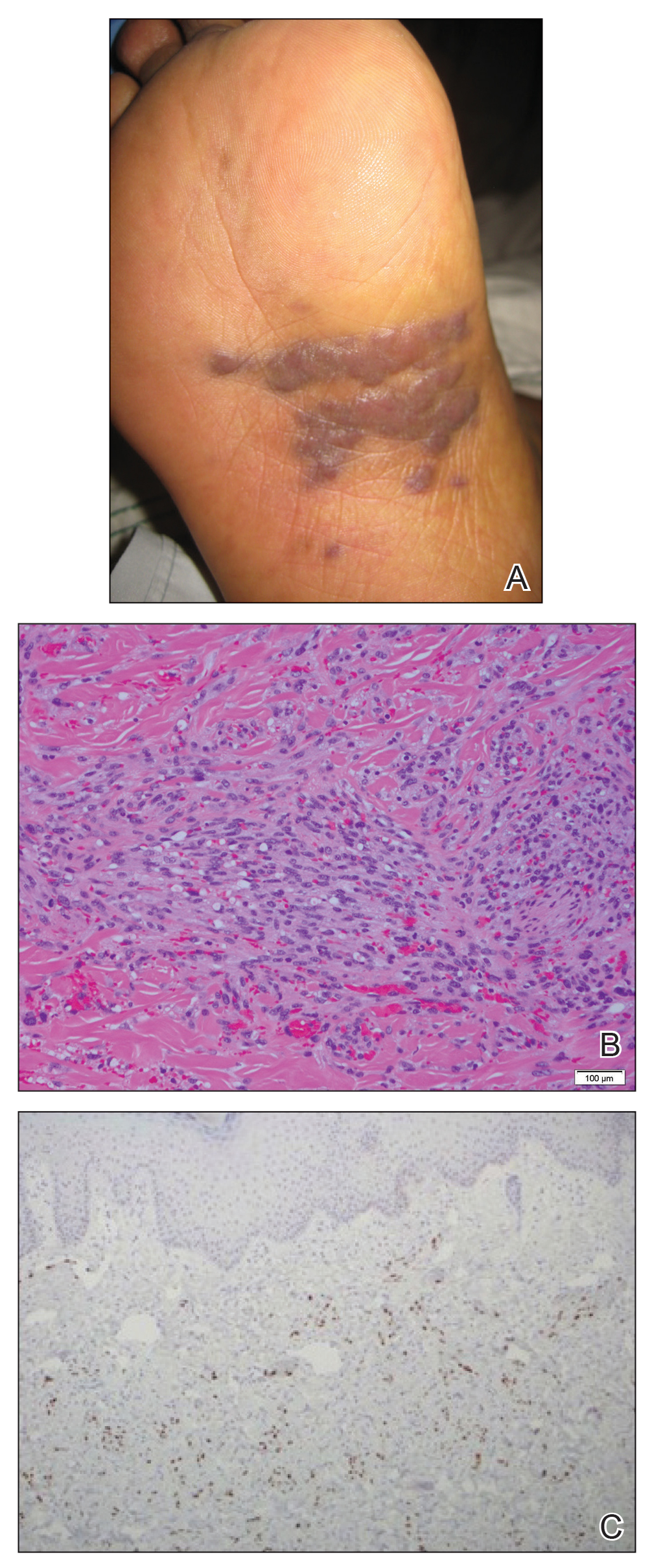
User login
Initial FLAG-Ida outperforms 7+3 for high-risk AML
A treatment commonly used as a salvage regimen for relapsed/refractory acute myelogenous leukemia (AML) showed better results than did standard treatment when used as initial induction therapy.
Researchers found that the use of fludarabine, high-dose cytarabine, with granulocyte-colony stimulating factor (FLAG) – with or without idarubicin (Ida) – resulted in higher remission rates for nonfavorable risk AML patients after one course of induction, compared with standard 7+3 (anthracycline plus cytarabine) treatment.
The use of FLAG+/-Ida also resulted in a shorter time to complete remission (CR) and a shorter time to transplant, compared with 7+3. Additionally, postremission overall survival (OS) and disease-free survival (DFS) were better after achieving CR from FLAG-Ida, compared with 7+3, Melhem Solh, MD, of the Blood & Marrow Transplant Program at Northside Hospital, Atlanta, and colleagues reported in Leukemia Research.
