressed Cassandra Brady, MD, assistant professor of pediatric endocrinology at Vanderbilt University School of Medicine, Memphis, Tennessee.
The bills come at a time when gender identity clinics for minors around the world have seen a significant uptick in cases. And, as widely reported by Medscape Medical News, some clinicians have begun to question whether treatment decisions are outpacing science.
Queries about use of puberty blockers and cross-sex hormones have embroiled the United Kingdom’s only publicly funded Gender Identity Development Service (GIDS) in controversy, for example, with five clinicians resigning last year over concerns about overuse of the treatments.
And earlier this month, the UK National Health Service (NHS) announced an independent review of services including the use of puberty blockers and cross-sex hormones in youth with gender dysphoria.
Meanwhile, the topic has ignited debate in Sweden, where a report from the Board of Health and Welfare confirmed a 1,500% rise between 2008 and 2018 in gender dysphoria diagnoses among 13- to 17-year-olds born as girls, as detailed by The Guardian.
Indeed, there is some indication of a so-called “rapid-onset gender dysphoria” in born females who say they wish to become males and some clinicians have said this represents a “social” phenomenon.
But guidelines from US clinical organizations – including the American Academy of Pediatrics issued in 2018, the Endocrine Society as reported by Medscape Medical News in 2017, and the US Professional Association for Transgender Health (USPATH) – all support the use of medical treatment in adolescents with gender dysphoria who have received mental health evaluations from appropriately trained professionals.
More data needed but evidence to intervene is compelling
Joshua Safer, MD, FACP, FACE, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York City, says the data “even if it’s rudimentary, are convincing that there is a biological component to gender identity.”
Attempts to manipulate gender identity in people who are born intersex, for example, have uniformly failed, he noted.
Yet it’s still not known what causes gender identity – whether it might be a result of a cluster of genes or a bundle in the brain, or some other biological process – said Safer, who treats transgender adults, but not children, and is also a coauthor of the aforementioned Endocrine Society Clinical Practice Guideline on Endocrine Treatment of Gender Dysphoric/Gender Incongruent Persons.
This is an area for future research, he noted.
Nevertheless, “The data for interventions for transgender people ... is compelling,” he added, noting evidence for improved mental health morbidity among those gender-questioning people who have medical interventions.
“Those data are modest at this point and we need better data, but they do all move in the same direction,” he asserted.
Meanwhile, a large group of around 1,800 parents of transgender and nonbinary children have called on legislators to withdraw the proposals in an open letter organized by the Human Rights Campaign.
“We know better than anyone what our children need in order to thrive: access to best practice, evidence-based gender-affirming healthcare,” the parents write.
“These healthcare decisions must be made on a case-by-case basis, in careful consultation with a medical team, and with the goal of reducing the physical and emotional distress experienced by many transgender children,” they continue.
“They should not be made by politicians who think they know better than medical professionals,” they add.
The American Academy of Child and Adolescent Psychiatry has also condemned state efforts “to block access to these recognized interventions,” it said in a statement.
Proponents of laws speak of harms
Most of the state proposals portray medical interventions as harmful to minors.
Missouri’s proposed legislation labels surgical or hormonal treatment for a child under age 18 “abuse or neglect”; a physician or anyone who assists or provides for the child would be charged with a felony.
One of the first bills was introduced in South Dakota in January. House bill 1057 would have charged clinicians providing gender-affirming care in anyone under age 16 with a misdemeanor punishable by up to a year in prison and a $2,000 fine.
The bill was defeated in the Senate after the South Dakota State Medical Association and several other physicians, families, and adolescents testified against the proposal, according to the Argus Leader.
The Endocrine Society applauded the failure and noted in a statement that it “supports physicians’ ability to provide the best evidence-based treatment to their patients,” and that “these decisions should be made by the family and physician, and not dictated by policymakers.”
Jack Turban, MD, a resident physician in child and adolescent psychiatry at Massachusetts General Hospital, Boston, who conducted a pivotal study of some 26,000 transgender adults showing that early administration of puberty blockers led to lower odds of lifetime suicidal ideation, also expressed dismay over the bills in an opinion piece for the New York Times.
“The potential benefits of providing gender-affirmative care typically outweigh the minor risks associated with treatment,” wrote Turban.
“State legislators need to educate themselves about these young people and their medical care before introducing legislation that will hurt them,” he added.
Few states seem to have approached clinicians for feedback
In Tennessee, lawmakers have approached some clinicians at Vanderbilt and have appreciated the feedback they’ve received so far, said Brady.
