User login
New York declares end to 2018 measles outbreak
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.
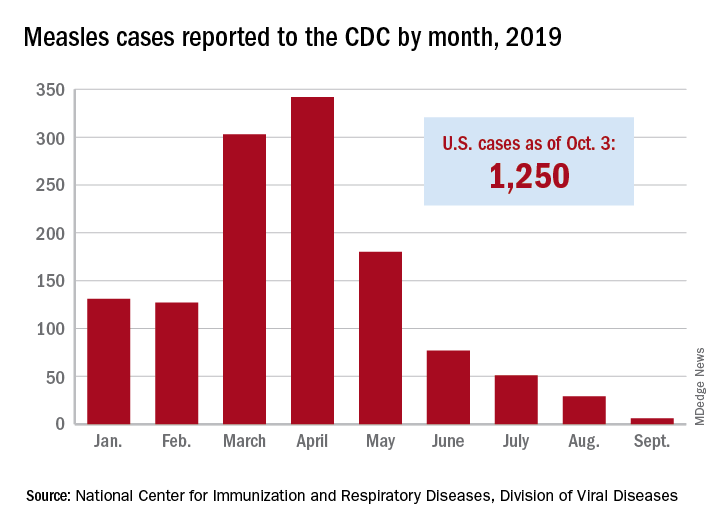
The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.

The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”
New York State has reported the end of all active measles cases related to the initial outbreak in 2018, but the state is now responding to new, unrelated cases in four counties, according to the Centers for Disease Control and Prevention.

The new cases – two in Nassau County and one each in Monroe, Putnam, and Rockland counties – are “related to measles exposures from international travel but not affiliated with the 2018 outbreak,” the New York State Department of Health said in a written statement. Officials in Rockland County had declared its 2018 measles outbreak, which involved 312 cases in 2018 and 2019, over on Sept. 25.
. Of those cases, 1,163 (93%) were associated with 22 outbreaks, with the two largest occurring in New York City and Rockland County. “These two almost year-long outbreaks placed the United States at risk for losing measles elimination status,” the CDC said in a separate report, but “robust responses … ended transmission before the 1-year mark.”
Hypoxia-related discoveries net Nobel Prize
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
C-Path and NORD team up to speed development of treatments for rare disorders
ROCKVILLE, MD – according to information provided at a launch event held Sept. 18, 2019.
By integrating data in a regulatory-grade format suitable for analytics, the RDCA-DAP hopes to accelerate the understanding of disease progression – including source of variability to optimize the characterization of subpopulations – develop clinical outcome measures and biomarkers, facilitate the development of mathematical models of disease, and promote innovative clinical trial designs.
The RDCA-DAP works as a database that will house patient-level data from a variety of sources, including clinical trials, longitudinal observational studies, patient registries, and other sources, such as real-world data collected from electronic health records across a wide range of rare diseases from all over the world. Data will then be made available to researchers to help speed the development of new treatments.
“The database and analytics we are creating will enable us to obtain new insight into these rare diseases,” C-Path President and CEO Joseph Scheeren, PharmD, said at the launch event. “Not only within specific diseases, but also we hope across related diseases.”
The key to the platform’s success will be data. “We need access to clinical data from the industry, patient groups, and academia,” Dr. Scheeren said. “Even more so, the RDCA-DAP will need to incorporate data from many sources. In addition to data from clinical trials conducted by industry and academia, we will want to access data from patients, hospitals, and any organization that can provide data.”
Dr. Scheeren said the data will take many forms, including numeric data, images, genomic information, and other forms of clinical information.
“The database will be able to handle these diverse datasets,” he said, adding that C-Path is preparing to be able to analyze the data sets “with the most sophisticated tools available.”
Dr. Scheeren made a call for all interested stakeholders with rare disease data to contribute to the platform.
NORD President and CEO Peter Saltonstall echoed that call. “We need data. We are accepting it immediately.”
Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, applauded the new platform. “I think foundations and patient advocacy groups and others that have been trying to help in this space have realized that simply funding basic research, although it is necessary and really important, it is not enough to get those therapies in the hands of doctors and patients,” she said. “You have to enable translation of research for that disease.”