The researchers retrospectively analyzed 304 consecutive AML patients, with nonfavorable National Comprehensive Cancer Network (NCCN) risk who received initial treatment at their center with either 7+3 (86 patients) or FLAG+/-Ida (218 patients). They found that patients in the FLAG+/-Ida group were more likely to achieve remission after one course of induction (74% vs. 62%; P less than .001) and had a faster time to achieve CR (30 days vs. 37.5 days; P less than .001), compared with patients receiving 7+3.
The time from diagnosis to allogeneic hematopoietic cell transplantation was shorter among CR patients after FLAG+/-Ida, compared with 7+3 (115 days vs. 151 days; P less than .003).
Additionally, the 3-year postremission OS and DFS were significantly better for patients receiving FLAG-Ida at 54% and 49%, compared with 39% and 32% for 7+3, respectively (P = .01).
Dr. Solh and colleagues found that the factors associated with postremission survival included age at first CR, NCCN risk, induction regimen (FLAG+/-Ida vs. 7+3; hazard ratio, 0.62; P = .01), and receipt of allogeneic hematopoietic cell transplantation.
“FLAG-Ida is a more efficacious regimen when used for initial induction compared to 3+7. It appears to produce better survival and improved postremission survival for AML patients with intermediate and poor risk AML without increasing toxicity,” Dr. Solh and colleagues wrote. “Further validation of these results in a well-designed randomized prospective trial will help define the best induction approaches for AML.”
There was no outside funding for this research and the authors reported that they had no relevant financial conflicts.
SOURCE: Solh MM et al. Leuk Res. 2020 Feb 14. doi: 10.1016/j.leukres.2020.106318.
A treatment commonly used as a salvage regimen for relapsed/refractory acute myelogenous leukemia (AML) showed better results than did standard treatment when used as initial induction therapy.
Researchers found that the use of fludarabine, high-dose cytarabine, with granulocyte-colony stimulating factor (FLAG) – with or without idarubicin (Ida) – resulted in higher remission rates for nonfavorable risk AML patients after one course of induction, compared with standard 7+3 (anthracycline plus cytarabine) treatment.
The use of FLAG+/-Ida also resulted in a shorter time to complete remission (CR) and a shorter time to transplant, compared with 7+3. Additionally, postremission overall survival (OS) and disease-free survival (DFS) were better after achieving CR from FLAG-Ida, compared with 7+3, Melhem Solh, MD, of the Blood & Marrow Transplant Program at Northside Hospital, Atlanta, and colleagues reported in Leukemia Research.
The researchers retrospectively analyzed 304 consecutive AML patients, with nonfavorable National Comprehensive Cancer Network (NCCN) risk who received initial treatment at their center with either 7+3 (86 patients) or FLAG+/-Ida (218 patients). They found that patients in the FLAG+/-Ida group were more likely to achieve remission after one course of induction (74% vs. 62%; P less than .001) and had a faster time to achieve CR (30 days vs. 37.5 days; P less than .001), compared with patients receiving 7+3.
The time from diagnosis to allogeneic hematopoietic cell transplantation was shorter among CR patients after FLAG+/-Ida, compared with 7+3 (115 days vs. 151 days; P less than .003).
Additionally, the 3-year postremission OS and DFS were significantly better for patients receiving FLAG-Ida at 54% and 49%, compared with 39% and 32% for 7+3, respectively (P = .01).
Dr. Solh and colleagues found that the factors associated with postremission survival included age at first CR, NCCN risk, induction regimen (FLAG+/-Ida vs. 7+3; hazard ratio, 0.62; P = .01), and receipt of allogeneic hematopoietic cell transplantation.
“FLAG-Ida is a more efficacious regimen when used for initial induction compared to 3+7. It appears to produce better survival and improved postremission survival for AML patients with intermediate and poor risk AML without increasing toxicity,” Dr. Solh and colleagues wrote. “Further validation of these results in a well-designed randomized prospective trial will help define the best induction approaches for AML.”
There was no outside funding for this research and the authors reported that they had no relevant financial conflicts.