But that may be an exception. It seems that few medical organizations have been consulted in the crafting of bills in the other states: Colorado, Florida, Idaho, Illinois, Kentucky, Mississippi, Missouri, New Hampshire, Oklahoma, South Carolina, South Dakota, and West Virginia. Lawmakers in Ohio and Utah also are drafting proposals.
Physicians could be charged with a felony in Florida, Idaho, Kentucky, Missouri, and reportedly, in the Ohio proposal under development.
The bills have been introduced at the behest of some conservative groups that doubt the existence of gender dysphoria or who have questions about treatment: the Eagle Forum, the Alliance Defending Freedom, and the Kelsey Coalition.
In a recent tweet clarifying its position on state efforts, the Kelsey Coalition said it “supports all bills that protect children, even those that may provide criminal penalties, because we believe these medical interventions should never be performed on children.”
“However, we do not support state bills that are not victim-led or used for political gain,” they added.
Existing knowledge imperfect but treatment indicated for some
The bills have also garnered support from some endocrinologists who have raised concerns about puberty blockers and other medical treatments for gender dysphoria.
One is Michael K. Laidlaw, MD, a Rocklin, California–based endocrinologist who has not treated transgender people but frequently writes about the subject, most recently calling the use of puberty blockers “a public health emergency.”
Laidlaw joined several other clinicians who do not treat transgender people in testifying in favor of the South Dakota bill.
Last year, as previously reported by Medscape Medical News, Laidlaw, along with others, criticized the Endocrine Society’s 2017 Clinical Practice Guideline on Treating Dysphoric/Gender-Incongruent Persons in a letter to the Journal of Clinical Endocrinology & Metabolism.
They stated that there is no lab, imaging, or other objective test to diagnose someone as transgender and that “the consequences of this gender-affirmative therapy are not trivial and include potential sterility, sexual dysfunction, thromboembolic and cardiovascular disease, and malignancy.”
Laidlaw told Medscape Medical News at the time that “If we’re talking about [transgender] adults [who have gone through puberty of their biological sex] and who can make a decision, if they have been truly notified of the risks and benefits [of cross-sex hormones] and have also had psychological evaluation, and they decide, ‘This is still the right course for me,’ then I don’t have any objection.”
But considering the use of cross-sex hormones in children and adolescents is “quite a different story,” he contended.
In May 2019, Rosenthal, Safer and colleagues responded to Laidlaw’s letter in the same journal, stating that for the right person, puberty blockers and cross-sex hormones are appropriate, and that medications can improve mental health outcomes.
“We agree that research to validate the safety and efficacy of all forms of treatment is desirable,” they wrote, noting some of that research is underway.
“However, we believe physicians would fall short in their duty of care if they withheld hormonal treatment of gender dysphoria/incongruence in pubertal youth, when indicated, given the existing state of knowledge, imperfect though it is.”
Research to validate safety and efficacy of transgender TX underway
Rosenthal’s center at UCSF is one of four in the United States that has been carrying out a National Institutes of Health-funded long-term observational study of the impact of early medical intervention on transgender adolescents.
It will take time to get those results, but in the meantime, clinicians should act on what is known now, said Rosenthal.
“We already have very compelling data to suggest that the benefits [of treatment] outweigh the potential harms,” he said.
Rosenthal told Medscape Medical News that Laidlaw has advanced the notion that clinicians who prescribe puberty blockers are forcing those individuals into a transgender outcome.
“We don’t push anybody down any path,” he said. “The guidelines make these treatments available in a very specific subset of people who are evaluated by skilled mental health professionals,” said Rosenthal.
Both he and Safer acknowledge that puberty blockers do have the potential for some harm. For instance, a frank discussion needs to happen about the likely lack of future fertility, said Rosenthal.
“Everything we do in medicine has a theoretical risk of harm,” noted Safer.
However, he said, to deny a puberty blocker to an individual approaching puberty who is distraught about growing breasts — but then to possibly have to surgically remove them later — is in itself doing harm.
“Puberty blockers are exactly the epitome of ‘do no harm’ in this case,” argued Safer.
The medications are reversible, he said, adding that they also give an individual and the family time to think through whether the adolescent is transgender, and, if yes, what they want to do in terms of taking cross-sex hormones in the future or getting other interventions.
Safer acknowledged that this doesn’t mean there aren’t still some concerns, however.
For instance, once puberty blockers — which have the potential to interfere with bone development — are started, “How much harm are you willing to risk? Maybe a couple of years is okay, but maybe 6 years is not,” he said.