Dr. Woodcock noted that the cures may not even come from that basic research, but rather from “left field” using research into cancer or another disease state, something that will be enabled by the disease-agnostic platform being created by C-Path and NORD. She said that the platform will not only put all the data in one spot, but will help to create a standardized set of disease definitions to help make the data useful across all research.
ROCKVILLE, MD – according to information provided at a launch event held Sept. 18, 2019.
By integrating data in a regulatory-grade format suitable for analytics, the RDCA-DAP hopes to accelerate the understanding of disease progression – including source of variability to optimize the characterization of subpopulations – develop clinical outcome measures and biomarkers, facilitate the development of mathematical models of disease, and promote innovative clinical trial designs.
The RDCA-DAP works as a database that will house patient-level data from a variety of sources, including clinical trials, longitudinal observational studies, patient registries, and other sources, such as real-world data collected from electronic health records across a wide range of rare diseases from all over the world. Data will then be made available to researchers to help speed the development of new treatments.
“The database and analytics we are creating will enable us to obtain new insight into these rare diseases,” C-Path President and CEO Joseph Scheeren, PharmD, said at the launch event. “Not only within specific diseases, but also we hope across related diseases.”
The key to the platform’s success will be data. “We need access to clinical data from the industry, patient groups, and academia,” Dr. Scheeren said. “Even more so, the RDCA-DAP will need to incorporate data from many sources. In addition to data from clinical trials conducted by industry and academia, we will want to access data from patients, hospitals, and any organization that can provide data.”
Dr. Scheeren said the data will take many forms, including numeric data, images, genomic information, and other forms of clinical information.
“The database will be able to handle these diverse datasets,” he said, adding that C-Path is preparing to be able to analyze the data sets “with the most sophisticated tools available.”
Dr. Scheeren made a call for all interested stakeholders with rare disease data to contribute to the platform.
NORD President and CEO Peter Saltonstall echoed that call. “We need data. We are accepting it immediately.”
Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, applauded the new platform. “I think foundations and patient advocacy groups and others that have been trying to help in this space have realized that simply funding basic research, although it is necessary and really important, it is not enough to get those therapies in the hands of doctors and patients,” she said. “You have to enable translation of research for that disease.”
Dr. Woodcock noted that the cures may not even come from that basic research, but rather from “left field” using research into cancer or another disease state, something that will be enabled by the disease-agnostic platform being created by C-Path and NORD. She said that the platform will not only put all the data in one spot, but will help to create a standardized set of disease definitions to help make the data useful across all research.
ROCKVILLE, MD – according to information provided at a launch event held Sept. 18, 2019.
By integrating data in a regulatory-grade format suitable for analytics, the RDCA-DAP hopes to accelerate the understanding of disease progression – including source of variability to optimize the characterization of subpopulations – develop clinical outcome measures and biomarkers, facilitate the development of mathematical models of disease, and promote innovative clinical trial designs.
The RDCA-DAP works as a database that will house patient-level data from a variety of sources, including clinical trials, longitudinal observational studies, patient registries, and other sources, such as real-world data collected from electronic health records across a wide range of rare diseases from all over the world. Data will then be made available to researchers to help speed the development of new treatments.
“The database and analytics we are creating will enable us to obtain new insight into these rare diseases,” C-Path President and CEO Joseph Scheeren, PharmD, said at the launch event. “Not only within specific diseases, but also we hope across related diseases.”
The key to the platform’s success will be data. “We need access to clinical data from the industry, patient groups, and academia,” Dr. Scheeren said. “Even more so, the RDCA-DAP will need to incorporate data from many sources. In addition to data from clinical trials conducted by industry and academia, we will want to access data from patients, hospitals, and any organization that can provide data.”
Dr. Scheeren said the data will take many forms, including numeric data, images, genomic information, and other forms of clinical information.
“The database will be able to handle these diverse datasets,” he said, adding that C-Path is preparing to be able to analyze the data sets “with the most sophisticated tools available.”
Dr. Scheeren made a call for all interested stakeholders with rare disease data to contribute to the platform.