SOURCE: Solh MM et al. Leuk Res. 2020 Feb 14. doi: 10.1016/j.leukres.2020.106318.
A treatment commonly used as a salvage regimen for relapsed/refractory acute myelogenous leukemia (AML) showed better results than did standard treatment when used as initial induction therapy.
Researchers found that the use of fludarabine, high-dose cytarabine, with granulocyte-colony stimulating factor (FLAG) – with or without idarubicin (Ida) – resulted in higher remission rates for nonfavorable risk AML patients after one course of induction, compared with standard 7+3 (anthracycline plus cytarabine) treatment.
The use of FLAG+/-Ida also resulted in a shorter time to complete remission (CR) and a shorter time to transplant, compared with 7+3. Additionally, postremission overall survival (OS) and disease-free survival (DFS) were better after achieving CR from FLAG-Ida, compared with 7+3, Melhem Solh, MD, of the Blood & Marrow Transplant Program at Northside Hospital, Atlanta, and colleagues reported in Leukemia Research.
The researchers retrospectively analyzed 304 consecutive AML patients, with nonfavorable National Comprehensive Cancer Network (NCCN) risk who received initial treatment at their center with either 7+3 (86 patients) or FLAG+/-Ida (218 patients). They found that patients in the FLAG+/-Ida group were more likely to achieve remission after one course of induction (74% vs. 62%; P less than .001) and had a faster time to achieve CR (30 days vs. 37.5 days; P less than .001), compared with patients receiving 7+3.
The time from diagnosis to allogeneic hematopoietic cell transplantation was shorter among CR patients after FLAG+/-Ida, compared with 7+3 (115 days vs. 151 days; P less than .003).
Additionally, the 3-year postremission OS and DFS were significantly better for patients receiving FLAG-Ida at 54% and 49%, compared with 39% and 32% for 7+3, respectively (P = .01).
Dr. Solh and colleagues found that the factors associated with postremission survival included age at first CR, NCCN risk, induction regimen (FLAG+/-Ida vs. 7+3; hazard ratio, 0.62; P = .01), and receipt of allogeneic hematopoietic cell transplantation.
“FLAG-Ida is a more efficacious regimen when used for initial induction compared to 3+7. It appears to produce better survival and improved postremission survival for AML patients with intermediate and poor risk AML without increasing toxicity,” Dr. Solh and colleagues wrote. “Further validation of these results in a well-designed randomized prospective trial will help define the best induction approaches for AML.”
There was no outside funding for this research and the authors reported that they had no relevant financial conflicts.
SOURCE: Solh MM et al. Leuk Res. 2020 Feb 14. doi: 10.1016/j.leukres.2020.106318.
FROM LEUKEMIA RESEARCH
New leaders at SKCC, Mount Sinai, NHF
The Sidney Kimmel Cancer Center–Jefferson Health (SKCC) has a new director of bone marrow transplant and cell-based therapy, Mount Sinai has a new head of clinical cancer research, and the National Hemophilia Foundation (NHF) has a new president and CEO.
Usama Gergis, MD, has joined SKCC in Philadelphia as director of the bone marrow transplant and immune cellular therapy program and as a professor in the department of medical oncology, division of hematological malignancies.
Dr. Gergis came to SKCC from Weill Cornell Medicine in New York, where he helped expand the use of umbilical cord blood transplant and established an immune cellular therapy program. He also established and led one of the largest oncology international medicine programs in the United States.
Dr. Gergis earned his medical degree from Cairo (Egypt) University. He completed an internal medicine residency and a hematology/oncology fellowship at the Brooklyn Hospital of Weill Cornell. He also completed a bone marrow transplant fellowship at the Moffitt Cancer Center in Tampa.
Karyn Aalami Goodman, MD, has been hired to lead clinical cancer research at Mount Sinai, New York. She is now the associate director for clinical research at the Tisch Cancer Institute and a professor and vice-chair for research and quality in the department of radiation oncology. In these roles, she will develop the infrastructure and resources to support clinical trials of cancer patients.
Dr. Goodman’s own research is focused on improving outcomes for patients with gastrointestinal malignancies. She has helped develop protocols combining radiation, chemotherapy, targeted agents, and immunotherapy.