“So, we do discuss how quickly...you have to get to your next decision point, whether it be to actually introduce hormones or not to introduce hormones,” he explained.
State proposals will have chilling effect on gender-questioning kids
Clinicians say that even if the proposals do not become law, just the fact of their existence could have a chilling effect on gender-questioning children, their families, and doctors considering whether to provide treatment.
“They’re already in a hard position,” Brady said of her patients.
“They’re coming here to seek something for a life that they’ve already not wanted to live and then we have people who are trying to put a real big block on that – I see that obviously affecting their mental health,” she observed.
“I can’t imagine how their lives would be without this care,” Brady said.
With the bills being out there, “two things can happen – one is, it can be very depressing and limiting, but it can also embolden people,” Rosenthal told Medscape Medical News.
“The people behind these things are the same people that have tried to stop our research at the National Institutes of Health (NIH),” he explained.
“These people are going to do everything they can, whether it’s to go state by state to try and exhaust us, or go to the NIH and try to get them to pull the plug on our research,” said Rosenthal.
Safer believes it’s ill-considered to try to legislate any aspect of medicine.
“The pitfalls of trying to legislate these things are myriad,” he said.
“Across all of medicine, interventions are very customized. Can you imagine a state legislature trying to legislate the optimal approach in medicines that can and cannot be given to people with diabetes? How crazy that would be,” he noted.
Rosenthal has served on an advisory panel for Endo Pharmaceuticals and is a grantee of the NIH. Safer has also served on an advisory panel for Endo Pharmaceuticals. Brady has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Many US endocrinologists are crying foul as a growing number of state lawmakers are attempting to enact legislation that would prohibit, and in some cases criminalize, medical treatment for minors with gender dysphoria.
As of press time, 13 states had introduced such bills, and legislators in two additional states said they were drafting bills. So far, one — in South Dakota — was defeated in a Senate committee, and another, in Florida, was essentially tabled without being enacted.
They all have a common goal of preventing minors from receiving puberty blockers, cross-sex hormones, or gender-affirmation surgery.
“These things are being proposed based on a lot of misinformation,” said Stephen Rosenthal, MD, professor of pediatrics at the University of California, San Francisco (UCSF), and a past president of the Pediatric Endocrine Society.
Lawmakers “are not looking at the scientific evidence that supports current clinical practice guidelines,” Rosenthal, who treats transgender children, told Medscape Medical News.
And “People just aren’t really understanding the harm that regulating this kind of medicine would do,” stressed Cassandra Brady, MD, assistant professor of pediatric endocrinology at Vanderbilt University School of Medicine, Memphis, Tennessee.
The bills come at a time when gender identity clinics for minors around the world have seen a significant uptick in cases. And, as widely reported by Medscape Medical News, some clinicians have begun to question whether treatment decisions are outpacing science.
Queries about use of puberty blockers and cross-sex hormones have embroiled the United Kingdom’s only publicly funded Gender Identity Development Service (GIDS) in controversy, for example, with five clinicians resigning last year over concerns about overuse of the treatments.
And earlier this month, the UK National Health Service (NHS) announced an independent review of services including the use of puberty blockers and cross-sex hormones in youth with gender dysphoria.
Meanwhile, the topic has ignited debate in Sweden, where a report from the Board of Health and Welfare confirmed a 1,500% rise between 2008 and 2018 in gender dysphoria diagnoses among 13- to 17-year-olds born as girls, as detailed by The Guardian.
Indeed, there is some indication of a so-called “rapid-onset gender dysphoria” in born females who say they wish to become males and some clinicians have said this represents a “social” phenomenon.
But guidelines from US clinical organizations – including the American Academy of Pediatrics issued in 2018, the Endocrine Society as reported by Medscape Medical News in 2017, and the US Professional Association for Transgender Health (USPATH) – all support the use of medical treatment in adolescents with gender dysphoria who have received mental health evaluations from appropriately trained professionals.
More data needed but evidence to intervene is compelling
Joshua Safer, MD, FACP, FACE, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York City, says the data “even if it’s rudimentary, are convincing that there is a biological component to gender identity.”
Attempts to manipulate gender identity in people who are born intersex, for example, have uniformly failed, he noted.
Yet it’s still not known what causes gender identity – whether it might be a result of a cluster of genes or a bundle in the brain, or some other biological process – said Safer, who treats transgender adults, but not children, and is also a coauthor of the aforementioned Endocrine Society Clinical Practice Guideline on Endocrine Treatment of Gender Dysphoric/Gender Incongruent Persons.
