User login
Screen all adults for hepatitis C, says USPSTF
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Use AGA patient education to help your patients better understand HCV, including their risk and treatment options, at https://www.gastro.org/
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Use AGA patient education to help your patients better understand HCV, including their risk and treatment options, at https://www.gastro.org/
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Use AGA patient education to help your patients better understand HCV, including their risk and treatment options, at https://www.gastro.org/
FROM JAMA
Upcoming vaccine may offset surge in polio subtypes
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
FROM AN ACIP MEETING
ERAS protocol for cesarean delivery reduces opioid usage
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
REPORTING FROM THE PREGNANCY MEETING
ACIP vaccination update
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
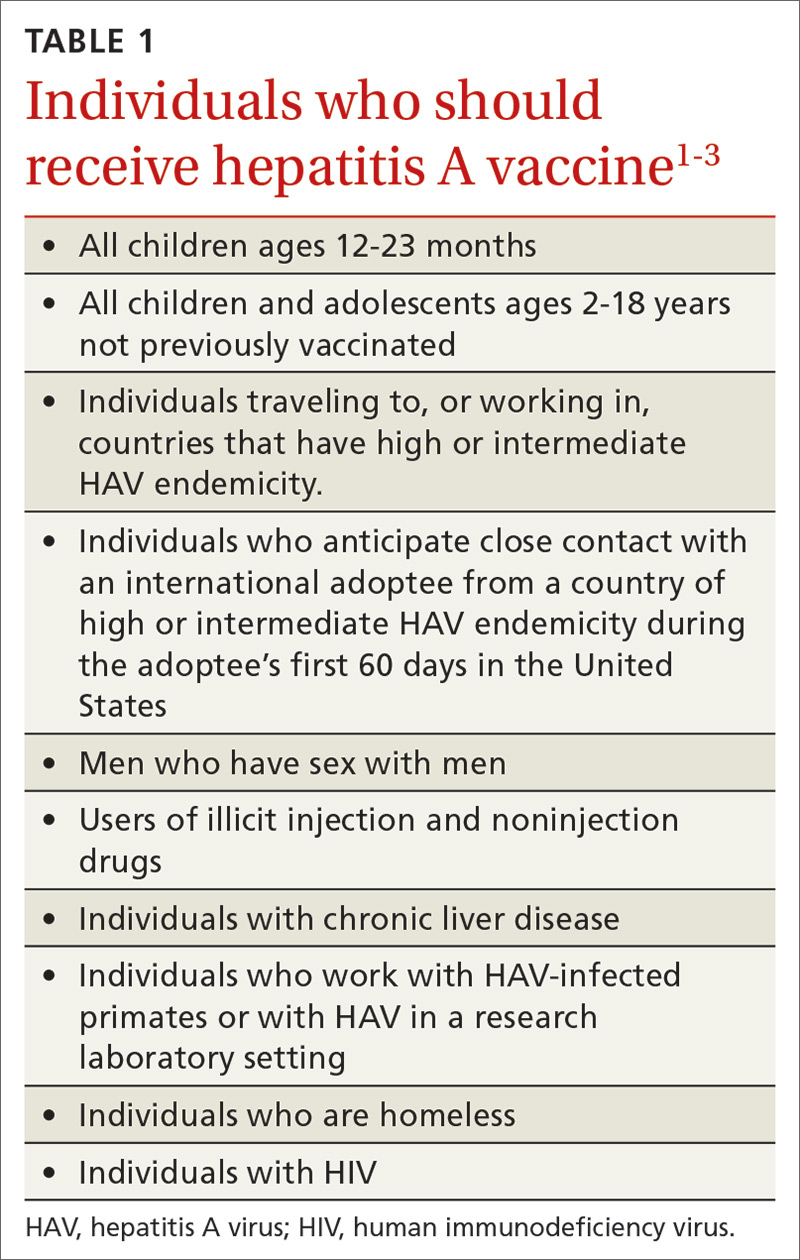
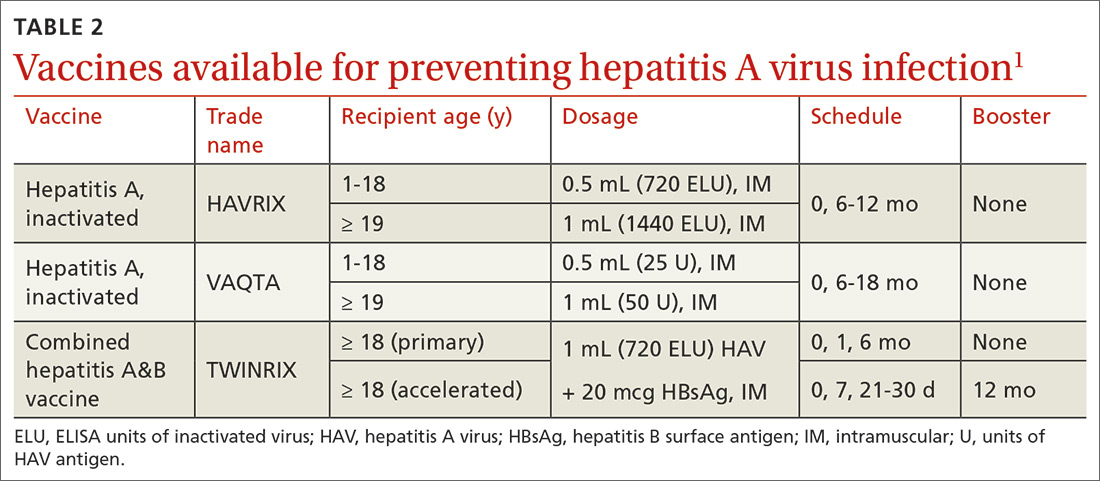
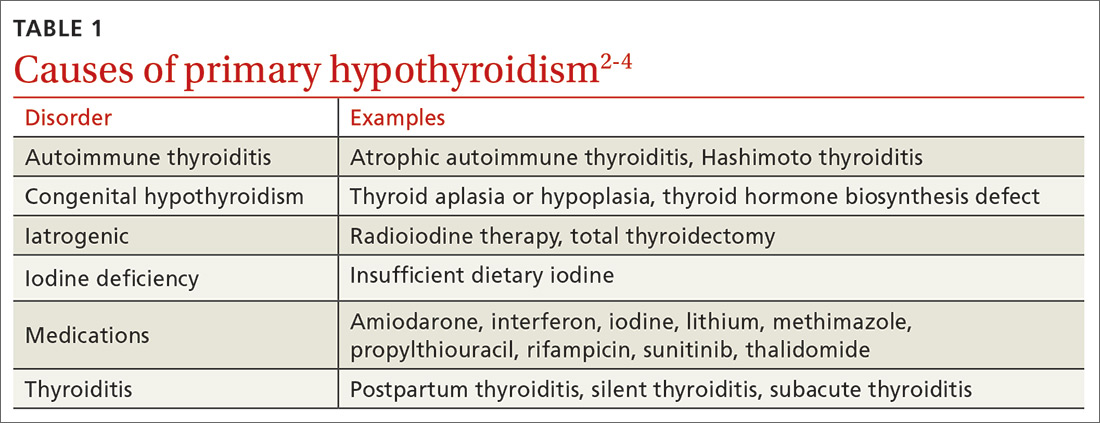
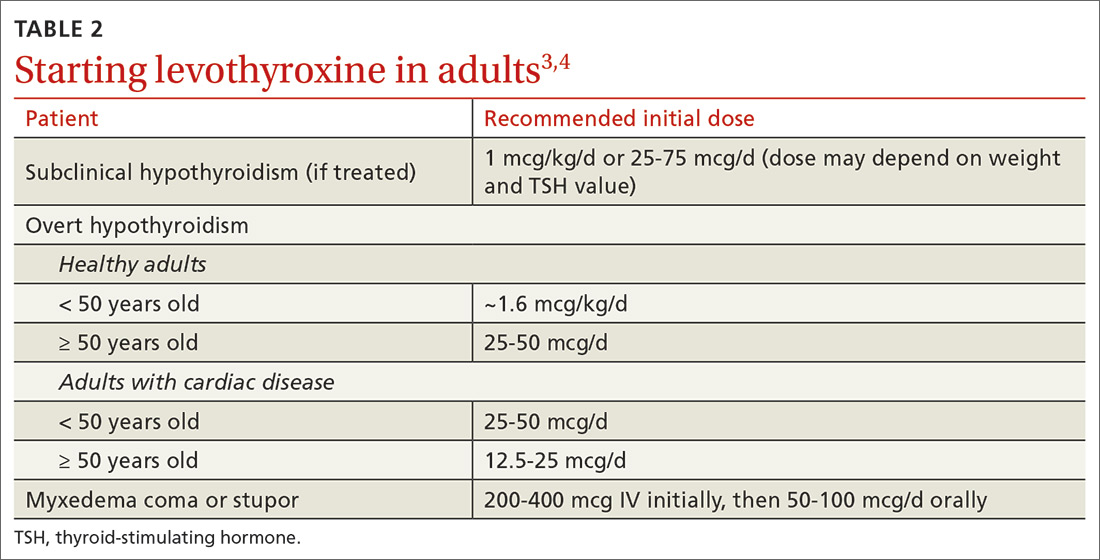
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
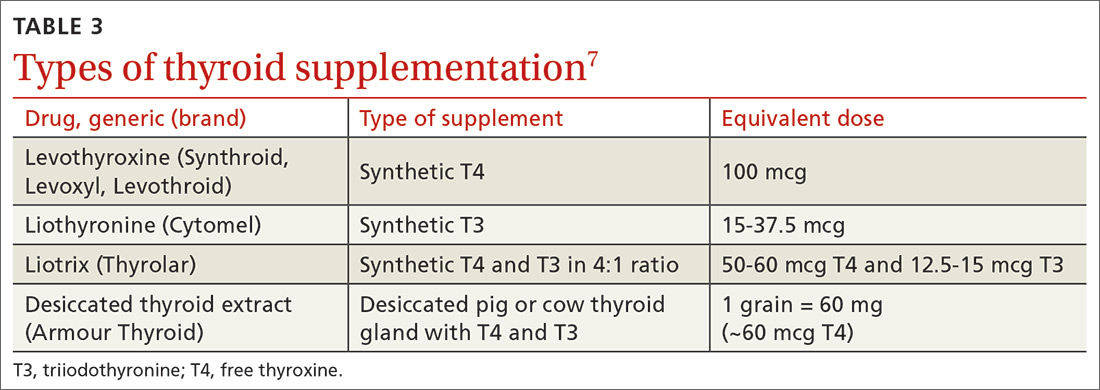
A new DTaP product, and substituting Tdap for Td is approved
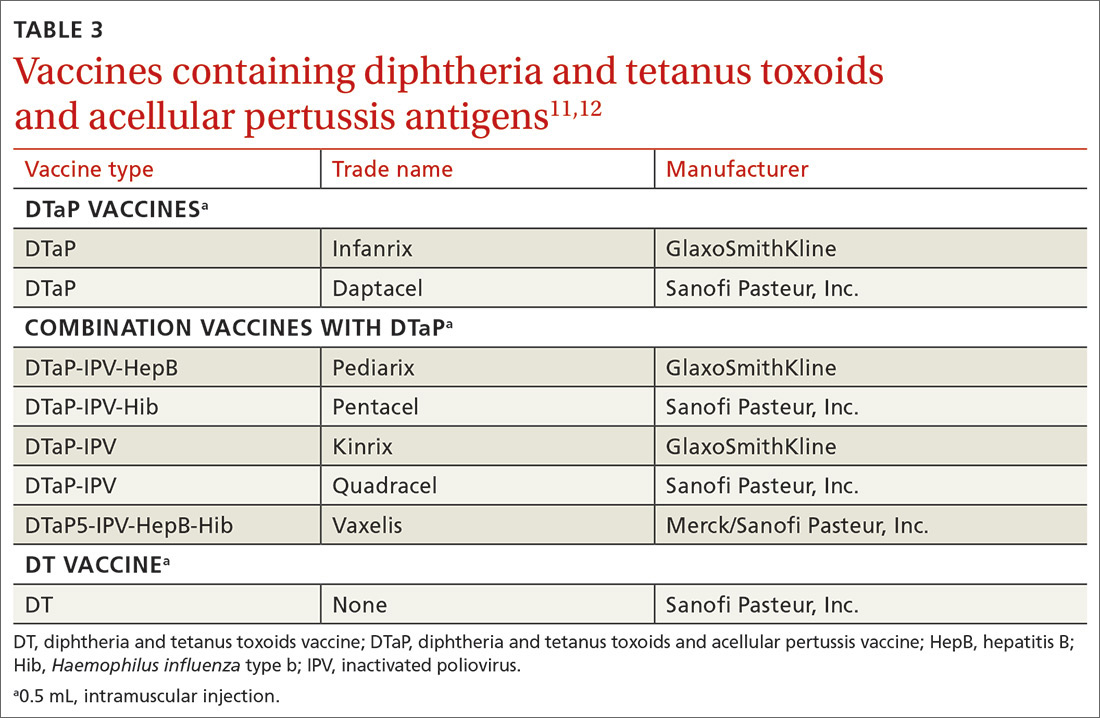
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Dengue vaccine deemed acceptable by most doctors, fewer parents
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
FROM AN ACIP MEETING
Salpingectomy adds little time and no complications to cesarean delivery
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
REPORTING FROM THE PREGNANCY MEETING
Borderline personality disorder common in chronic pain patients
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
REPORTING FROM THE AAPM 2020 ANNUAL MEETING
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
10-year data show no benefit when adding cetuximab to radiation and cisplatin
Adding cetuximab to radiotherapy and cisplatin does not improve outcomes in patients with locoregionally advanced head and neck carcinoma, according to 10-year follow-up from a phase 3 trial.
The addition of cetuximab did not reduce local-regional failure or distant metastasis, and it did not improve progression-free or overall survival.