NORD President and CEO Peter Saltonstall echoed that call. “We need data. We are accepting it immediately.”
Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, applauded the new platform. “I think foundations and patient advocacy groups and others that have been trying to help in this space have realized that simply funding basic research, although it is necessary and really important, it is not enough to get those therapies in the hands of doctors and patients,” she said. “You have to enable translation of research for that disease.”
Dr. Woodcock noted that the cures may not even come from that basic research, but rather from “left field” using research into cancer or another disease state, something that will be enabled by the disease-agnostic platform being created by C-Path and NORD. She said that the platform will not only put all the data in one spot, but will help to create a standardized set of disease definitions to help make the data useful across all research.
Federal Health Care Cancer Data Trends 2019
Click here to access Federal Health Care Cancer Data Trends 2019
Table of Contents
- Introduction
- Cancer in the VA: The Big Picture
- Hepatocellular Carcinoma
- Prostate Cancer
- Multiple Myeloma
- Lung Cancer in the VA at a National Level
- Lung Cancer – Regional Snapshot: Kansas City VA
- Colorectal Cancer
- Leukemias
- Lymphomas
- Renal Cell Carcinoma
- Bladder Cancer
- Afterword

Click here to access Federal Health Care Cancer Data Trends 2019
Table of Contents
- Introduction
- Cancer in the VA: The Big Picture
- Hepatocellular Carcinoma
- Prostate Cancer
- Multiple Myeloma
- Lung Cancer in the VA at a National Level
- Lung Cancer – Regional Snapshot: Kansas City VA
- Colorectal Cancer
- Leukemias
- Lymphomas
- Renal Cell Carcinoma
- Bladder Cancer
- Afterword

Click here to access Federal Health Care Cancer Data Trends 2019
Table of Contents
- Introduction
- Cancer in the VA: The Big Picture
- Hepatocellular Carcinoma
- Prostate Cancer
- Multiple Myeloma
- Lung Cancer in the VA at a National Level
- Lung Cancer – Regional Snapshot: Kansas City VA
- Colorectal Cancer
- Leukemias
- Lymphomas
- Renal Cell Carcinoma
- Bladder Cancer
- Afterword

Isolated iliac disease a marker for better health status?
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
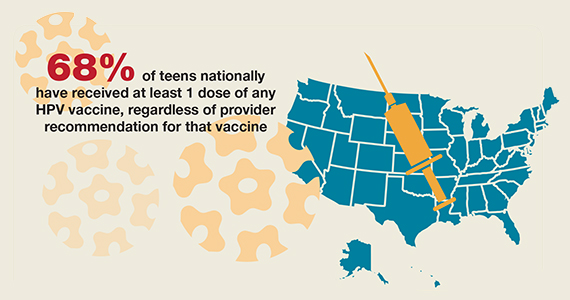
National HPV vaccination rates among teens according to provider recommendation



Romosozumab benefits prevail, despite renal insufficiency
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
ORLANDO – Use of romosozumab (Evenity) by patients with osteoporosis in various stages of renal insufficiency did not appear to affect increases in bone mineral density, the rate of new vertebral fractures, or the number of adverse events when compared with placebo, Paul D. Miller, MD, said at the annual meeting of the American Society for Bone and Mineral Research.
“Romosozumab could be considered a treatment option for osteoporotic patients with mild to moderate reductions in renal function,” said Dr. Miller, distinguished clinical professor of medicine at the University of Colorado at Denver, Aurora.
Since bisphosphonates are not recommended for use in patients with an estimated glomerular filtration rate (eGFR) of less than 30 or 35 mL/min/1.73 m2, other osteoporosis treatments such as romosozumab should also be examined. “It is important to evaluate other osteoporosis treatments in this setting, particularly in the context of monoclonal antibodies, which are not cleared in the kidneys and are metabolized in the reticular endothelial system, and have no FDA ... cut-off for their use,” Dr. Miller said.