This is an area for future research, he noted.
Nevertheless, “The data for interventions for transgender people ... is compelling,” he added, noting evidence for improved mental health morbidity among those gender-questioning people who have medical interventions.
“Those data are modest at this point and we need better data, but they do all move in the same direction,” he asserted.
Meanwhile, a large group of around 1,800 parents of transgender and nonbinary children have called on legislators to withdraw the proposals in an open letter organized by the Human Rights Campaign.
“We know better than anyone what our children need in order to thrive: access to best practice, evidence-based gender-affirming healthcare,” the parents write.
“These healthcare decisions must be made on a case-by-case basis, in careful consultation with a medical team, and with the goal of reducing the physical and emotional distress experienced by many transgender children,” they continue.
“They should not be made by politicians who think they know better than medical professionals,” they add.
The American Academy of Child and Adolescent Psychiatry has also condemned state efforts “to block access to these recognized interventions,” it said in a statement.
Proponents of laws speak of harms
Most of the state proposals portray medical interventions as harmful to minors.
Missouri’s proposed legislation labels surgical or hormonal treatment for a child under age 18 “abuse or neglect”; a physician or anyone who assists or provides for the child would be charged with a felony.
One of the first bills was introduced in South Dakota in January. House bill 1057 would have charged clinicians providing gender-affirming care in anyone under age 16 with a misdemeanor punishable by up to a year in prison and a $2,000 fine.
The bill was defeated in the Senate after the South Dakota State Medical Association and several other physicians, families, and adolescents testified against the proposal, according to the Argus Leader.
The Endocrine Society applauded the failure and noted in a statement that it “supports physicians’ ability to provide the best evidence-based treatment to their patients,” and that “these decisions should be made by the family and physician, and not dictated by policymakers.”
Jack Turban, MD, a resident physician in child and adolescent psychiatry at Massachusetts General Hospital, Boston, who conducted a pivotal study of some 26,000 transgender adults showing that early administration of puberty blockers led to lower odds of lifetime suicidal ideation, also expressed dismay over the bills in an opinion piece for the New York Times.
“The potential benefits of providing gender-affirmative care typically outweigh the minor risks associated with treatment,” wrote Turban.
“State legislators need to educate themselves about these young people and their medical care before introducing legislation that will hurt them,” he added.
Few states seem to have approached clinicians for feedback
In Tennessee, lawmakers have approached some clinicians at Vanderbilt and have appreciated the feedback they’ve received so far, said Brady.
But that may be an exception. It seems that few medical organizations have been consulted in the crafting of bills in the other states: Colorado, Florida, Idaho, Illinois, Kentucky, Mississippi, Missouri, New Hampshire, Oklahoma, South Carolina, South Dakota, and West Virginia. Lawmakers in Ohio and Utah also are drafting proposals.
Physicians could be charged with a felony in Florida, Idaho, Kentucky, Missouri, and reportedly, in the Ohio proposal under development.
The bills have been introduced at the behest of some conservative groups that doubt the existence of gender dysphoria or who have questions about treatment: the Eagle Forum, the Alliance Defending Freedom, and the Kelsey Coalition.
In a recent tweet clarifying its position on state efforts, the Kelsey Coalition said it “supports all bills that protect children, even those that may provide criminal penalties, because we believe these medical interventions should never be performed on children.”
“However, we do not support state bills that are not victim-led or used for political gain,” they added.
Existing knowledge imperfect but treatment indicated for some
The bills have also garnered support from some endocrinologists who have raised concerns about puberty blockers and other medical treatments for gender dysphoria.
One is Michael K. Laidlaw, MD, a Rocklin, California–based endocrinologist who has not treated transgender people but frequently writes about the subject, most recently calling the use of puberty blockers “a public health emergency.”
Laidlaw joined several other clinicians who do not treat transgender people in testifying in favor of the South Dakota bill.
Last year, as previously reported by Medscape Medical News, Laidlaw, along with others, criticized the Endocrine Society’s 2017 Clinical Practice Guideline on Treating Dysphoric/Gender-Incongruent Persons in a letter to the Journal of Clinical Endocrinology & Metabolism.
They stated that there is no lab, imaging, or other objective test to diagnose someone as transgender and that “the consequences of this gender-affirmative therapy are not trivial and include potential sterility, sexual dysfunction, thromboembolic and cardiovascular disease, and malignancy.”