Dr. Goodman earned her medical degree from Stanford (Calif.) University. She completed an internship in internal medicine at Stanford and residency training in radiation oncology at Memorial Sloan-Kettering Cancer Center in New York. She was previously associate director of clinical research at the University of Colorado Cancer Center in Aurora.
Leonard A. Valentino, MD, has assumed the role of president and CEO of the National Hemophilia Foundation in New York. In this role, Dr. Valentino will “work to advance the foundation’s mission of education, advocacy, and research,” according to the NHF.
Dr. Valentino previously held leadership roles at Spark Therapeutics, Shire, Baxalta, and Baxter Healthcare Corporation. Prior to that, he founded and led the Hemophilia and Thrombophilia Center at Rush University Medical Center in Chicago.
Dr. Valentino earned his undergraduate and medical degrees from Creighton University in Omaha, Neb. He completed his residency at the University of Illinois at Chicago and a fellowship in pediatric hematology-oncology at the University of California, Los Angeles .
The Sidney Kimmel Cancer Center–Jefferson Health (SKCC) has a new director of bone marrow transplant and cell-based therapy, Mount Sinai has a new head of clinical cancer research, and the National Hemophilia Foundation (NHF) has a new president and CEO.
Usama Gergis, MD, has joined SKCC in Philadelphia as director of the bone marrow transplant and immune cellular therapy program and as a professor in the department of medical oncology, division of hematological malignancies.
Dr. Gergis came to SKCC from Weill Cornell Medicine in New York, where he helped expand the use of umbilical cord blood transplant and established an immune cellular therapy program. He also established and led one of the largest oncology international medicine programs in the United States.
Dr. Gergis earned his medical degree from Cairo (Egypt) University. He completed an internal medicine residency and a hematology/oncology fellowship at the Brooklyn Hospital of Weill Cornell. He also completed a bone marrow transplant fellowship at the Moffitt Cancer Center in Tampa.
Karyn Aalami Goodman, MD, has been hired to lead clinical cancer research at Mount Sinai, New York. She is now the associate director for clinical research at the Tisch Cancer Institute and a professor and vice-chair for research and quality in the department of radiation oncology. In these roles, she will develop the infrastructure and resources to support clinical trials of cancer patients.
Dr. Goodman’s own research is focused on improving outcomes for patients with gastrointestinal malignancies. She has helped develop protocols combining radiation, chemotherapy, targeted agents, and immunotherapy.
Dr. Goodman earned her medical degree from Stanford (Calif.) University. She completed an internship in internal medicine at Stanford and residency training in radiation oncology at Memorial Sloan-Kettering Cancer Center in New York. She was previously associate director of clinical research at the University of Colorado Cancer Center in Aurora.
Leonard A. Valentino, MD, has assumed the role of president and CEO of the National Hemophilia Foundation in New York. In this role, Dr. Valentino will “work to advance the foundation’s mission of education, advocacy, and research,” according to the NHF.
Dr. Valentino previously held leadership roles at Spark Therapeutics, Shire, Baxalta, and Baxter Healthcare Corporation. Prior to that, he founded and led the Hemophilia and Thrombophilia Center at Rush University Medical Center in Chicago.
Dr. Valentino earned his undergraduate and medical degrees from Creighton University in Omaha, Neb. He completed his residency at the University of Illinois at Chicago and a fellowship in pediatric hematology-oncology at the University of California, Los Angeles .
The Sidney Kimmel Cancer Center–Jefferson Health (SKCC) has a new director of bone marrow transplant and cell-based therapy, Mount Sinai has a new head of clinical cancer research, and the National Hemophilia Foundation (NHF) has a new president and CEO.
Usama Gergis, MD, has joined SKCC in Philadelphia as director of the bone marrow transplant and immune cellular therapy program and as a professor in the department of medical oncology, division of hematological malignancies.
Dr. Gergis came to SKCC from Weill Cornell Medicine in New York, where he helped expand the use of umbilical cord blood transplant and established an immune cellular therapy program. He also established and led one of the largest oncology international medicine programs in the United States.
Dr. Gergis earned his medical degree from Cairo (Egypt) University. He completed an internal medicine residency and a hematology/oncology fellowship at the Brooklyn Hospital of Weill Cornell. He also completed a bone marrow transplant fellowship at the Moffitt Cancer Center in Tampa.