“With a median follow-up of over 10 years, this updated report confirms the addition of cetuximab to radiation/cisplatin did not improve any measured outcome in the entire cohort or when stratifying by p16 status,” said Jimmy J. Caudell, MD, of Moffitt Cancer Center in Tampa, Fla.
Dr. Caudell presented this update at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Dr. Caudell noted that cisplatin plus radiotherapy or cetuximab plus radiotherapy have been shown to improve overall survival in patients with locoregionally advanced head and neck carcinoma. Researchers conducted this phase 3 trial, RTOG 0522 (NCT00265941), to determine if adding cetuximab to radiotherapy and cisplatin would improve progression-free survival.
The trial included 891 evaluable patients with stage T2 N2a-3 M0 or T3-4 N0-3 M0 disease. They were randomized to receive radiotherapy and cisplatin without cetuximab (n = 447) or with cetuximab (n = 444). Most patients were assigned to intensity-modulated radiotherapy (86.8% in the radiotherapy/cisplatin arm and 89.2% in the cetuximab arm) rather than 3-D conformal radiotherapy (13.2% and 10.8%, respectively).
Baseline characteristics were balanced between the treatment arms. The median age was 57 years (range, 31-79 years) in the radiotherapy/cisplatin arm and 58 years (range, 34-76 years) in the cetuximab arm. Nearly 90% of patients in both arms were men, and the oropharynx was the primary site of disease in about 70% of patients in both arms.
More patients were p16-positive (35.7% in the radiotherapy/cisplatin arm and 39.4% in the cetuximab arm) than were p16-negative (14.4% and 13.1%, respectively). However, p16 status was unknown for about half of patients in each arm.
Long-term efficacy
At a median follow-up of 10.1 years, 452 patients were still alive.
The rate of local-regional failure was 28.5% in the radiotherapy/cisplatin arm and 34.8% in the cetuximab arm (hazard ratio, 1.21; P = .94). The rate of distant metastases was 15% and 11.8%, respectively (HR, 0.79; P = .10).
The 10-year progression-free survival rate was 43.6% in the radiotherapy/cisplatin arm and 40.2% in the cetuximab arm (HR, 1.06; P = .74). The 10-year overall survival rate was 49.9% and 50%, respectively (HR, 0.97; P = .36)
“As might be expected, patients who were p16-positive had a substantially improved progression-free survival as well as overall survival,” Dr. Caudell said. “Patients who had p16-negative oropharyngeal cancer or nonoropharyngeal cancer had equivalent progression-free survival and overall survival.”
However, the addition of cetuximab did not improve progression-free or overall survival in patients with p16-positive, p16-negative oropharyngeal, or nonoropharyngeal cancers.
“[These results] have proven conclusively that the addition of cetuximab to concurrent cisplatin and radiation therapy does not improve outcomes in stage III-IV head and neck cancer, regardless of the primary tumor site and p16 status,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
Late toxicity
Dr. Caudell noted that late toxicity was “substantial” in both treatment arms. Late toxicity was defined as adverse events occurring greater than 90 days from the start of radiotherapy.
The incidence of grade 3/4 late toxicity was 57.4% in the radiotherapy/cisplatin arm and 61.3% in the cetuximab arm (P = .26). The most common grade 3/4 late adverse event was dysphagia, occurring in 39.6% of patients in the radiotherapy/cisplatin arm and 38.2% of those in the cetuximab arm.
Other late grade 3/4 events (in the radiotherapy/cisplatin and cetuximab arms, respectively) included dry mouth (3% and 5%), radiation mucositis (5.3% and 7%), weight decrease (7.6% and 8.7%), hearing impairment (6% and 5%), pharynx mucositis/stomatitis (4.9% and 6%), and osteonecrosis (6% and 4.8%).
Feeding tube use was similar in both treatment arms over time. At 10 years, 14.3% of patients in the radiotherapy/cisplatin arm and 11% of those in the cetuximab arm used a feeding tube (P = .53).
“Despite the use of intensity-modulated radiotherapy, there was a high incidence of late grade 3 and higher toxicities, primarily related to dysphagia, which have substantial effects on the quality of life of our patients,” Dr. Sehgal noted. “These findings need to be considered carefully while designing future studies.
“Future directions for the management of locoregionally advanced head and neck cancer include evaluation of benefits from the addition of immune checkpoint inhibitors to cisplatin with concurrent radiation therapy (e.g., JAVELIN with avelumab [NCT01772004], KEYNOTE-412 with pembrolizumab [NCT03040999], and NCT03349710 with nivolumab) and whether immune checkpoint inhibitors can substitute for cisplatin in those being treated concurrently with radiation therapy (e.g., REACH trial comparing avelumab, cetuximab, and radiation therapy versus cisplatin plus radiation therapy [NCT02999087]).”