Laidlaw told Medscape Medical News at the time that “If we’re talking about [transgender] adults [who have gone through puberty of their biological sex] and who can make a decision, if they have been truly notified of the risks and benefits [of cross-sex hormones] and have also had psychological evaluation, and they decide, ‘This is still the right course for me,’ then I don’t have any objection.”
But considering the use of cross-sex hormones in children and adolescents is “quite a different story,” he contended.
In May 2019, Rosenthal, Safer and colleagues responded to Laidlaw’s letter in the same journal, stating that for the right person, puberty blockers and cross-sex hormones are appropriate, and that medications can improve mental health outcomes.
“We agree that research to validate the safety and efficacy of all forms of treatment is desirable,” they wrote, noting some of that research is underway.
“However, we believe physicians would fall short in their duty of care if they withheld hormonal treatment of gender dysphoria/incongruence in pubertal youth, when indicated, given the existing state of knowledge, imperfect though it is.”
Research to validate safety and efficacy of transgender TX underway
Rosenthal’s center at UCSF is one of four in the United States that has been carrying out a National Institutes of Health-funded long-term observational study of the impact of early medical intervention on transgender adolescents.
It will take time to get those results, but in the meantime, clinicians should act on what is known now, said Rosenthal.
“We already have very compelling data to suggest that the benefits [of treatment] outweigh the potential harms,” he said.
Rosenthal told Medscape Medical News that Laidlaw has advanced the notion that clinicians who prescribe puberty blockers are forcing those individuals into a transgender outcome.
“We don’t push anybody down any path,” he said. “The guidelines make these treatments available in a very specific subset of people who are evaluated by skilled mental health professionals,” said Rosenthal.
Both he and Safer acknowledge that puberty blockers do have the potential for some harm. For instance, a frank discussion needs to happen about the likely lack of future fertility, said Rosenthal.
“Everything we do in medicine has a theoretical risk of harm,” noted Safer.
However, he said, to deny a puberty blocker to an individual approaching puberty who is distraught about growing breasts — but then to possibly have to surgically remove them later — is in itself doing harm.
“Puberty blockers are exactly the epitome of ‘do no harm’ in this case,” argued Safer.
The medications are reversible, he said, adding that they also give an individual and the family time to think through whether the adolescent is transgender, and, if yes, what they want to do in terms of taking cross-sex hormones in the future or getting other interventions.
Safer acknowledged that this doesn’t mean there aren’t still some concerns, however.
For instance, once puberty blockers — which have the potential to interfere with bone development — are started, “How much harm are you willing to risk? Maybe a couple of years is okay, but maybe 6 years is not,” he said.
“So, we do discuss how quickly...you have to get to your next decision point, whether it be to actually introduce hormones or not to introduce hormones,” he explained.
State proposals will have chilling effect on gender-questioning kids
Clinicians say that even if the proposals do not become law, just the fact of their existence could have a chilling effect on gender-questioning children, their families, and doctors considering whether to provide treatment.
“They’re already in a hard position,” Brady said of her patients.
“They’re coming here to seek something for a life that they’ve already not wanted to live and then we have people who are trying to put a real big block on that – I see that obviously affecting their mental health,” she observed.
“I can’t imagine how their lives would be without this care,” Brady said.
With the bills being out there, “two things can happen – one is, it can be very depressing and limiting, but it can also embolden people,” Rosenthal told Medscape Medical News.
“The people behind these things are the same people that have tried to stop our research at the National Institutes of Health (NIH),” he explained.
“These people are going to do everything they can, whether it’s to go state by state to try and exhaust us, or go to the NIH and try to get them to pull the plug on our research,” said Rosenthal.
Safer believes it’s ill-considered to try to legislate any aspect of medicine.
“The pitfalls of trying to legislate these things are myriad,” he said.
“Across all of medicine, interventions are very customized. Can you imagine a state legislature trying to legislate the optimal approach in medicines that can and cannot be given to people with diabetes? How crazy that would be,” he noted.
Rosenthal has served on an advisory panel for Endo Pharmaceuticals and is a grantee of the NIH. Safer has also served on an advisory panel for Endo Pharmaceuticals. Brady has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Innovations to expect at HM20
Course director Dr. Benji Mathews offers highlights
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
Course director Dr. Benji Mathews offers highlights
Course director Dr. Benji Mathews offers highlights
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
Dulaglutide OK for primary, secondary CV risk reduction in U.S.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
Supreme Court roundup: Latest health care decisions
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
COVID-19: Time to ‘take the risk of scaring people’
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.