Karyn Aalami Goodman, MD, has been hired to lead clinical cancer research at Mount Sinai, New York. She is now the associate director for clinical research at the Tisch Cancer Institute and a professor and vice-chair for research and quality in the department of radiation oncology. In these roles, she will develop the infrastructure and resources to support clinical trials of cancer patients.
Dr. Goodman’s own research is focused on improving outcomes for patients with gastrointestinal malignancies. She has helped develop protocols combining radiation, chemotherapy, targeted agents, and immunotherapy.
Dr. Goodman earned her medical degree from Stanford (Calif.) University. She completed an internship in internal medicine at Stanford and residency training in radiation oncology at Memorial Sloan-Kettering Cancer Center in New York. She was previously associate director of clinical research at the University of Colorado Cancer Center in Aurora.
Leonard A. Valentino, MD, has assumed the role of president and CEO of the National Hemophilia Foundation in New York. In this role, Dr. Valentino will “work to advance the foundation’s mission of education, advocacy, and research,” according to the NHF.
Dr. Valentino previously held leadership roles at Spark Therapeutics, Shire, Baxalta, and Baxter Healthcare Corporation. Prior to that, he founded and led the Hemophilia and Thrombophilia Center at Rush University Medical Center in Chicago.
Dr. Valentino earned his undergraduate and medical degrees from Creighton University in Omaha, Neb. He completed his residency at the University of Illinois at Chicago and a fellowship in pediatric hematology-oncology at the University of California, Los Angeles .
Key to denervation response for hypertension may be in carotid body
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
REPORTING FROM CRT 2020
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
Vitamin D supplements in pregnancy boost bone health in offspring
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
FROM JAMA PEDIATRICS
FDA approves first IV migraine prevention drug
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
As previously reported by Medscape Medical News, the drug’s approval is based on results from two clinical studies – PROMISE-1 in episodic migraine and PROMISE-2 in chronic migraine.
The recommended dose is 100 mg every 3 months although some patients may benefit from a dose of 300 mg, the company notes. Lundbeck reports that the drug will likely be available in early April.
Roger Cady, MD, vice-president of neurology at Lundbeck, told Medscape Medical News the drug has almost immediate efficacy.
“Because it’s an IV [medication], it has very rapid benefit. In fact, we were able to demonstrate benefit on Day 1. Truly, it is going to impact on the unmet need for patients because of its profile, the way it’s delivered, and its uniqueness,” Cady said.
“Having preventive activity the day following an infusion is really important. We have in our data, if you take that time between the first day and the 28th day, whether they have episodic migraine or chronic migraine, that about 30% of the population had a 75% or more reduction in migraine days through that first month,” he added.
The clinical trial program demonstrated a treatment benefit over placebo that was observed for both doses of Vyepti as early as day 1 post-infusion, and the percentage of patients experiencing a migraine was lower for Vyepti than with placebo for most of the first 7 days, the company reports.
The safety of Vyepti was evaluated in 2076 patients with migraine who received at least one dose of the drug. The most common adverse reactions were nasopharyngitis and hypersensitivity. In PROMISE-1 and PROMISE-2, 1.9% of patients treated with Vyepti discontinued treatment as a result of adverse reactions.
“The PROMISE-2 data showed that many patients can achieve reduction in migraine days of at least 75% and experience a sustained migraine improvement through 6 months, which is clinically meaningful to both physicians and patients,” said Peter Goadsby, MD, professor of neurology at King’s College, London, UK, and the University of California, San Francisco, in a press release. “Vyepti is a valuable addition for the treatment of migraine, which can help reduce the burden of this serious disease.”
This article first appeared on Medscape.com.
FROM MEDSCAPE.COM
Irinotecan shows promise for triple-negative breast cancer
The topoisomerase I (TOP1) inhibitor irinotecan could be effective in treating triple-negative breast cancer (TNBC), according to results from a preclinical study published in Science Translational Medicine.
Florence Coussy, MD, PhD, of Institut Curie, Paris, and colleagues evaluated the antitumor activity of the Food and Drug Administration–approved TOP1 inhibitor irinotecan in 40 patient-derived xenografts (PDXs) of TNBC.