The current study was sponsored by the Radiation Therapy Oncology Group, the National Cancer Institute, NRG Oncology, and Eli Lilly. Dr. Caudell disclosed grants and fees from Varian Medical Systems. Dr. Sehgal had no conflicts of interest to disclose.
SOURCE: Caudell J et al. Head and Neck Cancers Symposium 2020, Abstract 6.
Adding cetuximab to radiotherapy and cisplatin does not improve outcomes in patients with locoregionally advanced head and neck carcinoma, according to 10-year follow-up from a phase 3 trial.
The addition of cetuximab did not reduce local-regional failure or distant metastasis, and it did not improve progression-free or overall survival.
“With a median follow-up of over 10 years, this updated report confirms the addition of cetuximab to radiation/cisplatin did not improve any measured outcome in the entire cohort or when stratifying by p16 status,” said Jimmy J. Caudell, MD, of Moffitt Cancer Center in Tampa, Fla.
Dr. Caudell presented this update at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Dr. Caudell noted that cisplatin plus radiotherapy or cetuximab plus radiotherapy have been shown to improve overall survival in patients with locoregionally advanced head and neck carcinoma. Researchers conducted this phase 3 trial, RTOG 0522 (NCT00265941), to determine if adding cetuximab to radiotherapy and cisplatin would improve progression-free survival.
The trial included 891 evaluable patients with stage T2 N2a-3 M0 or T3-4 N0-3 M0 disease. They were randomized to receive radiotherapy and cisplatin without cetuximab (n = 447) or with cetuximab (n = 444). Most patients were assigned to intensity-modulated radiotherapy (86.8% in the radiotherapy/cisplatin arm and 89.2% in the cetuximab arm) rather than 3-D conformal radiotherapy (13.2% and 10.8%, respectively).
Baseline characteristics were balanced between the treatment arms. The median age was 57 years (range, 31-79 years) in the radiotherapy/cisplatin arm and 58 years (range, 34-76 years) in the cetuximab arm. Nearly 90% of patients in both arms were men, and the oropharynx was the primary site of disease in about 70% of patients in both arms.
More patients were p16-positive (35.7% in the radiotherapy/cisplatin arm and 39.4% in the cetuximab arm) than were p16-negative (14.4% and 13.1%, respectively). However, p16 status was unknown for about half of patients in each arm.
Long-term efficacy
At a median follow-up of 10.1 years, 452 patients were still alive.
The rate of local-regional failure was 28.5% in the radiotherapy/cisplatin arm and 34.8% in the cetuximab arm (hazard ratio, 1.21; P = .94). The rate of distant metastases was 15% and 11.8%, respectively (HR, 0.79; P = .10).
The 10-year progression-free survival rate was 43.6% in the radiotherapy/cisplatin arm and 40.2% in the cetuximab arm (HR, 1.06; P = .74). The 10-year overall survival rate was 49.9% and 50%, respectively (HR, 0.97; P = .36)
“As might be expected, patients who were p16-positive had a substantially improved progression-free survival as well as overall survival,” Dr. Caudell said. “Patients who had p16-negative oropharyngeal cancer or nonoropharyngeal cancer had equivalent progression-free survival and overall survival.”
However, the addition of cetuximab did not improve progression-free or overall survival in patients with p16-positive, p16-negative oropharyngeal, or nonoropharyngeal cancers.
“[These results] have proven conclusively that the addition of cetuximab to concurrent cisplatin and radiation therapy does not improve outcomes in stage III-IV head and neck cancer, regardless of the primary tumor site and p16 status,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
Late toxicity
Dr. Caudell noted that late toxicity was “substantial” in both treatment arms. Late toxicity was defined as adverse events occurring greater than 90 days from the start of radiotherapy.
The incidence of grade 3/4 late toxicity was 57.4% in the radiotherapy/cisplatin arm and 61.3% in the cetuximab arm (P = .26). The most common grade 3/4 late adverse event was dysphagia, occurring in 39.6% of patients in the radiotherapy/cisplatin arm and 38.2% of those in the cetuximab arm.
Other late grade 3/4 events (in the radiotherapy/cisplatin and cetuximab arms, respectively) included dry mouth (3% and 5%), radiation mucositis (5.3% and 7%), weight decrease (7.6% and 8.7%), hearing impairment (6% and 5%), pharynx mucositis/stomatitis (4.9% and 6%), and osteonecrosis (6% and 4.8%).
Feeding tube use was similar in both treatment arms over time. At 10 years, 14.3% of patients in the radiotherapy/cisplatin arm and 11% of those in the cetuximab arm used a feeding tube (P = .53).