Dr. Miller and colleagues performed a post hoc analysis of patients in the FRAME study, which enrolled 3,589 patients who received a monthly dose of subcutaneous romosozumab (215 mg) and 3,591 patients who received placebo in a double-blinded study for 12 months before moving to a 12-month, open-label portion of the study where all patients received 60 mg of subcutaneous denosumab every 6 months. After 12 months, the researchers analyzed the least squares mean (LSM) percentage change in bone mineral density (BMD) at the total hip, lumbar spine, and femoral neck as well as whether patients had any new vertebral fractures or experienced adverse events from treatment.
Patients were postmenopausal women between ages 55 and 90 years with a BMD T-score between –2.5 and –3.5 at the total hip or femoral neck. Researchers divided patients into four eGFR groups based chronic kidney disease (CKD) stage: normal (90 mL/min/1.73 m2 or higher; 848 patients), mild CKD (60-89 mL/min/1.73 m2; 4,939 patients), moderate CKD (30-59 mL/min/1.73 m2; 1,360 patients), and severe (15-29 mL/min/1.73 m2; 18 patients).
The LSM percentage change was 13.1% in the lumbar spine (95% confidence interval, 12.8%-13.3%) for the romosozumab group, compared with 0.4% in the placebo group (95% CI, 0.2%-0.5%). The LSM percentage change for total hip was 6.0% in the romosozumab group (95% CI, 5.9%-6.2%), compared with 0.3% in the placebo group (95% CI, 0.1%-0.4%), while the LSM percentage change for the femoral neck was 5.5% in the romosozumab group (95% CI, 5.2%-5.7%) and 0.3% in the placebo group (0.1%-0.5%).
“[The] risk of new vertebral fractures was decreased in all eGFR subgroups and did not appear to be affected by eGFR level,” he said.
Specifically, vertebral fracture incidence was 0.5% in the romosozumab group, compared with 3.0% in the placebo group, for patients with normal renal function, 0.4% in the romosozumab group, compared with 1.5% in the placebo group, for patients with mild chronic kidney disease, and 0.6% in the romosozumab group vs. 2.1% in the placebo group for patients with moderate chronic kidney disease. The incidence of adverse events, serious adverse events, and positively adjudicated cardiovascular events were similar between patients in the romosozumab group regardless of renal function status. The researchers reported 1 patient in the romosozumab group who experienced grade 2 hypocalcemia, and 14 patients in the romosozumab group who experienced mild to moderate decreases in calcium, compared with 4 patients in the placebo group.
Dr. Miller noted the study was limited by having few patients with an eGFR of less than 30 mL/min/1.73 m2 and no patients with an eGFR of less than 15 mL/min/1.73 m2, but said the study strengths were its large randomized nature and well-balanced baseline characteristics between each group.
This study was sponsored in part by Amgen, Astellas, and UCB Pharma. Dr. Miller reported receiving grants from Alexion, Amgen, Radius, Regeneron, UCB, and Ultragenyx. Amgen and UCB assisted in and provided financial assistance for the preparation of Dr. Miller’s presentation.
SOURCE: Miller P et al. ASBMR 2019. Abstract 1085.
REPORTING FROM ASBMR 2019
Study questions preemptive TEVAR for extended type A dissections
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
i-HOPE study engages patients, families to improve quality of hospital stays
Make patients ‘equal members of the team.’
Hospitalization can be a challenging and vulnerable time for patients and their families. While challenges associated with the quality and safety of hospital care are well documented, perspectives of patients, families, caregivers, and other stakeholders are not as easily understood and are important targets of improvement research.
This led to the initiation of the i-HOPE Patient Engagement Study, a collaboration including the Society for Hospital Medicine’s Center for Quality Improvement. The team completed a systematic and broad engagement process with patients, families, and caregivers, followed by an in-person prioritization meeting to generate a priority list of research topics that describe the most important gaps in the care of hospitalized patients.
The Hospitalist recently spoke with Luci Leykum, MD, MSc, MBA, SFHM, principal investigator for the i-HOPE Study, professor of medicine and investigator in the South Texas Veterans Health Care System and incoming associate chair for clinical innovation at the University of Texas at Austin.
Why is it so important to include the perspective of the patient during a hospital stay?