Other treatments, such as noncamptothecin TOP1 inhibitors (indotecan and indimitecan) and irinotecan plus VE-822 (an ataxia telangiectasia and Rad3-related protein [ATR] inhibitor), were also evaluated in the PDX models.
Tumor samples were collected from patients with primary breast cancer at the time of surgery (55%), residual breast cancer after neoadjuvant treatment (40%), or axillary lymph node metastases (5%). The patients had a mean age of 56 years (range, 29-89 years).
The researchers assessed BRCAness using the homologous recombination deficiency–large-scale state transition assay. Additional potential markers of response, including expression of retinoblastoma transcriptional corepressor 1 (RB1) and Schlafen family member 11 (SLFN11), were detected on transcriptomic analysis and validated using immunohistochemistry analyses.
The researchers found that 37.5% (n = 15) of TNBC PDX models achieved a partial or complete response to irinotecan therapy, while 22.5% (n = 9) of models had stable disease. BRCAness, RB1 loss, and high SLFN11 expression were deemed potential markers of response to irinotecan and other clinical TOP1 inhibitors.
The researchers then evaluated 250 breast cancer patients treated with anthracycline-based chemotherapy and found that lower expression of SLFN11 was associated with worse survival.
“We also [found] that, in the absence of SLFN11, response to irinotecan can be increased by adding an ATR inhibitor and that the clinical TOP1 inhibitors are highly efficient in BRCA1-mutant and BRCAness-positive TNBC PDXs,” the researchers reported.
They acknowledged that a key limitation of this study was the absence of tumor samples from patients who had received a TOP1 inhibitor.
“Overall, our findings are in line with the notion that concomitant defects in DNA repair and checkpoints render cancer cells highly vulnerable to TOP1 inhibitors,” the researchers concluded.
The study was funded by the National Cancer Institute, PIC3i NCI-Curie, and Site de Recherche Intégrée sur le Cancer. One author is an inventor of indotecan and indimitecan. Two authors are coinventors of the method used to detect inactivation of the homologous recombination pathway, which is licensed to Myriad Genetics.
SOURCE: Coussy F et al. Sci Transl Med. 2020 Feb 19. doi: 10.1126/scitranslmed.aax2625.
The topoisomerase I (TOP1) inhibitor irinotecan could be effective in treating triple-negative breast cancer (TNBC), according to results from a preclinical study published in Science Translational Medicine.
Florence Coussy, MD, PhD, of Institut Curie, Paris, and colleagues evaluated the antitumor activity of the Food and Drug Administration–approved TOP1 inhibitor irinotecan in 40 patient-derived xenografts (PDXs) of TNBC.
Other treatments, such as noncamptothecin TOP1 inhibitors (indotecan and indimitecan) and irinotecan plus VE-822 (an ataxia telangiectasia and Rad3-related protein [ATR] inhibitor), were also evaluated in the PDX models.
Tumor samples were collected from patients with primary breast cancer at the time of surgery (55%), residual breast cancer after neoadjuvant treatment (40%), or axillary lymph node metastases (5%). The patients had a mean age of 56 years (range, 29-89 years).
The researchers assessed BRCAness using the homologous recombination deficiency–large-scale state transition assay. Additional potential markers of response, including expression of retinoblastoma transcriptional corepressor 1 (RB1) and Schlafen family member 11 (SLFN11), were detected on transcriptomic analysis and validated using immunohistochemistry analyses.
The researchers found that 37.5% (n = 15) of TNBC PDX models achieved a partial or complete response to irinotecan therapy, while 22.5% (n = 9) of models had stable disease. BRCAness, RB1 loss, and high SLFN11 expression were deemed potential markers of response to irinotecan and other clinical TOP1 inhibitors.
The researchers then evaluated 250 breast cancer patients treated with anthracycline-based chemotherapy and found that lower expression of SLFN11 was associated with worse survival.
“We also [found] that, in the absence of SLFN11, response to irinotecan can be increased by adding an ATR inhibitor and that the clinical TOP1 inhibitors are highly efficient in BRCA1-mutant and BRCAness-positive TNBC PDXs,” the researchers reported.