“Despite the use of intensity-modulated radiotherapy, there was a high incidence of late grade 3 and higher toxicities, primarily related to dysphagia, which have substantial effects on the quality of life of our patients,” Dr. Sehgal noted. “These findings need to be considered carefully while designing future studies.
“Future directions for the management of locoregionally advanced head and neck cancer include evaluation of benefits from the addition of immune checkpoint inhibitors to cisplatin with concurrent radiation therapy (e.g., JAVELIN with avelumab [NCT01772004], KEYNOTE-412 with pembrolizumab [NCT03040999], and NCT03349710 with nivolumab) and whether immune checkpoint inhibitors can substitute for cisplatin in those being treated concurrently with radiation therapy (e.g., REACH trial comparing avelumab, cetuximab, and radiation therapy versus cisplatin plus radiation therapy [NCT02999087]).”
The current study was sponsored by the Radiation Therapy Oncology Group, the National Cancer Institute, NRG Oncology, and Eli Lilly. Dr. Caudell disclosed grants and fees from Varian Medical Systems. Dr. Sehgal had no conflicts of interest to disclose.
SOURCE: Caudell J et al. Head and Neck Cancers Symposium 2020, Abstract 6.
Adding cetuximab to radiotherapy and cisplatin does not improve outcomes in patients with locoregionally advanced head and neck carcinoma, according to 10-year follow-up from a phase 3 trial.
The addition of cetuximab did not reduce local-regional failure or distant metastasis, and it did not improve progression-free or overall survival.
“With a median follow-up of over 10 years, this updated report confirms the addition of cetuximab to radiation/cisplatin did not improve any measured outcome in the entire cohort or when stratifying by p16 status,” said Jimmy J. Caudell, MD, of Moffitt Cancer Center in Tampa, Fla.
Dr. Caudell presented this update at the Multidisciplinary Head and Neck Cancer Symposium, sponsored by the American Society for Radiation Oncology.
Dr. Caudell noted that cisplatin plus radiotherapy or cetuximab plus radiotherapy have been shown to improve overall survival in patients with locoregionally advanced head and neck carcinoma. Researchers conducted this phase 3 trial, RTOG 0522 (NCT00265941), to determine if adding cetuximab to radiotherapy and cisplatin would improve progression-free survival.
The trial included 891 evaluable patients with stage T2 N2a-3 M0 or T3-4 N0-3 M0 disease. They were randomized to receive radiotherapy and cisplatin without cetuximab (n = 447) or with cetuximab (n = 444). Most patients were assigned to intensity-modulated radiotherapy (86.8% in the radiotherapy/cisplatin arm and 89.2% in the cetuximab arm) rather than 3-D conformal radiotherapy (13.2% and 10.8%, respectively).
Baseline characteristics were balanced between the treatment arms. The median age was 57 years (range, 31-79 years) in the radiotherapy/cisplatin arm and 58 years (range, 34-76 years) in the cetuximab arm. Nearly 90% of patients in both arms were men, and the oropharynx was the primary site of disease in about 70% of patients in both arms.
More patients were p16-positive (35.7% in the radiotherapy/cisplatin arm and 39.4% in the cetuximab arm) than were p16-negative (14.4% and 13.1%, respectively). However, p16 status was unknown for about half of patients in each arm.
Long-term efficacy
At a median follow-up of 10.1 years, 452 patients were still alive.
The rate of local-regional failure was 28.5% in the radiotherapy/cisplatin arm and 34.8% in the cetuximab arm (hazard ratio, 1.21; P = .94). The rate of distant metastases was 15% and 11.8%, respectively (HR, 0.79; P = .10).
The 10-year progression-free survival rate was 43.6% in the radiotherapy/cisplatin arm and 40.2% in the cetuximab arm (HR, 1.06; P = .74). The 10-year overall survival rate was 49.9% and 50%, respectively (HR, 0.97; P = .36)
“As might be expected, patients who were p16-positive had a substantially improved progression-free survival as well as overall survival,” Dr. Caudell said. “Patients who had p16-negative oropharyngeal cancer or nonoropharyngeal cancer had equivalent progression-free survival and overall survival.”
However, the addition of cetuximab did not improve progression-free or overall survival in patients with p16-positive, p16-negative oropharyngeal, or nonoropharyngeal cancers.
“[These results] have proven conclusively that the addition of cetuximab to concurrent cisplatin and radiation therapy does not improve outcomes in stage III-IV head and neck cancer, regardless of the primary tumor site and p16 status,” said Kartik Sehgal, MD, of Beth Israel Deaconess Medical Center in Boston, who was not involved in this study.
Late toxicity
Dr. Caudell noted that late toxicity was “substantial” in both treatment arms. Late toxicity was defined as adverse events occurring greater than 90 days from the start of radiotherapy.