We cannot optimally improve outcomes of hospitalized patients if we don’t have patients’ perspectives on what needs to be improved. Hearing these perspectives also provides insights into how we can address gaps in hospital care.
How were patients and other stakeholders engaged during the i-HOPE program?
Patients, caregivers, and stakeholders were engaged throughout the entire project, from conceptualization to dissemination of results.
We worked with seven patient partners to develop the proposal that we submitted to the Patient-Centered Outcomes Research Institute. They were involved in all phases of the project, from developing the informational webinars and surveys to analyzing our results.
We engaged additional patients, caregivers, and stakeholders to submit their highest priority unanswered research questions for improving hospital care. A total of 117 patients and 127 caregivers submitted questions. Our patient partners and more than 30 stakeholders were involved in prioritizing those research questions to develop our final agenda.
What is unique about the approach in the i-HOPE project, compared with other projects that may have had similar intended objectives?
Our project is unique in several respects. First, it was completely patient partnered. Having patients as equal members of the team changed our approach at every level – from how we communicated with patients and stakeholders to how we analyzed and presented our data. Second, we worked with a larger number of stakeholders representing a broad range of constituencies, from professional societies to health care delivery systems to payers.
How has SHM’s Center for Quality Improvement helped the i-HOPE program to realize its goals?
The Center for Quality Improvement helped considerably with the execution of the project. The researchers involved in i-HOPE were all members of the SHM Research Committee and were familiar with SHM’s capability as a partner in these larger-scale projects. The SHM Meetings team was instrumental in making our in-person patient and stakeholder prioritization meeting happen as well.
How can the findings of the i-HOPE program be applied?
We hope everyone can utilize our findings. Patients, families, and caregivers can use our results to improve their own care. Providers and delivery systems can target their improvement efforts using our findings to ensure that their work has the greatest impact on patients. Policy makers and funders can use our findings to direct work to the priority areas we identified. And finally, we hope the hospital research community uses our results to develop novel interventions to improve care.
For more information on the i-HOPE Patient Engagement Study, visit hospitalmedicine.org/ihope.
Make patients ‘equal members of the team.’
Make patients ‘equal members of the team.’
Hospitalization can be a challenging and vulnerable time for patients and their families. While challenges associated with the quality and safety of hospital care are well documented, perspectives of patients, families, caregivers, and other stakeholders are not as easily understood and are important targets of improvement research.
This led to the initiation of the i-HOPE Patient Engagement Study, a collaboration including the Society for Hospital Medicine’s Center for Quality Improvement. The team completed a systematic and broad engagement process with patients, families, and caregivers, followed by an in-person prioritization meeting to generate a priority list of research topics that describe the most important gaps in the care of hospitalized patients.
The Hospitalist recently spoke with Luci Leykum, MD, MSc, MBA, SFHM, principal investigator for the i-HOPE Study, professor of medicine and investigator in the South Texas Veterans Health Care System and incoming associate chair for clinical innovation at the University of Texas at Austin.
Why is it so important to include the perspective of the patient during a hospital stay?
We cannot optimally improve outcomes of hospitalized patients if we don’t have patients’ perspectives on what needs to be improved. Hearing these perspectives also provides insights into how we can address gaps in hospital care.
How were patients and other stakeholders engaged during the i-HOPE program?
Patients, caregivers, and stakeholders were engaged throughout the entire project, from conceptualization to dissemination of results.
We worked with seven patient partners to develop the proposal that we submitted to the Patient-Centered Outcomes Research Institute. They were involved in all phases of the project, from developing the informational webinars and surveys to analyzing our results.
We engaged additional patients, caregivers, and stakeholders to submit their highest priority unanswered research questions for improving hospital care. A total of 117 patients and 127 caregivers submitted questions. Our patient partners and more than 30 stakeholders were involved in prioritizing those research questions to develop our final agenda.
What is unique about the approach in the i-HOPE project, compared with other projects that may have had similar intended objectives?
Our project is unique in several respects. First, it was completely patient partnered. Having patients as equal members of the team changed our approach at every level – from how we communicated with patients and stakeholders to how we analyzed and presented our data. Second, we worked with a larger number of stakeholders representing a broad range of constituencies, from professional societies to health care delivery systems to payers.
How has SHM’s Center for Quality Improvement helped the i-HOPE program to realize its goals?
The Center for Quality Improvement helped considerably with the execution of the project. The researchers involved in i-HOPE were all members of the SHM Research Committee and were familiar with SHM’s capability as a partner in these larger-scale projects. The SHM Meetings team was instrumental in making our in-person patient and stakeholder prioritization meeting happen as well.
How can the findings of the i-HOPE program be applied?
We hope everyone can utilize our findings. Patients, families, and caregivers can use our results to improve their own care. Providers and delivery systems can target their improvement efforts using our findings to ensure that their work has the greatest impact on patients. Policy makers and funders can use our findings to direct work to the priority areas we identified. And finally, we hope the hospital research community uses our results to develop novel interventions to improve care.
For more information on the i-HOPE Patient Engagement Study, visit hospitalmedicine.org/ihope.
Hospitalization can be a challenging and vulnerable time for patients and their families. While challenges associated with the quality and safety of hospital care are well documented, perspectives of patients, families, caregivers, and other stakeholders are not as easily understood and are important targets of improvement research.
This led to the initiation of the i-HOPE Patient Engagement Study, a collaboration including the Society for Hospital Medicine’s Center for Quality Improvement. The team completed a systematic and broad engagement process with patients, families, and caregivers, followed by an in-person prioritization meeting to generate a priority list of research topics that describe the most important gaps in the care of hospitalized patients.
The Hospitalist recently spoke with Luci Leykum, MD, MSc, MBA, SFHM, principal investigator for the i-HOPE Study, professor of medicine and investigator in the South Texas Veterans Health Care System and incoming associate chair for clinical innovation at the University of Texas at Austin.
Why is it so important to include the perspective of the patient during a hospital stay?
We cannot optimally improve outcomes of hospitalized patients if we don’t have patients’ perspectives on what needs to be improved. Hearing these perspectives also provides insights into how we can address gaps in hospital care.
How were patients and other stakeholders engaged during the i-HOPE program?
Patients, caregivers, and stakeholders were engaged throughout the entire project, from conceptualization to dissemination of results.
We worked with seven patient partners to develop the proposal that we submitted to the Patient-Centered Outcomes Research Institute. They were involved in all phases of the project, from developing the informational webinars and surveys to analyzing our results.
We engaged additional patients, caregivers, and stakeholders to submit their highest priority unanswered research questions for improving hospital care. A total of 117 patients and 127 caregivers submitted questions. Our patient partners and more than 30 stakeholders were involved in prioritizing those research questions to develop our final agenda.
What is unique about the approach in the i-HOPE project, compared with other projects that may have had similar intended objectives?
Our project is unique in several respects. First, it was completely patient partnered. Having patients as equal members of the team changed our approach at every level – from how we communicated with patients and stakeholders to how we analyzed and presented our data. Second, we worked with a larger number of stakeholders representing a broad range of constituencies, from professional societies to health care delivery systems to payers.
How has SHM’s Center for Quality Improvement helped the i-HOPE program to realize its goals?
The Center for Quality Improvement helped considerably with the execution of the project. The researchers involved in i-HOPE were all members of the SHM Research Committee and were familiar with SHM’s capability as a partner in these larger-scale projects. The SHM Meetings team was instrumental in making our in-person patient and stakeholder prioritization meeting happen as well.
How can the findings of the i-HOPE program be applied?
We hope everyone can utilize our findings. Patients, families, and caregivers can use our results to improve their own care. Providers and delivery systems can target their improvement efforts using our findings to ensure that their work has the greatest impact on patients. Policy makers and funders can use our findings to direct work to the priority areas we identified. And finally, we hope the hospital research community uses our results to develop novel interventions to improve care.
For more information on the i-HOPE Patient Engagement Study, visit hospitalmedicine.org/ihope.
Eltrombopag elicits positive responses in secondary ITP
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
FROM BLOOD