They acknowledged that a key limitation of this study was the absence of tumor samples from patients who had received a TOP1 inhibitor.
“Overall, our findings are in line with the notion that concomitant defects in DNA repair and checkpoints render cancer cells highly vulnerable to TOP1 inhibitors,” the researchers concluded.
The study was funded by the National Cancer Institute, PIC3i NCI-Curie, and Site de Recherche Intégrée sur le Cancer. One author is an inventor of indotecan and indimitecan. Two authors are coinventors of the method used to detect inactivation of the homologous recombination pathway, which is licensed to Myriad Genetics.
SOURCE: Coussy F et al. Sci Transl Med. 2020 Feb 19. doi: 10.1126/scitranslmed.aax2625.
The topoisomerase I (TOP1) inhibitor irinotecan could be effective in treating triple-negative breast cancer (TNBC), according to results from a preclinical study published in Science Translational Medicine.
Florence Coussy, MD, PhD, of Institut Curie, Paris, and colleagues evaluated the antitumor activity of the Food and Drug Administration–approved TOP1 inhibitor irinotecan in 40 patient-derived xenografts (PDXs) of TNBC.
Other treatments, such as noncamptothecin TOP1 inhibitors (indotecan and indimitecan) and irinotecan plus VE-822 (an ataxia telangiectasia and Rad3-related protein [ATR] inhibitor), were also evaluated in the PDX models.
Tumor samples were collected from patients with primary breast cancer at the time of surgery (55%), residual breast cancer after neoadjuvant treatment (40%), or axillary lymph node metastases (5%). The patients had a mean age of 56 years (range, 29-89 years).
The researchers assessed BRCAness using the homologous recombination deficiency–large-scale state transition assay. Additional potential markers of response, including expression of retinoblastoma transcriptional corepressor 1 (RB1) and Schlafen family member 11 (SLFN11), were detected on transcriptomic analysis and validated using immunohistochemistry analyses.
The researchers found that 37.5% (n = 15) of TNBC PDX models achieved a partial or complete response to irinotecan therapy, while 22.5% (n = 9) of models had stable disease. BRCAness, RB1 loss, and high SLFN11 expression were deemed potential markers of response to irinotecan and other clinical TOP1 inhibitors.
The researchers then evaluated 250 breast cancer patients treated with anthracycline-based chemotherapy and found that lower expression of SLFN11 was associated with worse survival.
“We also [found] that, in the absence of SLFN11, response to irinotecan can be increased by adding an ATR inhibitor and that the clinical TOP1 inhibitors are highly efficient in BRCA1-mutant and BRCAness-positive TNBC PDXs,” the researchers reported.
They acknowledged that a key limitation of this study was the absence of tumor samples from patients who had received a TOP1 inhibitor.
“Overall, our findings are in line with the notion that concomitant defects in DNA repair and checkpoints render cancer cells highly vulnerable to TOP1 inhibitors,” the researchers concluded.
The study was funded by the National Cancer Institute, PIC3i NCI-Curie, and Site de Recherche Intégrée sur le Cancer. One author is an inventor of indotecan and indimitecan. Two authors are coinventors of the method used to detect inactivation of the homologous recombination pathway, which is licensed to Myriad Genetics.
SOURCE: Coussy F et al. Sci Transl Med. 2020 Feb 19. doi: 10.1126/scitranslmed.aax2625.
FROM SCIENCE TRANSLATIONAL MEDICINE
Testing phagocytes might better characterize IBD dysbiosis
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Should routine colon cancer screening start at 45, not 50?
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Not So Classic, Classic Kaposi Sarcoma
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.
A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.
Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.
A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.
Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
To the Editor:
Kaposi sarcoma (KS) is a lymphatic endothelial cell neoplasm that frequently presents as multiple vascular cutaneous and mucosal nodules.1 The classic KS variant typically is described as the presentation of KS in otherwise healthy elderly men of Jewish or Mediterranean descent.2 We present 2 cases of classic KS presenting in Mexican women living in Los Angeles County, California, with atypical clinical features.
A 65-year-old woman of Mexican descent presented to the dermatology clinic for evaluation of an asymptomatic growth on the right ventral forearm of 4 months’ duration. Biopsy of the lesion demonstrated a spindle cell proliferation suspicious for KS. Physical examination several months following the initial biopsy revealed a mildly indurated scar on the right ventral forearm with an adjacent faintly erythematous papule (Figure 1A). Repeat biopsy revealed a dermal spindle cell proliferation suggestive of KS (Figures 1B and 1C). S-100 and cytokeratin stains were negative, but a latent nuclear antigen 1 stain for human herpesvirus 8 was positive. Human immunodeficiency virus screening also was negative. Given the isolated findings, the oncology service determined that observation alone was the most appropriate management. Over time, the patient developed several similar scattered erythematous papules, and treatment with imiquimod cream 5% was initiated for 1 month without improvement. She was subsequently given a trial of alitretinoin ointment 0.1% twice daily. The lesions improved, and she continues to be well controlled on this topical therapy alone.
A 62-year-old woman of Mexican descent with end-stage cryptogenic cirrhosis was admitted to the hospital for evaluation of transplant candidacy. Dermatology was consulted to assess a 5×3-cm, asymptomatic, solitary, violaceous plaque on the right plantar foot (Figure 2A) with no palpable lymph nodes. Biopsy revealed a dermal proliferation of slit-like vascular channels infiltrating through the collagen and surrounding preexisting vascular spaces and adnexal structures (Figure 2B). Extravasation of erythrocytes and plasma cells also was appreciated. Latent nuclear antigen 1 staining showed strong nuclear positivity consistent with KS (Figure 2C), and a human immunodeficiency virus test and workup for underlying immunosuppression were negative. The patient had no history of treatment with immunosuppressive medications. Further workup revealed involvement of the lymphatic system. The patient was removed from the transplant list and was not a candidate for chemotherapy due to liver failure.
Kaposi sarcoma is a vascular neoplasm associated with human herpesvirus 8. It typically presents as erythematous to violaceous papules and plaques on the extremities.1 At least 10 morphologic variants of KS have been identified.3 The indolent classic variant of KS most commonly is found in immunocompetent individuals and has been reported to primarily affect elderly men of Jewish or Mediterranean descent.
Epidemiologic analyses of this disease in the South American population are rare. More than 250 cases have been published from South American countries with the largest series published from patients in Argentina, Peru, and Colombia.4 The incidence of classic KS in Peru is 2.54 per 10,000 individuals,5 and the disease has been diagnosed in 1 of 1000 malignant neoplasms at the National Cancer Institute of Colombia.6
A search of the Armed Forces Institute of Pathology Soft Tissue Pathology registry for classic KS patients (1980-2000) showed that 18% of 438 cases of classic KS were diagnosed in South American Hispanics,7 a percentage that nearly approximates the proportion of patients with KS of Mediterranean descent. Interestingly, in an analysis conducted within Los Angeles County, classic KS was most frequently diagnosed in Jewish, Eastern European–born men who were 55 years or older, followed by European-born women. In this study, white, Spanish, and black populations were diagnosed with classic KS with equal frequency.8
Our 2 cases of classic KS from Los Angeles County had several notable features. Both patients were women from Mexico, a demographic not previously associated with classic KS,8 and they did not have risk factors commonly associated with classic KS. We emphasize that classic KS likely is underreported and understudied in the Mexican and South American populations with need for further epidemiological and clinical analyses.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265-272.
- Akasbi Y, Awada A, Arifi S, et al. Non-HIV Kaposi’s sarcoma: a review and therapeutic perspectives. Bull Cancer. 2012;99:92-99.
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:2392-2350.
- Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi’s sarcoma in Peru. J Am Acad Dermatol. 2005;53:435-441.
- García A, Olivella F, Valderrama S, et al. Kaposi’s sarcoma in Colombia. Cancer. 1989;64:2393-2398.
- Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi Sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma. Mod Pathol. 2008;21:572-582.
- Ross RK, Casagrande JT, Dworsky RL, et al. Kaposi’s sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011-1015.
Practice Points
- Classic Kaposi sarcoma is an indolent neoplasm that can be diagnosed in immunocompetent females of Mexican descent.
- Common diagnoses appearing in skin of color may appear morphologically disparate, and a high index of suspicion is needed for correct diagnosis.