The incidence of grade 3/4 late toxicity was 57.4% in the radiotherapy/cisplatin arm and 61.3% in the cetuximab arm (P = .26). The most common grade 3/4 late adverse event was dysphagia, occurring in 39.6% of patients in the radiotherapy/cisplatin arm and 38.2% of those in the cetuximab arm.
Other late grade 3/4 events (in the radiotherapy/cisplatin and cetuximab arms, respectively) included dry mouth (3% and 5%), radiation mucositis (5.3% and 7%), weight decrease (7.6% and 8.7%), hearing impairment (6% and 5%), pharynx mucositis/stomatitis (4.9% and 6%), and osteonecrosis (6% and 4.8%).
Feeding tube use was similar in both treatment arms over time. At 10 years, 14.3% of patients in the radiotherapy/cisplatin arm and 11% of those in the cetuximab arm used a feeding tube (P = .53).
“Despite the use of intensity-modulated radiotherapy, there was a high incidence of late grade 3 and higher toxicities, primarily related to dysphagia, which have substantial effects on the quality of life of our patients,” Dr. Sehgal noted. “These findings need to be considered carefully while designing future studies.
“Future directions for the management of locoregionally advanced head and neck cancer include evaluation of benefits from the addition of immune checkpoint inhibitors to cisplatin with concurrent radiation therapy (e.g., JAVELIN with avelumab [NCT01772004], KEYNOTE-412 with pembrolizumab [NCT03040999], and NCT03349710 with nivolumab) and whether immune checkpoint inhibitors can substitute for cisplatin in those being treated concurrently with radiation therapy (e.g., REACH trial comparing avelumab, cetuximab, and radiation therapy versus cisplatin plus radiation therapy [NCT02999087]).”
The current study was sponsored by the Radiation Therapy Oncology Group, the National Cancer Institute, NRG Oncology, and Eli Lilly. Dr. Caudell disclosed grants and fees from Varian Medical Systems. Dr. Sehgal had no conflicts of interest to disclose.
SOURCE: Caudell J et al. Head and Neck Cancers Symposium 2020, Abstract 6.
REPORTING FROM HEAD AND NECK CANCERS SYMPOSIUM 2020
Review reveals shorter OS, RFS in young colorectal cancer patients
Colorectal cancer patients aged 10-25 years have significantly worse survival rates than those of older patients, findings from a retrospective review suggest.
Overall survival (OS) at 3 years was 42% among patients aged 10-25 years and 90% among patients older than 25 years of age (P less than .0001). Recurrence-free survival (RFS) rates were 32% and 78%, respectively (P less than .0001).
Andrea A. Hayes-Jordan, MD, of the University of North Carolina at Chapel Hill, and colleagues reported these findings in the Journal of the American College of Surgeons.
The researchers reviewed patients treated at MD Anderson Cancer Center in Houston between 1991 and 2017 who were part of a prospectively maintained database. The cohort comprised 94 patients ages 10-25 years and 765 patients who were older than 25 years of age.
RFS and OS data were available for all of the older patients. For the younger group, RFS data were available for 77 patients, and OS data were available for 80 patients.
Five-year OS rates by disease stage in the younger and older groups, respectively, were:
100% and 96% for stage 1.
64% and 90% for stage 2 (P less than .0001).
58% and 85% for stage 3 (P less than .0001).
16% and 55% for stage 4 (P less than .0001).
Five-year RFS rates by disease stage in the younger and older groups, respectively, were:
100% and 95% for stage 1.
55% and 85% for stage 2 (P = .0002).
31% and 73% for stage 3 (P less than .0001).
5% and 27% for stage 4 (P less than .0001).
On multivariate analysis, patients aged 10-25 years had a significantly higher risk of recurrence or death (hazard ratio, 2.312; P less than .0001) and a higher risk of death (HR, 3.592; P less than .0001) compared with older patients.
Of note, peritoneal metastasis occurred significantly more often in the younger group (P = .00001), but predisposing conditions such as polyposis or congenital colon disease did not account for this difference.
“Besides age, mucinous and signet ring histology, poorly differentiated histology, [and] microsatellite instability were statistically significantly more prevalent in this [young] population,” the researchers wrote. “Although other authors have noted similar differences, none have identified peritoneal metastasis to be statistically significantly more prevalent in patients under 25 years.”
The reason for this finding “most certainly lies in the biology in this age group and propensity to develop peritoneal metastasis based on a yet unknown genetic factor,” the researchers added. They reported having no disclosures.
SOURCE: Hayes-Jordan A et al. J Am Coll Surg. 2020 Feb 21. doi: 10.1016/j.jamcollsurg.2019.12.043.
Colorectal cancer patients aged 10-25 years have significantly worse survival rates than those of older patients, findings from a retrospective review suggest.
Overall survival (OS) at 3 years was 42% among patients aged 10-25 years and 90% among patients older than 25 years of age (P less than .0001). Recurrence-free survival (RFS) rates were 32% and 78%, respectively (P less than .0001).
Andrea A. Hayes-Jordan, MD, of the University of North Carolina at Chapel Hill, and colleagues reported these findings in the Journal of the American College of Surgeons.
The researchers reviewed patients treated at MD Anderson Cancer Center in Houston between 1991 and 2017 who were part of a prospectively maintained database. The cohort comprised 94 patients ages 10-25 years and 765 patients who were older than 25 years of age.
RFS and OS data were available for all of the older patients. For the younger group, RFS data were available for 77 patients, and OS data were available for 80 patients.
Five-year OS rates by disease stage in the younger and older groups, respectively, were:
100% and 96% for stage 1.
64% and 90% for stage 2 (P less than .0001).
58% and 85% for stage 3 (P less than .0001).
16% and 55% for stage 4 (P less than .0001).
Five-year RFS rates by disease stage in the younger and older groups, respectively, were:
100% and 95% for stage 1.
55% and 85% for stage 2 (P = .0002).
31% and 73% for stage 3 (P less than .0001).
5% and 27% for stage 4 (P less than .0001).
On multivariate analysis, patients aged 10-25 years had a significantly higher risk of recurrence or death (hazard ratio, 2.312; P less than .0001) and a higher risk of death (HR, 3.592; P less than .0001) compared with older patients.
Of note, peritoneal metastasis occurred significantly more often in the younger group (P = .00001), but predisposing conditions such as polyposis or congenital colon disease did not account for this difference.
“Besides age, mucinous and signet ring histology, poorly differentiated histology, [and] microsatellite instability were statistically significantly more prevalent in this [young] population,” the researchers wrote. “Although other authors have noted similar differences, none have identified peritoneal metastasis to be statistically significantly more prevalent in patients under 25 years.”
The reason for this finding “most certainly lies in the biology in this age group and propensity to develop peritoneal metastasis based on a yet unknown genetic factor,” the researchers added. They reported having no disclosures.
SOURCE: Hayes-Jordan A et al. J Am Coll Surg. 2020 Feb 21. doi: 10.1016/j.jamcollsurg.2019.12.043.
Colorectal cancer patients aged 10-25 years have significantly worse survival rates than those of older patients, findings from a retrospective review suggest.
Overall survival (OS) at 3 years was 42% among patients aged 10-25 years and 90% among patients older than 25 years of age (P less than .0001). Recurrence-free survival (RFS) rates were 32% and 78%, respectively (P less than .0001).
Andrea A. Hayes-Jordan, MD, of the University of North Carolina at Chapel Hill, and colleagues reported these findings in the Journal of the American College of Surgeons.
The researchers reviewed patients treated at MD Anderson Cancer Center in Houston between 1991 and 2017 who were part of a prospectively maintained database. The cohort comprised 94 patients ages 10-25 years and 765 patients who were older than 25 years of age.
RFS and OS data were available for all of the older patients. For the younger group, RFS data were available for 77 patients, and OS data were available for 80 patients.
Five-year OS rates by disease stage in the younger and older groups, respectively, were:
100% and 96% for stage 1.
64% and 90% for stage 2 (P less than .0001).
58% and 85% for stage 3 (P less than .0001).
16% and 55% for stage 4 (P less than .0001).
Five-year RFS rates by disease stage in the younger and older groups, respectively, were:
100% and 95% for stage 1.
55% and 85% for stage 2 (P = .0002).
31% and 73% for stage 3 (P less than .0001).
5% and 27% for stage 4 (P less than .0001).
On multivariate analysis, patients aged 10-25 years had a significantly higher risk of recurrence or death (hazard ratio, 2.312; P less than .0001) and a higher risk of death (HR, 3.592; P less than .0001) compared with older patients.
Of note, peritoneal metastasis occurred significantly more often in the younger group (P = .00001), but predisposing conditions such as polyposis or congenital colon disease did not account for this difference.
“Besides age, mucinous and signet ring histology, poorly differentiated histology, [and] microsatellite instability were statistically significantly more prevalent in this [young] population,” the researchers wrote. “Although other authors have noted similar differences, none have identified peritoneal metastasis to be statistically significantly more prevalent in patients under 25 years.”
The reason for this finding “most certainly lies in the biology in this age group and propensity to develop peritoneal metastasis based on a yet unknown genetic factor,” the researchers added. They reported having no disclosures.
SOURCE: Hayes-Jordan A et al. J Am Coll Surg. 2020 Feb 21. doi: 10.1016/j.jamcollsurg.2019.12.043.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS