User login
FDA’s low-risk TAVR okay set to propel case volume
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
FDA approves Taltz for treatment of ankylosing spondylitis
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
(AS), according to a press release from Eli Lilly.
AS is the third indication for ixekizumab, along with moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and active psoriatic arthritis in adults.
Approval of the humanized interleukin-17A antagonist was based on results from a pair of randomized, double-blind, placebo-controlled, phase 3 studies involving 657 adult patients with active AS: the COAST-V trial in those naive to biologic disease-modifying antirheumatic drugs (bDMARDs) and the COAST-W trial in those who were intolerant or had inadequate response to tumor necrosis factor (TNF) inhibitors. The primary endpoint in both trials was achievement of 40% improvement in Assessment of Spondyloarthritis International Society criteria (ASAS40) at 16 weeks, compared with placebo.
In COAST-V, 48% of patients who received ixekizumab achieved ASAS40, compared with 18% of controls (P less than .0001). In COAST-W, 25% of patients who received ixekizumab achieved ASAS40 versus 13% of controls (P less than .05). The adverse events reported during both trials were consistent with the safety profile in patients who receive ixekizumab for the treatment of plaque psoriasis, including injection-site reactions, upper respiratory tract infections, nausea, and tinea infections.
“Results from the phase 3 clinical trial program in ankylosing spondylitis show that Taltz helped reduce pain and inflammation and improve function in patients who had never been treated with a bDMARD as well as those who previously failed TNF inhibitors. This approval is an important milestone for patients and physicians who are looking for a much-needed alternative to address symptoms of AS,” said Philip Mease, MD, of Providence St. Joseph Health and the University of Washington, both in Seattle.
Malignant Pleural Effusion: Therapeutic Options and Strategies
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
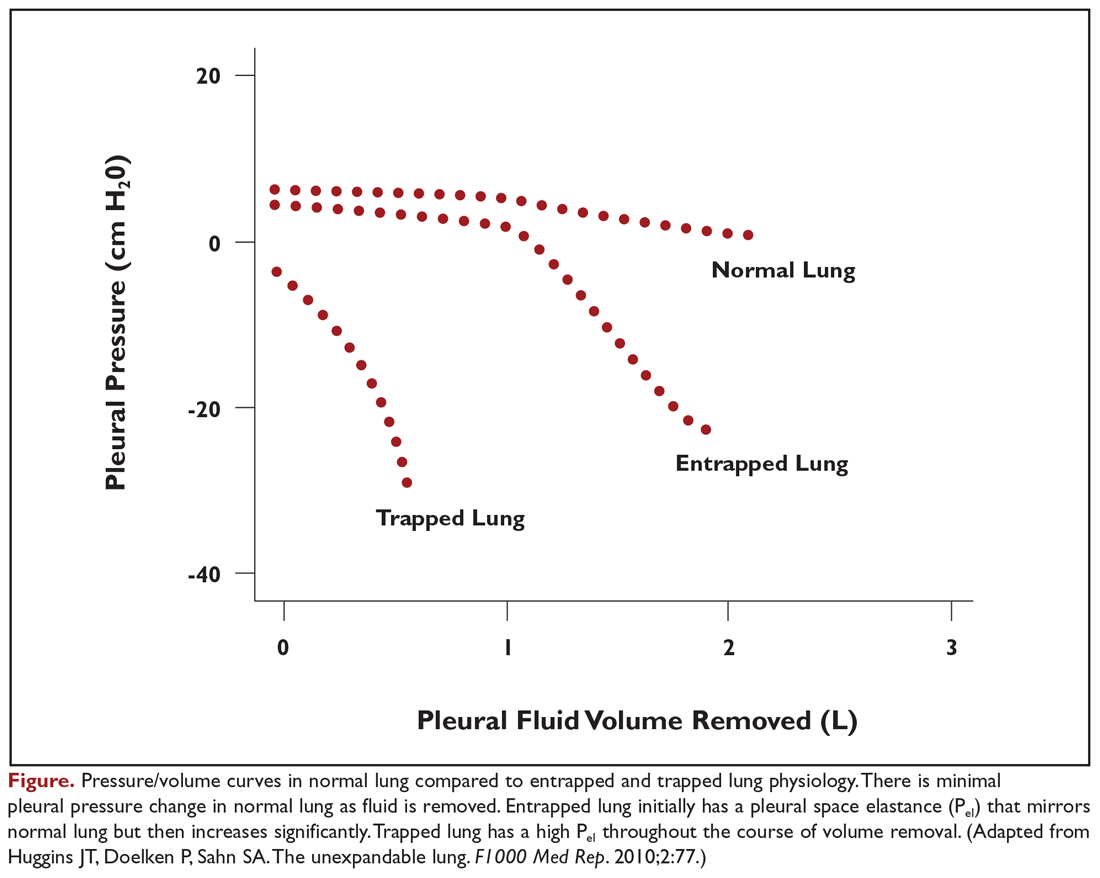
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
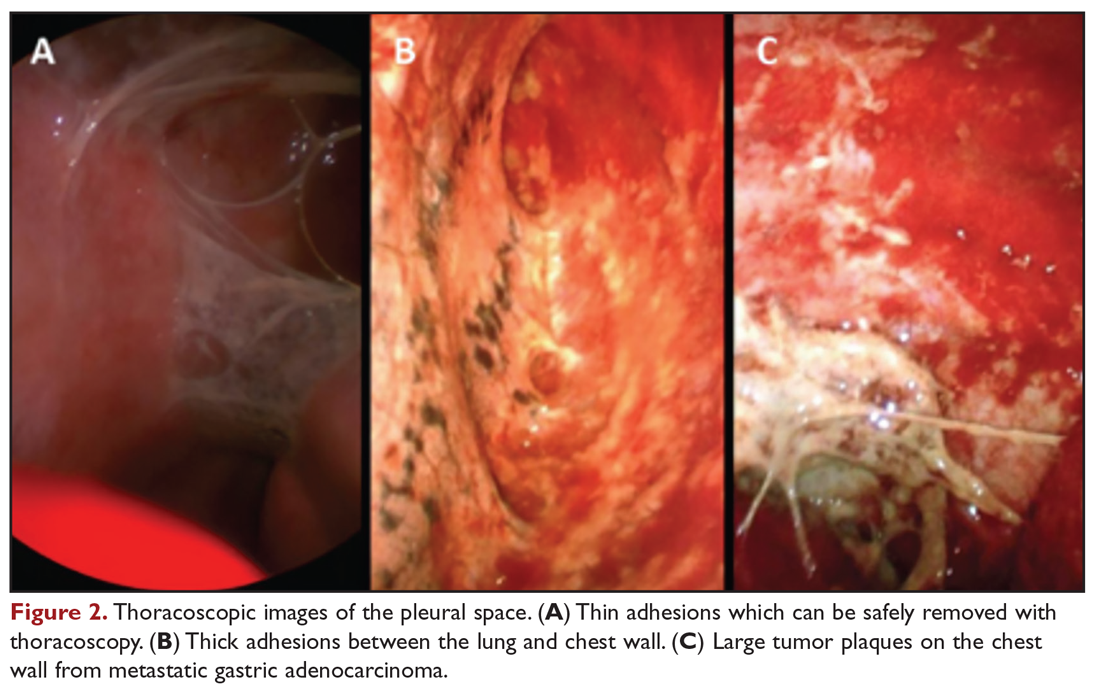
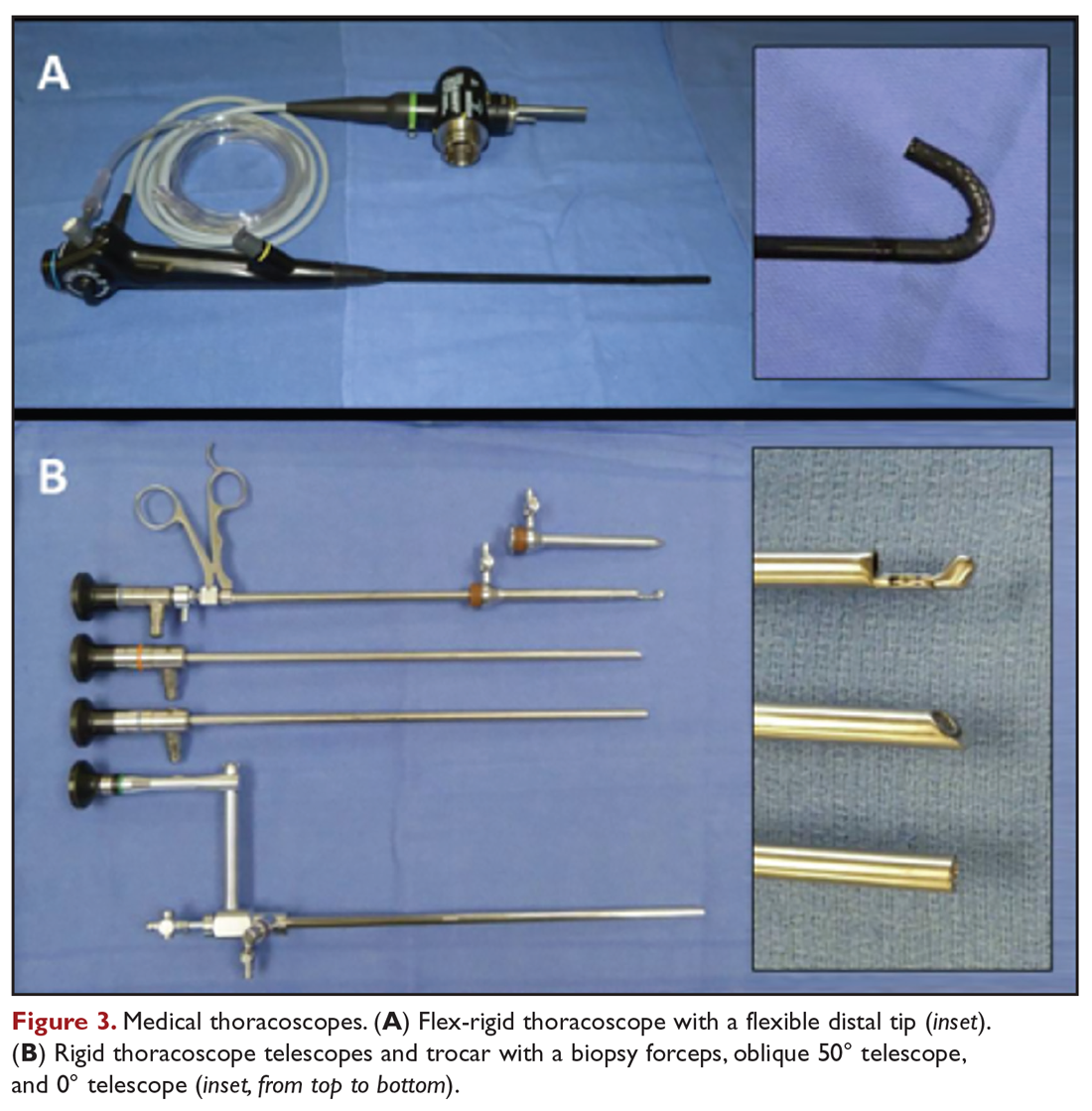
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
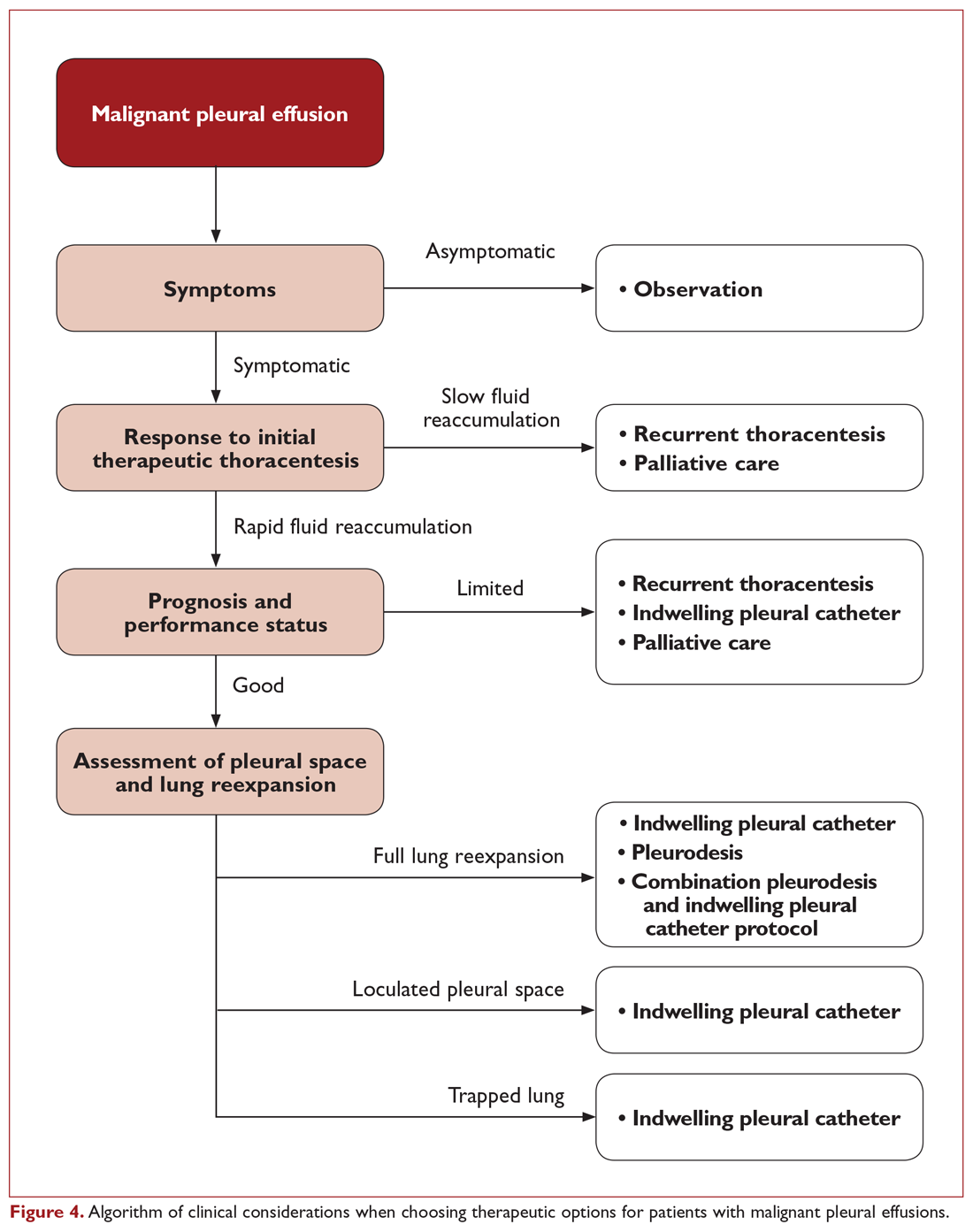
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
Malignant Pleural Effusion: Evaluation and Diagnosis
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4
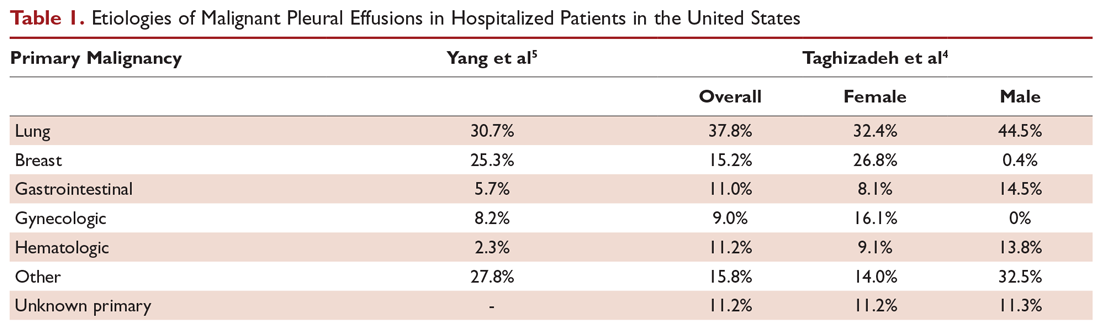
Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23
Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4

Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23
Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4

Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23
Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
The ‘fun’ in leader-fun-ship
Add value to relationships, loyalty, commitment
Leadership and “fun” are not often linked in the same sentence, let alone in the same word. However, as a student, observer, and teacher of leadership, I find that leaders who are having fun in their practice deftly share the energy, engagement, appeal, dedication, exuberance, and pleasure with others.
Imagine going to work and meeting all those qualities at the front door. Leaders who are having fun impart that same joy to others. It’s a great source of motivation, problem solving capacity, and morale enhancement. And when the going gets tough, it helps you and others make it through.
What takes the fun out of leadership? There are difficult decisions, complicated personalities, messy histories, conflict, and, of course, the “buck stops here” responsibility. Leadership is a lot of work, going above and beyond your clinical duties. Many arrive at leadership positions without the requisite training and preparation, and success at leading can be elusive for reasons you can’t control. There are budget constraints, difficult personalities, laws, and rules. For some leaders, it is an oxymoron to place leadership and fun together. For them, leadership is not fun.
At the 2018 Society of Hospital Medicine Leadership Academy in Vancouver, this combination of fun and leadership arose in a number of my conversations. I asked people if they were having fun. I heard the enjoyment, excitement, amusement, and playfulness of leading. And I could see these leaders – who found fun in their work – were transmitting those very qualities to their followers. They talked about exceptional productivity, expanding programs, heightened commitment, and a knack for overcoming occasional setbacks. In many ways, “work” works better when people are having fun.
How might putting fun into your leadership style, practices, and assessment make you a more effective leader? Start with our definition of leadership: “People follow you.” Whether people follow you, in fact, has to do with a lot more than just fun. Your clinical expertise and skills, your management capabilities, and your devotion to the job all are ingredients in what makes you an effective leader. Add fun into the equation and relationships, loyalty, and commitment assume new value. That value translates into the joy, fulfillment, and pleasure of doing important work with people who matter to you.
I once asked a C-suite leader at Southwest Airlines about fun and leadership. He told me that fun was incorporated into the airline’s company culture. It was also included in his annual performance review: He is responsible for ensuring that his subordinates find working for him to be fun. That week he was hosting a barbecue and fun was on the menu. He explained that this attitude is baked into Southwest philosophy. It transmits out to frontline employees, flight attendants, and gate agents. Their job is making the passengers’ experience safe, comfortable, and, at the same time, fun. That combination has made the company consistently profitable and remarkably resilient. (My wife and her university friend – now both therapists – call this a “fun unit,” which made their grueling graduate school work far more tolerable.)
How do you translate this lesson into your leadership practices? First, don’t expect others to have fun working and following you if you aren’t having fun yourself, or if you are not fun to be with. Assess your own work experience. What is it that you truly enjoy? What tasks and responsibilities detract from that engagement and delight? What provides you that sense of fulfillment and value in what you are doing and the direction you are leading? Dissect your priorities and ask whether your allotment of time and attention track to what is really important. What changes could you make?
Second, ask those same questions of the group of people whom you lead. Assess their experiences, what supports their sense of accomplishment, their satisfaction with their job, and their engagement with the people with whom they work. Every one of your followers is different. However, on the whole, have you built, encouraged, and rewarded team spirit among people who value being together, who are committed to the shared mission, and who together take pride in their achievements?
Finally, ask yourself what would make your work experience and that of your followers more fun? Similarly, what would better engage the patients, family members, and colleagues you serve? Ask a leader you respect – a leader enthusiast – what they find fun in their leading. As you become more engaged, you likely will become a more effective leader, and those who follow you will be so too. What could you do to elevate the work experiences of others and thereby the value, success, and meaning of their work? Fun has many ways to express itself.
Bottom line, ask yourself: Are you someone who others want to work for? Do you care? Can you bring out the best in people because of who you are and what you do?
Your work is as serious as it gets. You are at the cusp of life and death, quality of life decisions, and medical care. The fun comes in putting your all into it and getting the satisfaction and interpersonal bonds that make that effort worthwhile. Often, you have the privilege of making people healthier and happier. What a gift! Excellence can be fun.
Keep an appropriate sense of humor in your pocket and an ample supply of personal and professional curiosity in your backpack. Relish the delight of something or someone new and pleasantly unexpected. The fun for others comes in your rewarding flash of a smile, your laugh, or your approval when it matters most.
Your job as leader is tough. Health care is hard work and the changes and shifts in the health care system are only making it more so. Imagine how a dash of humanity and relationships can make that all far more bearable.
And have fun finding out.
Dr. Marcus is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition” (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution at Harvard School of Public Health, Boston. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at ljmarcus@hsph.harvard.edu.
Add value to relationships, loyalty, commitment
Add value to relationships, loyalty, commitment
Leadership and “fun” are not often linked in the same sentence, let alone in the same word. However, as a student, observer, and teacher of leadership, I find that leaders who are having fun in their practice deftly share the energy, engagement, appeal, dedication, exuberance, and pleasure with others.
Imagine going to work and meeting all those qualities at the front door. Leaders who are having fun impart that same joy to others. It’s a great source of motivation, problem solving capacity, and morale enhancement. And when the going gets tough, it helps you and others make it through.
What takes the fun out of leadership? There are difficult decisions, complicated personalities, messy histories, conflict, and, of course, the “buck stops here” responsibility. Leadership is a lot of work, going above and beyond your clinical duties. Many arrive at leadership positions without the requisite training and preparation, and success at leading can be elusive for reasons you can’t control. There are budget constraints, difficult personalities, laws, and rules. For some leaders, it is an oxymoron to place leadership and fun together. For them, leadership is not fun.
At the 2018 Society of Hospital Medicine Leadership Academy in Vancouver, this combination of fun and leadership arose in a number of my conversations. I asked people if they were having fun. I heard the enjoyment, excitement, amusement, and playfulness of leading. And I could see these leaders – who found fun in their work – were transmitting those very qualities to their followers. They talked about exceptional productivity, expanding programs, heightened commitment, and a knack for overcoming occasional setbacks. In many ways, “work” works better when people are having fun.
How might putting fun into your leadership style, practices, and assessment make you a more effective leader? Start with our definition of leadership: “People follow you.” Whether people follow you, in fact, has to do with a lot more than just fun. Your clinical expertise and skills, your management capabilities, and your devotion to the job all are ingredients in what makes you an effective leader. Add fun into the equation and relationships, loyalty, and commitment assume new value. That value translates into the joy, fulfillment, and pleasure of doing important work with people who matter to you.
I once asked a C-suite leader at Southwest Airlines about fun and leadership. He told me that fun was incorporated into the airline’s company culture. It was also included in his annual performance review: He is responsible for ensuring that his subordinates find working for him to be fun. That week he was hosting a barbecue and fun was on the menu. He explained that this attitude is baked into Southwest philosophy. It transmits out to frontline employees, flight attendants, and gate agents. Their job is making the passengers’ experience safe, comfortable, and, at the same time, fun. That combination has made the company consistently profitable and remarkably resilient. (My wife and her university friend – now both therapists – call this a “fun unit,” which made their grueling graduate school work far more tolerable.)
How do you translate this lesson into your leadership practices? First, don’t expect others to have fun working and following you if you aren’t having fun yourself, or if you are not fun to be with. Assess your own work experience. What is it that you truly enjoy? What tasks and responsibilities detract from that engagement and delight? What provides you that sense of fulfillment and value in what you are doing and the direction you are leading? Dissect your priorities and ask whether your allotment of time and attention track to what is really important. What changes could you make?
Second, ask those same questions of the group of people whom you lead. Assess their experiences, what supports their sense of accomplishment, their satisfaction with their job, and their engagement with the people with whom they work. Every one of your followers is different. However, on the whole, have you built, encouraged, and rewarded team spirit among people who value being together, who are committed to the shared mission, and who together take pride in their achievements?
Finally, ask yourself what would make your work experience and that of your followers more fun? Similarly, what would better engage the patients, family members, and colleagues you serve? Ask a leader you respect – a leader enthusiast – what they find fun in their leading. As you become more engaged, you likely will become a more effective leader, and those who follow you will be so too. What could you do to elevate the work experiences of others and thereby the value, success, and meaning of their work? Fun has many ways to express itself.
Bottom line, ask yourself: Are you someone who others want to work for? Do you care? Can you bring out the best in people because of who you are and what you do?
Your work is as serious as it gets. You are at the cusp of life and death, quality of life decisions, and medical care. The fun comes in putting your all into it and getting the satisfaction and interpersonal bonds that make that effort worthwhile. Often, you have the privilege of making people healthier and happier. What a gift! Excellence can be fun.
Keep an appropriate sense of humor in your pocket and an ample supply of personal and professional curiosity in your backpack. Relish the delight of something or someone new and pleasantly unexpected. The fun for others comes in your rewarding flash of a smile, your laugh, or your approval when it matters most.
Your job as leader is tough. Health care is hard work and the changes and shifts in the health care system are only making it more so. Imagine how a dash of humanity and relationships can make that all far more bearable.
And have fun finding out.
Dr. Marcus is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition” (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution at Harvard School of Public Health, Boston. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at ljmarcus@hsph.harvard.edu.
Leadership and “fun” are not often linked in the same sentence, let alone in the same word. However, as a student, observer, and teacher of leadership, I find that leaders who are having fun in their practice deftly share the energy, engagement, appeal, dedication, exuberance, and pleasure with others.
Imagine going to work and meeting all those qualities at the front door. Leaders who are having fun impart that same joy to others. It’s a great source of motivation, problem solving capacity, and morale enhancement. And when the going gets tough, it helps you and others make it through.
What takes the fun out of leadership? There are difficult decisions, complicated personalities, messy histories, conflict, and, of course, the “buck stops here” responsibility. Leadership is a lot of work, going above and beyond your clinical duties. Many arrive at leadership positions without the requisite training and preparation, and success at leading can be elusive for reasons you can’t control. There are budget constraints, difficult personalities, laws, and rules. For some leaders, it is an oxymoron to place leadership and fun together. For them, leadership is not fun.
At the 2018 Society of Hospital Medicine Leadership Academy in Vancouver, this combination of fun and leadership arose in a number of my conversations. I asked people if they were having fun. I heard the enjoyment, excitement, amusement, and playfulness of leading. And I could see these leaders – who found fun in their work – were transmitting those very qualities to their followers. They talked about exceptional productivity, expanding programs, heightened commitment, and a knack for overcoming occasional setbacks. In many ways, “work” works better when people are having fun.
How might putting fun into your leadership style, practices, and assessment make you a more effective leader? Start with our definition of leadership: “People follow you.” Whether people follow you, in fact, has to do with a lot more than just fun. Your clinical expertise and skills, your management capabilities, and your devotion to the job all are ingredients in what makes you an effective leader. Add fun into the equation and relationships, loyalty, and commitment assume new value. That value translates into the joy, fulfillment, and pleasure of doing important work with people who matter to you.
I once asked a C-suite leader at Southwest Airlines about fun and leadership. He told me that fun was incorporated into the airline’s company culture. It was also included in his annual performance review: He is responsible for ensuring that his subordinates find working for him to be fun. That week he was hosting a barbecue and fun was on the menu. He explained that this attitude is baked into Southwest philosophy. It transmits out to frontline employees, flight attendants, and gate agents. Their job is making the passengers’ experience safe, comfortable, and, at the same time, fun. That combination has made the company consistently profitable and remarkably resilient. (My wife and her university friend – now both therapists – call this a “fun unit,” which made their grueling graduate school work far more tolerable.)
How do you translate this lesson into your leadership practices? First, don’t expect others to have fun working and following you if you aren’t having fun yourself, or if you are not fun to be with. Assess your own work experience. What is it that you truly enjoy? What tasks and responsibilities detract from that engagement and delight? What provides you that sense of fulfillment and value in what you are doing and the direction you are leading? Dissect your priorities and ask whether your allotment of time and attention track to what is really important. What changes could you make?
Second, ask those same questions of the group of people whom you lead. Assess their experiences, what supports their sense of accomplishment, their satisfaction with their job, and their engagement with the people with whom they work. Every one of your followers is different. However, on the whole, have you built, encouraged, and rewarded team spirit among people who value being together, who are committed to the shared mission, and who together take pride in their achievements?
Finally, ask yourself what would make your work experience and that of your followers more fun? Similarly, what would better engage the patients, family members, and colleagues you serve? Ask a leader you respect – a leader enthusiast – what they find fun in their leading. As you become more engaged, you likely will become a more effective leader, and those who follow you will be so too. What could you do to elevate the work experiences of others and thereby the value, success, and meaning of their work? Fun has many ways to express itself.
Bottom line, ask yourself: Are you someone who others want to work for? Do you care? Can you bring out the best in people because of who you are and what you do?
Your work is as serious as it gets. You are at the cusp of life and death, quality of life decisions, and medical care. The fun comes in putting your all into it and getting the satisfaction and interpersonal bonds that make that effort worthwhile. Often, you have the privilege of making people healthier and happier. What a gift! Excellence can be fun.
Keep an appropriate sense of humor in your pocket and an ample supply of personal and professional curiosity in your backpack. Relish the delight of something or someone new and pleasantly unexpected. The fun for others comes in your rewarding flash of a smile, your laugh, or your approval when it matters most.
Your job as leader is tough. Health care is hard work and the changes and shifts in the health care system are only making it more so. Imagine how a dash of humanity and relationships can make that all far more bearable.
And have fun finding out.
Dr. Marcus is coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration, Second Edition” (San Francisco: Jossey-Bass Publishers, 2011) and is director of the program for health care negotiation and conflict resolution at Harvard School of Public Health, Boston. Dr. Marcus teaches regularly in the SHM Leadership Academy. He can be reached at ljmarcus@hsph.harvard.edu.
Predictive model estimates likelihood of failing induction of labor in obese patients
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
FROM OBSTETRICS & GYNECOLOGY
Endometriosis is linked to adverse pregnancy outcomes
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
FROM OBSTETRICS & GYNECOLOGY
Genotype may affect lifestyle’s influence on dementia risk
But among people at high genetic risk for dementia, these potentially modifiable factors – not smoking, not having depression or diabetes, getting regular physical activity, avoiding social isolation, and following a healthy diet – may not have protective associations, according to research published in Nature Medicine.
Recent analyses have indicated that eliminating known modifiable risk factors for dementia at a population level could prevent one-third of dementia cases, but prevention trials “have yielded inconsistent results so far,” wrote first author Silvan Licher, MD, of the department of epidemiology at Erasmus University Medical Center Rotterdam (the Netherlands) and his colleagues.
“Prior studies have mostly focused on the risk of dementia associated with an individual protective factor, yet the combination of multiple factors may yield more beneficial effects than the individual parts,” they wrote. “Combining data about a number of factors is also important because it takes into account the multifactorial nature of late-life dementia. We used data from the Rotterdam Study to determine to what extent a favorable profile based on modifiable risk factors is associated with a lower risk of dementia among individuals at low, intermediate, or high genetic risk.”
Grouped by APOE genotype
Patients who were apolipoprotein E epsilon-4 allele (APOE4) carriers (i.e., APOE2 and 4, APOE3 and 4, or two APOE4) were classified as having high genetic risk (n = 1,747). Other APOE genotypes were considered intermediate risk (n = 3,718 with two APOE3 alleles) or low risk (n = 887 with either two APOE2 alleles or APOE2 and 3).
The researchers measured six potentially modifiable lifestyle or health factors that “have been implicated in a lower risk of dementia.” Modifiable risk scores ranged from 0 to 6. Participants were classified as having an unfavorable profile (0-2 protective factors), an intermediate profile (3-4 protective factors), or a favorable profile (5-6 factors).
The researchers calculated the relative risk of developing dementia using a Cox proportional hazards model and the absolutely risk using competing risk models.
In all, 56.2% of the participants were women, the average age was about 69 years, and patient characteristics were similar across the categories of APOE risk. APOE4 carriers received dementia diagnoses at a younger age, more often had a parental history of dementia, and had higher total cholesterol levels, compared with noncarriers. In all, 915 people received a dementia diagnosis, of whom 739 received a diagnosis of Alzheimer’s disease. The other 2,644 participants died free from dementia. The median follow-up was 14.1 years.
“Dementia risk was significantly higher among participants at high or intermediate APOE risk, compared with those at low APOE risk,” the researchers said. In addition, the risk of dementia increased in participants who had fewer protective factors. Those with 0-2 protective factors had a 29% higher risk of dementia, compared with participants with 5 or 6 protective factors, after adjusting for age, sex, level of education, parental history of dementia, history of stroke, systolic blood pressure, and total and high-density lipoprotein cholesterol.
Lifestyle benefits tended to be greater in younger participants
“APOE genotype significantly modified the association between protective factors and dementia,” the authors said. Compared with participants with protective modifiable risk profiles, participants with unfavorable modifiable risk profiles had greater risk for dementia in the low–APOE risk group (hazard ratio, 2.51) and intermediate–APOE risk group (HR, 1.39), but not in the high–APOE risk group.
“Protective associations of favorable risk profiles against dementia tended to be stronger in younger individuals than in older individuals and were most pronounced for younger individuals at low APOE risk,” Dr. Licher and colleagues said. In a sensitivity analysis that used a polygenic risk score for Alzheimer’s disease based on 27 variants other than APOE to determine participants’ genetic risk, the patterns were “attenuated yet largely comparable,” they wrote. Patterns also remained consistent when the researchers used an ideal cardiovascular health score to indicate modifiable health profiles.
“Our results confirm that individuals with a favorable profile have a lower risk of dementia than those with intermediate or unfavorable profiles based on modifiable risk factors,” they said. Unlike in a subgroup analysis of data from the FINGER study, however, “this study found that a favorable profile could not offset high APOE risk.”
The findings may have implications for clinical trial design and suggest that APOE4 carriers may need to be targeted earlier in the disease process to influence their risk for dementia.
“On the positive side, results from this study show that avoiding an unhealthy lifestyle could potentially prevent or postpone the onset of dementia in most individuals in the population (73%), namely those at low and intermediate genetic risk,” the investigators wrote. “Among these, the majority were categorized has having a favorable profile (66%), yet room for improvement is still substantial.”
The study lacked data on hearing impairment and did not capture shifts to more adverse or optimal lifestyles during follow-up. In addition, the results are based on relatively small samples in each risk category, and the estimates had wide confidence intervals. The population was older and mostly of European descent, which limits the generalizability of the findings, the authors noted.
Lifestyle factors may benefit only people with low genetic risk
“The authors’ key finding was that modifiable lifestyle risk factors were able to reduce dementia risk only in people who did not have an APOE4 allele and hence were at lower genetic risk,” said Kenneth Rockwood, MD, professor of geriatric medicine and neurology at Dalhousie University in Halifax, N.S., and his coauthors, in an accompanying editorial.
The findings contrast with those of another recent population-based study using data from the UK Biobank (JAMA. 2019;322[5]:430-7. doi: 10.1001/jama.2019.9879), which suggested that modifiable factors affect dementia risk regardless of genetic risk.
Together, these studies “tell us that ... we must better understand outcomes in those most at risk,” they said. “We might begin by recognizing that aging is essential, rather than incidental, to dementia disease expression.” Future research should focus on people living with frailty, who often are excluded from trials and are at high risk for dementia. Older adults who develop delirium also may be an ideal target group of patients at increased risk for dementia.
“Reducing the extent of disease expression in people prone to developing dementia late in life is difficult. Studies investigating whether dementia can be prevented at all, such as the Rotterdam study, and then whether it can be prevented in the people at greatest risk, can be commended for their clear-eyed approach,” Dr. Rockwood and colleagues said.
The Rotterdam Study is funded by Erasmus Medical Center and University, as well as a variety of Dutch organizations, institutes, and government ministries, and the European Commission. The authors had no competing interests.
Dr. Rockwood is president and chief science officer of DGI Clinical, which has contracts with pharmaceutical and device manufacturers related to individualized outcome measurement.
SOURCES: Licher S et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0547-7; and Rockwood K et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0575-3.
The study by Dr. Licher and associates shows a clinically significant impact of a healthy lifestyle in reducing dementia. But what is surprising is that the effect was not seen in genetically higher-risk people.
If anything, the results strengthen our recommendations to people interested in lowering their risk for dementia with lifestyle modification. Bear in mind that APOE testing is not done routinely, so the vast majority of our patients do not know their APOE genotype. Since a healthy lifestyle can benefit the majority of the population (around 75%), even if it is less or ineffective in the APOE4 carrier group (about 25% of the population), it is certainly something to recommend. Of course, health care professionals already recommend heart healthy habits, which have an equivalent benefit, and sadly, adherence is relatively low. Adding that lifestyle modification may help prevent dementia might improve patient compliance. Starting healthy lifestyles as early in life as possible may be the key. It is less effective if we wait until we already have memory loss.
Finally, the study results regard relative risk, a concept that many fail to fully grasp. A person can still get dementia in any of the categories, including the “best one” (low genetic and lifestyle risk). It’s a matter of the odds being better or worse, but there is no guarantee of a positive or negative outcome.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center, Phoenix. He made these comments in an interview.
The study by Dr. Licher and associates shows a clinically significant impact of a healthy lifestyle in reducing dementia. But what is surprising is that the effect was not seen in genetically higher-risk people.
If anything, the results strengthen our recommendations to people interested in lowering their risk for dementia with lifestyle modification. Bear in mind that APOE testing is not done routinely, so the vast majority of our patients do not know their APOE genotype. Since a healthy lifestyle can benefit the majority of the population (around 75%), even if it is less or ineffective in the APOE4 carrier group (about 25% of the population), it is certainly something to recommend. Of course, health care professionals already recommend heart healthy habits, which have an equivalent benefit, and sadly, adherence is relatively low. Adding that lifestyle modification may help prevent dementia might improve patient compliance. Starting healthy lifestyles as early in life as possible may be the key. It is less effective if we wait until we already have memory loss.
Finally, the study results regard relative risk, a concept that many fail to fully grasp. A person can still get dementia in any of the categories, including the “best one” (low genetic and lifestyle risk). It’s a matter of the odds being better or worse, but there is no guarantee of a positive or negative outcome.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center, Phoenix. He made these comments in an interview.
The study by Dr. Licher and associates shows a clinically significant impact of a healthy lifestyle in reducing dementia. But what is surprising is that the effect was not seen in genetically higher-risk people.
If anything, the results strengthen our recommendations to people interested in lowering their risk for dementia with lifestyle modification. Bear in mind that APOE testing is not done routinely, so the vast majority of our patients do not know their APOE genotype. Since a healthy lifestyle can benefit the majority of the population (around 75%), even if it is less or ineffective in the APOE4 carrier group (about 25% of the population), it is certainly something to recommend. Of course, health care professionals already recommend heart healthy habits, which have an equivalent benefit, and sadly, adherence is relatively low. Adding that lifestyle modification may help prevent dementia might improve patient compliance. Starting healthy lifestyles as early in life as possible may be the key. It is less effective if we wait until we already have memory loss.
Finally, the study results regard relative risk, a concept that many fail to fully grasp. A person can still get dementia in any of the categories, including the “best one” (low genetic and lifestyle risk). It’s a matter of the odds being better or worse, but there is no guarantee of a positive or negative outcome.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center, Phoenix. He made these comments in an interview.
But among people at high genetic risk for dementia, these potentially modifiable factors – not smoking, not having depression or diabetes, getting regular physical activity, avoiding social isolation, and following a healthy diet – may not have protective associations, according to research published in Nature Medicine.
Recent analyses have indicated that eliminating known modifiable risk factors for dementia at a population level could prevent one-third of dementia cases, but prevention trials “have yielded inconsistent results so far,” wrote first author Silvan Licher, MD, of the department of epidemiology at Erasmus University Medical Center Rotterdam (the Netherlands) and his colleagues.
“Prior studies have mostly focused on the risk of dementia associated with an individual protective factor, yet the combination of multiple factors may yield more beneficial effects than the individual parts,” they wrote. “Combining data about a number of factors is also important because it takes into account the multifactorial nature of late-life dementia. We used data from the Rotterdam Study to determine to what extent a favorable profile based on modifiable risk factors is associated with a lower risk of dementia among individuals at low, intermediate, or high genetic risk.”
Grouped by APOE genotype
Patients who were apolipoprotein E epsilon-4 allele (APOE4) carriers (i.e., APOE2 and 4, APOE3 and 4, or two APOE4) were classified as having high genetic risk (n = 1,747). Other APOE genotypes were considered intermediate risk (n = 3,718 with two APOE3 alleles) or low risk (n = 887 with either two APOE2 alleles or APOE2 and 3).
The researchers measured six potentially modifiable lifestyle or health factors that “have been implicated in a lower risk of dementia.” Modifiable risk scores ranged from 0 to 6. Participants were classified as having an unfavorable profile (0-2 protective factors), an intermediate profile (3-4 protective factors), or a favorable profile (5-6 factors).
The researchers calculated the relative risk of developing dementia using a Cox proportional hazards model and the absolutely risk using competing risk models.
In all, 56.2% of the participants were women, the average age was about 69 years, and patient characteristics were similar across the categories of APOE risk. APOE4 carriers received dementia diagnoses at a younger age, more often had a parental history of dementia, and had higher total cholesterol levels, compared with noncarriers. In all, 915 people received a dementia diagnosis, of whom 739 received a diagnosis of Alzheimer’s disease. The other 2,644 participants died free from dementia. The median follow-up was 14.1 years.
“Dementia risk was significantly higher among participants at high or intermediate APOE risk, compared with those at low APOE risk,” the researchers said. In addition, the risk of dementia increased in participants who had fewer protective factors. Those with 0-2 protective factors had a 29% higher risk of dementia, compared with participants with 5 or 6 protective factors, after adjusting for age, sex, level of education, parental history of dementia, history of stroke, systolic blood pressure, and total and high-density lipoprotein cholesterol.
Lifestyle benefits tended to be greater in younger participants
“APOE genotype significantly modified the association between protective factors and dementia,” the authors said. Compared with participants with protective modifiable risk profiles, participants with unfavorable modifiable risk profiles had greater risk for dementia in the low–APOE risk group (hazard ratio, 2.51) and intermediate–APOE risk group (HR, 1.39), but not in the high–APOE risk group.
“Protective associations of favorable risk profiles against dementia tended to be stronger in younger individuals than in older individuals and were most pronounced for younger individuals at low APOE risk,” Dr. Licher and colleagues said. In a sensitivity analysis that used a polygenic risk score for Alzheimer’s disease based on 27 variants other than APOE to determine participants’ genetic risk, the patterns were “attenuated yet largely comparable,” they wrote. Patterns also remained consistent when the researchers used an ideal cardiovascular health score to indicate modifiable health profiles.
“Our results confirm that individuals with a favorable profile have a lower risk of dementia than those with intermediate or unfavorable profiles based on modifiable risk factors,” they said. Unlike in a subgroup analysis of data from the FINGER study, however, “this study found that a favorable profile could not offset high APOE risk.”
The findings may have implications for clinical trial design and suggest that APOE4 carriers may need to be targeted earlier in the disease process to influence their risk for dementia.
“On the positive side, results from this study show that avoiding an unhealthy lifestyle could potentially prevent or postpone the onset of dementia in most individuals in the population (73%), namely those at low and intermediate genetic risk,” the investigators wrote. “Among these, the majority were categorized has having a favorable profile (66%), yet room for improvement is still substantial.”
The study lacked data on hearing impairment and did not capture shifts to more adverse or optimal lifestyles during follow-up. In addition, the results are based on relatively small samples in each risk category, and the estimates had wide confidence intervals. The population was older and mostly of European descent, which limits the generalizability of the findings, the authors noted.
Lifestyle factors may benefit only people with low genetic risk
“The authors’ key finding was that modifiable lifestyle risk factors were able to reduce dementia risk only in people who did not have an APOE4 allele and hence were at lower genetic risk,” said Kenneth Rockwood, MD, professor of geriatric medicine and neurology at Dalhousie University in Halifax, N.S., and his coauthors, in an accompanying editorial.
The findings contrast with those of another recent population-based study using data from the UK Biobank (JAMA. 2019;322[5]:430-7. doi: 10.1001/jama.2019.9879), which suggested that modifiable factors affect dementia risk regardless of genetic risk.
Together, these studies “tell us that ... we must better understand outcomes in those most at risk,” they said. “We might begin by recognizing that aging is essential, rather than incidental, to dementia disease expression.” Future research should focus on people living with frailty, who often are excluded from trials and are at high risk for dementia. Older adults who develop delirium also may be an ideal target group of patients at increased risk for dementia.
“Reducing the extent of disease expression in people prone to developing dementia late in life is difficult. Studies investigating whether dementia can be prevented at all, such as the Rotterdam study, and then whether it can be prevented in the people at greatest risk, can be commended for their clear-eyed approach,” Dr. Rockwood and colleagues said.
The Rotterdam Study is funded by Erasmus Medical Center and University, as well as a variety of Dutch organizations, institutes, and government ministries, and the European Commission. The authors had no competing interests.
Dr. Rockwood is president and chief science officer of DGI Clinical, which has contracts with pharmaceutical and device manufacturers related to individualized outcome measurement.
SOURCES: Licher S et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0547-7; and Rockwood K et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0575-3.
But among people at high genetic risk for dementia, these potentially modifiable factors – not smoking, not having depression or diabetes, getting regular physical activity, avoiding social isolation, and following a healthy diet – may not have protective associations, according to research published in Nature Medicine.
Recent analyses have indicated that eliminating known modifiable risk factors for dementia at a population level could prevent one-third of dementia cases, but prevention trials “have yielded inconsistent results so far,” wrote first author Silvan Licher, MD, of the department of epidemiology at Erasmus University Medical Center Rotterdam (the Netherlands) and his colleagues.
“Prior studies have mostly focused on the risk of dementia associated with an individual protective factor, yet the combination of multiple factors may yield more beneficial effects than the individual parts,” they wrote. “Combining data about a number of factors is also important because it takes into account the multifactorial nature of late-life dementia. We used data from the Rotterdam Study to determine to what extent a favorable profile based on modifiable risk factors is associated with a lower risk of dementia among individuals at low, intermediate, or high genetic risk.”
Grouped by APOE genotype
Patients who were apolipoprotein E epsilon-4 allele (APOE4) carriers (i.e., APOE2 and 4, APOE3 and 4, or two APOE4) were classified as having high genetic risk (n = 1,747). Other APOE genotypes were considered intermediate risk (n = 3,718 with two APOE3 alleles) or low risk (n = 887 with either two APOE2 alleles or APOE2 and 3).
The researchers measured six potentially modifiable lifestyle or health factors that “have been implicated in a lower risk of dementia.” Modifiable risk scores ranged from 0 to 6. Participants were classified as having an unfavorable profile (0-2 protective factors), an intermediate profile (3-4 protective factors), or a favorable profile (5-6 factors).
The researchers calculated the relative risk of developing dementia using a Cox proportional hazards model and the absolutely risk using competing risk models.
In all, 56.2% of the participants were women, the average age was about 69 years, and patient characteristics were similar across the categories of APOE risk. APOE4 carriers received dementia diagnoses at a younger age, more often had a parental history of dementia, and had higher total cholesterol levels, compared with noncarriers. In all, 915 people received a dementia diagnosis, of whom 739 received a diagnosis of Alzheimer’s disease. The other 2,644 participants died free from dementia. The median follow-up was 14.1 years.
“Dementia risk was significantly higher among participants at high or intermediate APOE risk, compared with those at low APOE risk,” the researchers said. In addition, the risk of dementia increased in participants who had fewer protective factors. Those with 0-2 protective factors had a 29% higher risk of dementia, compared with participants with 5 or 6 protective factors, after adjusting for age, sex, level of education, parental history of dementia, history of stroke, systolic blood pressure, and total and high-density lipoprotein cholesterol.
Lifestyle benefits tended to be greater in younger participants
“APOE genotype significantly modified the association between protective factors and dementia,” the authors said. Compared with participants with protective modifiable risk profiles, participants with unfavorable modifiable risk profiles had greater risk for dementia in the low–APOE risk group (hazard ratio, 2.51) and intermediate–APOE risk group (HR, 1.39), but not in the high–APOE risk group.
“Protective associations of favorable risk profiles against dementia tended to be stronger in younger individuals than in older individuals and were most pronounced for younger individuals at low APOE risk,” Dr. Licher and colleagues said. In a sensitivity analysis that used a polygenic risk score for Alzheimer’s disease based on 27 variants other than APOE to determine participants’ genetic risk, the patterns were “attenuated yet largely comparable,” they wrote. Patterns also remained consistent when the researchers used an ideal cardiovascular health score to indicate modifiable health profiles.
“Our results confirm that individuals with a favorable profile have a lower risk of dementia than those with intermediate or unfavorable profiles based on modifiable risk factors,” they said. Unlike in a subgroup analysis of data from the FINGER study, however, “this study found that a favorable profile could not offset high APOE risk.”
The findings may have implications for clinical trial design and suggest that APOE4 carriers may need to be targeted earlier in the disease process to influence their risk for dementia.
“On the positive side, results from this study show that avoiding an unhealthy lifestyle could potentially prevent or postpone the onset of dementia in most individuals in the population (73%), namely those at low and intermediate genetic risk,” the investigators wrote. “Among these, the majority were categorized has having a favorable profile (66%), yet room for improvement is still substantial.”
The study lacked data on hearing impairment and did not capture shifts to more adverse or optimal lifestyles during follow-up. In addition, the results are based on relatively small samples in each risk category, and the estimates had wide confidence intervals. The population was older and mostly of European descent, which limits the generalizability of the findings, the authors noted.
Lifestyle factors may benefit only people with low genetic risk
“The authors’ key finding was that modifiable lifestyle risk factors were able to reduce dementia risk only in people who did not have an APOE4 allele and hence were at lower genetic risk,” said Kenneth Rockwood, MD, professor of geriatric medicine and neurology at Dalhousie University in Halifax, N.S., and his coauthors, in an accompanying editorial.
The findings contrast with those of another recent population-based study using data from the UK Biobank (JAMA. 2019;322[5]:430-7. doi: 10.1001/jama.2019.9879), which suggested that modifiable factors affect dementia risk regardless of genetic risk.
Together, these studies “tell us that ... we must better understand outcomes in those most at risk,” they said. “We might begin by recognizing that aging is essential, rather than incidental, to dementia disease expression.” Future research should focus on people living with frailty, who often are excluded from trials and are at high risk for dementia. Older adults who develop delirium also may be an ideal target group of patients at increased risk for dementia.
“Reducing the extent of disease expression in people prone to developing dementia late in life is difficult. Studies investigating whether dementia can be prevented at all, such as the Rotterdam study, and then whether it can be prevented in the people at greatest risk, can be commended for their clear-eyed approach,” Dr. Rockwood and colleagues said.
The Rotterdam Study is funded by Erasmus Medical Center and University, as well as a variety of Dutch organizations, institutes, and government ministries, and the European Commission. The authors had no competing interests.
Dr. Rockwood is president and chief science officer of DGI Clinical, which has contracts with pharmaceutical and device manufacturers related to individualized outcome measurement.
SOURCES: Licher S et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0547-7; and Rockwood K et al. Nat Med. 2019 Aug 26. doi: 10.1038/s41591-019-0575-3.
FROM NATURE MEDICINE
ObGyn compensation: Strides in the gender wage gap indicate closure possible
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3
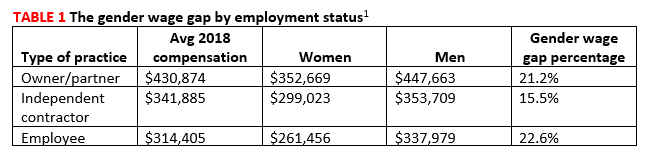
Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6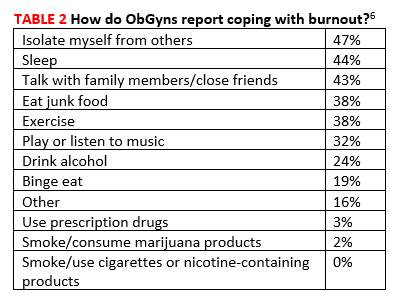
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6
Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3

Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6

Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
The gender wage gap in physician compensation persists but is narrowing. According to information gleaned from self-reported compensation surveys, collected by Doximity and completed by 90,000 full-time, US-licensed physicians, while wages for men idled between 2017 and 2018, they increased for women by 2%.1 So, whereas the gender wage gap was 27.7% in 2017, it dropped to 25.2% in 2018. This translates to female physicians making $90,490 less than male counterparts in 2018 vs $105,000 less in 2017.1
Gender wage gap and geography. Metropolitan areas with the smallest gender wage gaps according to the Doximity report include Birmingham, Alabama (9%); Bridgeport, Connecticut (10%); and Seattle, Washington (15%). Areas with the largest gender wage gap include Louisville/Jefferson County, Kentucky-Indiana (40%); New Orleans, Louisiana (32%); and Austin, Texas (31%).1
Gender wage gap and specialty. Specialties with the widest gender wage gaps are pediatric pulmonology (23%), otolaryngology (22%), and urology (22%). Those with the narrowest gaps are hematology (4%), rheumatology (8%), and radiation oncology (9%).1
Interestingly, although female physicians continue to earn less than men across the board, women were the slight majority of US medical school applicants (50.9%) and matriculants (51.6%) in 2018.2
What are physicians earning?
The overall average salary for physicians in 2019 is $313,000, according to a Medscape report, and the average annual compensation for ObGyns is $303,000, up from $300,000 in 2018.3 Doximity’s figure was slightly different; it reported average annual compensation for ObGyns to be $335,000 in 2018, ranking ObGyns 20th in specialties with the highest annual compensation.1
Compensation by specialty. The specialties with the highest average annual compensation in 2018 according to the Doximity report were neurosurgery ($617K), thoracic surgery ($584K), and orthopedic surgery ($526K). Those with the lowest were pediatric infectious disease ($186K), pediatric endocrinology ($201K), and general pediatrics ($223K).1
While women make up 61% of the ObGyn workforce, fewer than 15% of cardiologists, urologists, and orthopedists—some of the highest paying specialties—are women, although this alone does not explain the gender wage gap.3
Compensation by employment type. While average annual compensation increased from 2017 to 2018 for physicians working in single specialty groups (1%), multispecialty groups (1%), solo practices (3%), and industry/pharmaceutical (17%), compensation decreased for those working in health maintenance organizations (-1%), hospitals (-7%), and academia (-9%).1 Only 14% of private practices are owned by female physicians (TABLE 1).1
Satisfaction with compensation. Exactly half (50%) of ObGyns report feeling fairly compensated.3 Those physicians working in public health and preventive medicine are the most likely to feel fairly compensated (73%), while those working in infectious disease are least likely (42%).3

Location matters and may surprise you
Contrary to what many believe, less populated metropolitan areas tend to pay better than larger, more populated cities.1 This may be because metropolitan areas without academic institutions or nationally renowned health systems tend to offer slightly higher compensation than those with such facilities. The reason? The presence of large or prestigious medical schools ensures a pipeline of viable physician candidates for limited jobs, resulting in institutions and practices needing to pay less for qualified applicants.1
The 5 markets paying the highest physician salaries in 2018 were (from highest to lowest) Milwaukee; New Orleans; Riverside, California; Minneapolis; and Charlotte, North Carolina. Those paying the lowest were Durham, North Carolina; Providence, Rhode Island; San Antonio; Virginia Beach; and New Haven, Connecticut.1 Rural areas continue to have problems luring physicians (see “Cures for the famine of rural physicians?”3,4).
Job satisfaction
ObGyns rank 16th in terms of specialists who are happiest at work; 27% responded that they were very or extremely happy. Plastic surgeons ranked first in happiness on the job (41%), while those in physical medicine and rehabilitation ranked last (19%).5
Physicians as a whole report that the most rewarding part of the job is the gratitude from and relationships with patients, followed by “being good at a what I do”/finding answers/diagnoses, and “knowing that I’m making the world a better place.”3 Three-quarters (74%) of ObGyns would choose medicine again, and 75% would choose the same specialty. Those most likely to choose medicine again are those in infectious disease (84%), while those least likely work in physical medicine and rehabilitation (62%). Those most satisfied with their chosen specialty are ophthalmologists; 96% would choose the specialty again, whereas only 62% of internists would do so.3
Burnout. In a Medscape survey of 15,000 physicians in 29 specialties, 45% of ObGyns reported being burned out.5 Another 15% reported being “colloquially” depressed (sad, despondent, but not clinically depressed), and 7% reported clinical depression. While physicians overall most frequently engage in exercise as a coping mechanism, ObGyns most frequently report isolating themselves from others (47%)(TABLE 2).6
Across all specialties, more female physicians report being burned out than men (50% vs 39%). The 3 highest contributors to burnout are too many bureaucratic tasks (charting, paperwork), spending too many hours at work/insufficient compensation, and the increasing computerization of practices (electronic health records [EHRs])(TABLE 3).6 While 44% of ObGyns report that their feelings of being burned out or depressed do not affect their interactions with patients, 39% say such feelings make them easily exasperated with their patients.6 One in five (20%) responding ObGyns reported having had thoughts of suicide (vs 14% for physicians as a whole).5,6

Fortunately, ObGyns are the third most likely type of specialists to seek help for burnout or depression (37%), following psychiatrists (45%) and public health and preventive medicine specialists (45%).6 Those least likely to seek help are allergists/immunologists (13%).5
Sources of frustration on the job
Long hours. Physicians responding to the Medscape survey say that the most frustrating part of their job is having so many rules and regulations, followed by having to work with an EHR, and having to work long hours.3
As for the latter, 60% of responding ObGyns reported working long hours, which places obstetrics/gynecology in the 11th position on a list of specialties with physicians reporting working too many hours.5 Surgeons were number 1 with 77% reporting working long hours, and emergency medicine physicians were last with only 13% reporting working long hours.
Paper and administrative tasks. Thirty-eight percent of the physicians responding to the Medscape survey report spending 10 to 19 hours per week on paperwork; another 36% report spending 20 hours or more.3 This is almost identical to last year when the figures were 38% and 32%, respectively. However, the trend in the last few years has been dramatic. In 2017, the total percentage of physicians spending 10 of more hours on paperwork per week was 57%, compared with this year’s 74%.3
According to the latest Medscape report, 50% of responding physicians employ nurse practitioners (NPs) and 36% employ physician assistants (PAs); 38% employ neither. Almost half (47%) of respondents report increased profitability as a result of employing NPs/PAs.1
NPs and PAs may be increasingly important in rural America, suggests Skinner and colleagues in an article in New England Journal of Medicine.2,3 They report that the total number of rural physicians grew only 3% between 2000 and 2017 (from 61,000 to 62,700) and that the number of physicians under 50 years of age living in rural areas decreased by 25% during the same time period (from 39,200 to 29,600). As a result, the rural physician workforce is aging. In 2017, only about 25% of rural physicians were under the age of 50 years. Without a sizeable influx of younger physicians, the size of the rural physician workforce will decrease by 23% by 2030, as all of the current rural physicians retire.
To help offset the difference, the authors suggest that the rapidly growing NP workforce is poised to help. NPs provide cost-effective, high-quality care, and many more go into primary care in rural areas than do physicians. The authors suggest that sites training primary care clinicians, particularly those in or near rural areas, should work with programs educating NPs to develop ways to make it conducive for rural NPs to consult with physicians and other rural health specialists, and, in this way, help to stave off the coming dearth of physicians in rural America.
In addition to utilizing an NP workforce, Skinner and colleagues suggest that further strategies will be needed to address the rural physician shortfall and greater patient workload. Although certain actions instituted in the past have been helpful, including physician loan repayment, expansion of the national health service corps, medical school grants, and funding of rural teaching clinics, they have not done enough to address the growing needs of rural patient populations. The authors additionally suggest2:
- expansion of graduate medical education programs in rural hospitals
- higher payments for physicians in rural areas
- expanding use of mobile health vans equipped with diagnostic and treatment technology
- overcoming barriers that have slowed adoption of telehealth services.
References
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, Buerhaus PI. Implications of an aging rural physician workforce. N Engl J Med. 2019;381(4):299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Morr M. Nurse practitioners may alleviate dwindling physician workforce in rural populations. Clinical Advisor.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
- Doximity. 2019 Physician Compensation Report. Third annual study. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round4.pdf Color/Word_R0_G0_B255 March 2019. Accessed August 19, 2019.
- Association of American Medical Colleges (AAMC). Women were majority of US medical school applicants in 2018. Press release, December 4, 2018. Color/Word_R0_G0_B255https://news.aamc.org/press-releases/article/applicant-data-2018/. Accessed August 19, 2019.
- Kane L. Medscape physician compensation report 2019. Color/Word_R0_G0_B255https://www.medscape.com/slideshow/2019-compensation-overview-6011286#30. Accessed August 19, 2019.
- Skinner L, Staiger DO, Auerbach DI, et al. Implications of an aging rural physician workforce. N Engl J Med. 2019;381:299-300. https://www.nejm.org/doi/pdf/10.1056/NEJMp1900808?articleTools=true. Accessed August 19, 2019.
- Kane L. Medscape national physician burnout, depression and suicide report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056. Accessed August 19, 2019.
- Kane L. Medscape obstetrician and gynecologist lifestyle, happiness and burnout report 2019. February 20, 2019. https://www.medscape.com/slideshow/2019-lifestyle-obgyn-6011131Color/Word_R0_G0_B255. Accessed August 20, 2019.
Primary care psychiatry fellowship seeks to fill ‘void’
Program’s aim is to ‘optimize and expand the psychiatric workforce’
ORANGE, CALIF. – When Shannon Suo, MD, reached her family medicine rotation as a medical student at the University of Cincinnati, she recalls that the faculty “seemed a little lost” when they encountered patients who presented with psychiatric issues.
“If it were diabetes, high blood pressure, or back pain, they knew what to do; they had a plan,” said Dr. Suo, who is now training director for the combined family medicine/psychiatry residency at the University of California, Davis. “But when it came to depression, anxiety, maybe even some psychosis, they kind of looked a little nervous. Maybe they prescribed an antidepressant, but then they were like, ‘I don’t know what else to do for you.’ I felt uncomfortable with that.”
Eric Eschweiler, DNP, can identify with that sentiment. After he assumed the medical directorship of a federally qualified health center that opened 4 years ago in San Bernardino, Calif., nearly every person who presented for treatment had an underlying psychiatric disorder, from depression and anxiety to bipolar disorder and substance abuse stemming from childhood trauma.
“I really felt unprepared to take care of that population because with those mental health disorders, we would get a lot of concomitant chronic medical conditions,” Dr. Eschweiler said. “But they were not adherent, not compliant because they couldn’t control the mental health aspects, or we couldn’t get a handle on those issues.
“In [nurse practitioner] training, we really don’t get a whole lot of education related to the psychiatric component of health care,” he said. “I have a doctorate, but even with that, we didn’t get very much.”
When Dr. Eschweiler learned about a yearlong clinical fellowship designed for primary care–oriented trainees and clinicians who wish to receive advanced training in primary care psychiatry, he applied, “hoping I’d be first in line,” he said. He earned a spot in the 2018 class.
The program, known as the UC Irvine/UC Davis Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship, launched in 2016 as a way to train primary care clinicians to diagnose and treat the most common psychiatric conditions found in the primary care setting. The fellowship is aimed at educating not only MDs and DOs, but physician assistants, nurse practitioners, and pharmacists as well.
“We want to give high-quality training to as many people as we can in order to optimize and expand the psychiatric workforce,” said Robert M. McCarron, DO, founding codirector of the fellowship program, and professor and vice chair of education and integrated care at the University of California, Irvine. “That’s really what we’re trying to do here.”
According to Dr. McCarron, who founded the fellowship with seed money during his tenure as a faculty member at UC Davis, up to 60% of all behavioral health care is provided in the busy primary setting, and as many as 80% of antidepressants are prescribed by nonpsychiatrists. Couple that with the current psychiatric workforce shortage “and you have a perfect storm,” he said.
“The toughest part is the volume of patients primary care providers have to see, comingled with a high frequency of comorbid mental illness. Added on to that, they didn’t get the training for much of the conditions they’re trying to address or treat. A primary care provider can refer to a psychiatrist, but there is not a large referral base. In addition, plenty of studies show that about half of those patients don’t end up seeing a mental health provider. Resources are limited in many areas of the country.”
TNT PCP fellows participate in the year-long program during nonclinic hours and accrue more than 45 CME units. Much of the training involves online courses framed around the textbook Primary Care Psychiatry (Wolters Kluwer, 2019), but fellows also are required to attend two intensive weekend sessions where they drill down on core topics, such as how to conduct an effective psychiatric interview, techniques, and how to screen and treat for psychiatric disorders.
“It’s not just about medicine options,” said Jaesu Han, MD, a fellowship faculty member who is board certified in psychiatry and family medicine. “For example, we try to focus on basic principles of cognitive-behavioral therapy, the role of psychotherapy, and the role of motivational interviewing. We try to help the providers recognize that there are other tools available [besides medication]. Those principles are going to help them engage with their patients more and have a larger impact.”
During one of the intensive weekend sessions, fellows are required to give a PowerPoint lecture on a topic of their choosing, with the idea of getting them comfortable with training their primary care colleagues on principles they learn.
“Some fellows are training their staff on their lunch breaks, or they’re implementing different tools they learned in our program to their organization,” said Wendy Cant, MBA, who is TNT PCP’s administrative director.
During a PowerPoint presentation a few years ago, one fellow chose to lecture about how to administer long-term antipsychotic medication in the primary care setting, a topic that the TNT PCP faculty had not thought to recommend. “We thought that was something a primary care provider would punt on,” said Dr. Suo, who is codirector of the fellowship program. “The fellow said, ‘No. This makes absolute sense. Our offices are equipped to do IM injections; our medical staff are trained to do that. It’s something that improves adherence and outcomes. It stabilizes people, and it makes us better able to take care of that population and not have to punt.’ That was unexpected and gratifying to know that we increased his comfort level to that point.”
Fellows also participate in two interactive learning sessions per month with faculty members, and each is paired with a faculty mentor, with whom they meet monthly in person or by video call or phone call.
“One of my fellows is feeling overwhelmed because she’s been tasked with taking care of the general psychiatric population, to do initial triage before she refers off to one of very few psychiatrists,” said Matthew Reed, MD, MSPH, one of the faculty mentors who serves as director of education for TNT PCP. “Her biggest concern is, ‘How do I not hate what I’m doing with my job because it’s so stressful and all patients want are opioid analgesics, or all they want are some benzodiazepines? They always want something, and I can’t get them to do what I want them to do.’ ” Together, they discuss various tools that might help her situation, such as using motivational interviewing to foster a sense of collaboration with patients.
Dr. Reed used the analogy of skeet shooting to illustrate the point that, without inviting patients to share in decision making, they are more likely to “shoot down” interventions that clinicians suggest to them.
Currently, about 80% of the program’s 118 fellows practice in California, there is a similar proportion of men and women, and their experience levels range from trainees to seasoned clinicians.
“They have 10, 15, and in some cases 25 years of experience, and they’re saying, ‘I‘ve been practicing for years and have been struggling when it comes to psychiatric issues. I want to do better,’ ” Dr. Suo noted.
Tuition is $15,500, and it usually is covered by outside funding sources. Last year, the program received a $1 million grant from California’s Office of Statewide Health Planning and Development, which was enough to fund tuition for 62 fellows. TNT PCP administrators and faculty also formed sponsor partnerships with several health plans and counties, including Inland Empire Health Plan, LA Care, Alameda Health Consortium, and Fresno County.
Employers also have stepped up. Cedars-Sinai Medical Center recently supplied a grant to fund tuition for 30 affiliated clinicians from different service planning areas. Other organizations that support funding for the fellowship include the American Academy of Pediatrics, the California Academy of Family Physicians, the California Behavioral Health Directors Association, and the California Psychiatric Association.
Meanwhile, word about the TNT PCP program is spreading to clinicians in other states. “By far, the hardest part is helping fellows from out of state find funding in their area,” Ms. Cant said. “That’s new territory we are starting to look into, but typically it’s not out of pocket. We only had three people pay out of pocket last year.”
Program faculty hope to not only improve the fellows’ knowledge in psychiatry and better treatment for depression, anxiety, and substance abuse, but also decrease prescription of opioids and decrease the stigma that some clinicians might perceive in mental illness. “People come to our fellowship feeling like there’s a void in their training,” Dr. Suo said. “We’re able to fill that void. In the first year, we had a fellow who said that, after completing the program, she felt physically less tired at the end of the day, because she had been so anxious about what to do for her patients who came in with mental health complaints.
“Now she knows what to do, so she can relax and listen to them – and be more connected.”
In the spirit of paying his TNT PCP education forward, last year Dr. Eschweiler secured a Song Brown grant to implement a 2-day psychiatric course for family medicine NP students at Western University of Health Sciences in Pomona, Calif., where he holds a faculty post. After they completed the course, students spent 80 hours with Dr. Eschweiler or with a designated clinician in the clinic, where they applied what they learned.
“By the time we shared information with students who I received in the TNT PCP fellowship, their comfort level went up by 80%-90%,” he said.
Such stories are heartening to TNT PCP faculty. “We simply do not have enough psychiatrists in the country to provide mental health care for everybody who needs it,” Dr. Suo said. “Unfortunately, that means primary care will have to take up the slack. We want to prepare people to be able to do that.”
For information about the 2020 TNT PCP Fellowship, visit www.psychiatry.uci.edu/tnt.
dbrunk@mdedge.com
Program’s aim is to ‘optimize and expand the psychiatric workforce’
Program’s aim is to ‘optimize and expand the psychiatric workforce’
ORANGE, CALIF. – When Shannon Suo, MD, reached her family medicine rotation as a medical student at the University of Cincinnati, she recalls that the faculty “seemed a little lost” when they encountered patients who presented with psychiatric issues.
“If it were diabetes, high blood pressure, or back pain, they knew what to do; they had a plan,” said Dr. Suo, who is now training director for the combined family medicine/psychiatry residency at the University of California, Davis. “But when it came to depression, anxiety, maybe even some psychosis, they kind of looked a little nervous. Maybe they prescribed an antidepressant, but then they were like, ‘I don’t know what else to do for you.’ I felt uncomfortable with that.”
Eric Eschweiler, DNP, can identify with that sentiment. After he assumed the medical directorship of a federally qualified health center that opened 4 years ago in San Bernardino, Calif., nearly every person who presented for treatment had an underlying psychiatric disorder, from depression and anxiety to bipolar disorder and substance abuse stemming from childhood trauma.
“I really felt unprepared to take care of that population because with those mental health disorders, we would get a lot of concomitant chronic medical conditions,” Dr. Eschweiler said. “But they were not adherent, not compliant because they couldn’t control the mental health aspects, or we couldn’t get a handle on those issues.
“In [nurse practitioner] training, we really don’t get a whole lot of education related to the psychiatric component of health care,” he said. “I have a doctorate, but even with that, we didn’t get very much.”
When Dr. Eschweiler learned about a yearlong clinical fellowship designed for primary care–oriented trainees and clinicians who wish to receive advanced training in primary care psychiatry, he applied, “hoping I’d be first in line,” he said. He earned a spot in the 2018 class.
The program, known as the UC Irvine/UC Davis Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship, launched in 2016 as a way to train primary care clinicians to diagnose and treat the most common psychiatric conditions found in the primary care setting. The fellowship is aimed at educating not only MDs and DOs, but physician assistants, nurse practitioners, and pharmacists as well.
“We want to give high-quality training to as many people as we can in order to optimize and expand the psychiatric workforce,” said Robert M. McCarron, DO, founding codirector of the fellowship program, and professor and vice chair of education and integrated care at the University of California, Irvine. “That’s really what we’re trying to do here.”
According to Dr. McCarron, who founded the fellowship with seed money during his tenure as a faculty member at UC Davis, up to 60% of all behavioral health care is provided in the busy primary setting, and as many as 80% of antidepressants are prescribed by nonpsychiatrists. Couple that with the current psychiatric workforce shortage “and you have a perfect storm,” he said.
“The toughest part is the volume of patients primary care providers have to see, comingled with a high frequency of comorbid mental illness. Added on to that, they didn’t get the training for much of the conditions they’re trying to address or treat. A primary care provider can refer to a psychiatrist, but there is not a large referral base. In addition, plenty of studies show that about half of those patients don’t end up seeing a mental health provider. Resources are limited in many areas of the country.”
TNT PCP fellows participate in the year-long program during nonclinic hours and accrue more than 45 CME units. Much of the training involves online courses framed around the textbook Primary Care Psychiatry (Wolters Kluwer, 2019), but fellows also are required to attend two intensive weekend sessions where they drill down on core topics, such as how to conduct an effective psychiatric interview, techniques, and how to screen and treat for psychiatric disorders.
“It’s not just about medicine options,” said Jaesu Han, MD, a fellowship faculty member who is board certified in psychiatry and family medicine. “For example, we try to focus on basic principles of cognitive-behavioral therapy, the role of psychotherapy, and the role of motivational interviewing. We try to help the providers recognize that there are other tools available [besides medication]. Those principles are going to help them engage with their patients more and have a larger impact.”
During one of the intensive weekend sessions, fellows are required to give a PowerPoint lecture on a topic of their choosing, with the idea of getting them comfortable with training their primary care colleagues on principles they learn.
“Some fellows are training their staff on their lunch breaks, or they’re implementing different tools they learned in our program to their organization,” said Wendy Cant, MBA, who is TNT PCP’s administrative director.
During a PowerPoint presentation a few years ago, one fellow chose to lecture about how to administer long-term antipsychotic medication in the primary care setting, a topic that the TNT PCP faculty had not thought to recommend. “We thought that was something a primary care provider would punt on,” said Dr. Suo, who is codirector of the fellowship program. “The fellow said, ‘No. This makes absolute sense. Our offices are equipped to do IM injections; our medical staff are trained to do that. It’s something that improves adherence and outcomes. It stabilizes people, and it makes us better able to take care of that population and not have to punt.’ That was unexpected and gratifying to know that we increased his comfort level to that point.”
Fellows also participate in two interactive learning sessions per month with faculty members, and each is paired with a faculty mentor, with whom they meet monthly in person or by video call or phone call.
“One of my fellows is feeling overwhelmed because she’s been tasked with taking care of the general psychiatric population, to do initial triage before she refers off to one of very few psychiatrists,” said Matthew Reed, MD, MSPH, one of the faculty mentors who serves as director of education for TNT PCP. “Her biggest concern is, ‘How do I not hate what I’m doing with my job because it’s so stressful and all patients want are opioid analgesics, or all they want are some benzodiazepines? They always want something, and I can’t get them to do what I want them to do.’ ” Together, they discuss various tools that might help her situation, such as using motivational interviewing to foster a sense of collaboration with patients.
Dr. Reed used the analogy of skeet shooting to illustrate the point that, without inviting patients to share in decision making, they are more likely to “shoot down” interventions that clinicians suggest to them.
Currently, about 80% of the program’s 118 fellows practice in California, there is a similar proportion of men and women, and their experience levels range from trainees to seasoned clinicians.
“They have 10, 15, and in some cases 25 years of experience, and they’re saying, ‘I‘ve been practicing for years and have been struggling when it comes to psychiatric issues. I want to do better,’ ” Dr. Suo noted.
Tuition is $15,500, and it usually is covered by outside funding sources. Last year, the program received a $1 million grant from California’s Office of Statewide Health Planning and Development, which was enough to fund tuition for 62 fellows. TNT PCP administrators and faculty also formed sponsor partnerships with several health plans and counties, including Inland Empire Health Plan, LA Care, Alameda Health Consortium, and Fresno County.
Employers also have stepped up. Cedars-Sinai Medical Center recently supplied a grant to fund tuition for 30 affiliated clinicians from different service planning areas. Other organizations that support funding for the fellowship include the American Academy of Pediatrics, the California Academy of Family Physicians, the California Behavioral Health Directors Association, and the California Psychiatric Association.
Meanwhile, word about the TNT PCP program is spreading to clinicians in other states. “By far, the hardest part is helping fellows from out of state find funding in their area,” Ms. Cant said. “That’s new territory we are starting to look into, but typically it’s not out of pocket. We only had three people pay out of pocket last year.”
Program faculty hope to not only improve the fellows’ knowledge in psychiatry and better treatment for depression, anxiety, and substance abuse, but also decrease prescription of opioids and decrease the stigma that some clinicians might perceive in mental illness. “People come to our fellowship feeling like there’s a void in their training,” Dr. Suo said. “We’re able to fill that void. In the first year, we had a fellow who said that, after completing the program, she felt physically less tired at the end of the day, because she had been so anxious about what to do for her patients who came in with mental health complaints.
“Now she knows what to do, so she can relax and listen to them – and be more connected.”
In the spirit of paying his TNT PCP education forward, last year Dr. Eschweiler secured a Song Brown grant to implement a 2-day psychiatric course for family medicine NP students at Western University of Health Sciences in Pomona, Calif., where he holds a faculty post. After they completed the course, students spent 80 hours with Dr. Eschweiler or with a designated clinician in the clinic, where they applied what they learned.
“By the time we shared information with students who I received in the TNT PCP fellowship, their comfort level went up by 80%-90%,” he said.
Such stories are heartening to TNT PCP faculty. “We simply do not have enough psychiatrists in the country to provide mental health care for everybody who needs it,” Dr. Suo said. “Unfortunately, that means primary care will have to take up the slack. We want to prepare people to be able to do that.”
For information about the 2020 TNT PCP Fellowship, visit www.psychiatry.uci.edu/tnt.
dbrunk@mdedge.com
ORANGE, CALIF. – When Shannon Suo, MD, reached her family medicine rotation as a medical student at the University of Cincinnati, she recalls that the faculty “seemed a little lost” when they encountered patients who presented with psychiatric issues.
“If it were diabetes, high blood pressure, or back pain, they knew what to do; they had a plan,” said Dr. Suo, who is now training director for the combined family medicine/psychiatry residency at the University of California, Davis. “But when it came to depression, anxiety, maybe even some psychosis, they kind of looked a little nervous. Maybe they prescribed an antidepressant, but then they were like, ‘I don’t know what else to do for you.’ I felt uncomfortable with that.”
Eric Eschweiler, DNP, can identify with that sentiment. After he assumed the medical directorship of a federally qualified health center that opened 4 years ago in San Bernardino, Calif., nearly every person who presented for treatment had an underlying psychiatric disorder, from depression and anxiety to bipolar disorder and substance abuse stemming from childhood trauma.
“I really felt unprepared to take care of that population because with those mental health disorders, we would get a lot of concomitant chronic medical conditions,” Dr. Eschweiler said. “But they were not adherent, not compliant because they couldn’t control the mental health aspects, or we couldn’t get a handle on those issues.
“In [nurse practitioner] training, we really don’t get a whole lot of education related to the psychiatric component of health care,” he said. “I have a doctorate, but even with that, we didn’t get very much.”
When Dr. Eschweiler learned about a yearlong clinical fellowship designed for primary care–oriented trainees and clinicians who wish to receive advanced training in primary care psychiatry, he applied, “hoping I’d be first in line,” he said. He earned a spot in the 2018 class.
The program, known as the UC Irvine/UC Davis Train New Trainers (TNT) Primary Care Psychiatry (PCP) Fellowship, launched in 2016 as a way to train primary care clinicians to diagnose and treat the most common psychiatric conditions found in the primary care setting. The fellowship is aimed at educating not only MDs and DOs, but physician assistants, nurse practitioners, and pharmacists as well.
“We want to give high-quality training to as many people as we can in order to optimize and expand the psychiatric workforce,” said Robert M. McCarron, DO, founding codirector of the fellowship program, and professor and vice chair of education and integrated care at the University of California, Irvine. “That’s really what we’re trying to do here.”
According to Dr. McCarron, who founded the fellowship with seed money during his tenure as a faculty member at UC Davis, up to 60% of all behavioral health care is provided in the busy primary setting, and as many as 80% of antidepressants are prescribed by nonpsychiatrists. Couple that with the current psychiatric workforce shortage “and you have a perfect storm,” he said.
“The toughest part is the volume of patients primary care providers have to see, comingled with a high frequency of comorbid mental illness. Added on to that, they didn’t get the training for much of the conditions they’re trying to address or treat. A primary care provider can refer to a psychiatrist, but there is not a large referral base. In addition, plenty of studies show that about half of those patients don’t end up seeing a mental health provider. Resources are limited in many areas of the country.”
TNT PCP fellows participate in the year-long program during nonclinic hours and accrue more than 45 CME units. Much of the training involves online courses framed around the textbook Primary Care Psychiatry (Wolters Kluwer, 2019), but fellows also are required to attend two intensive weekend sessions where they drill down on core topics, such as how to conduct an effective psychiatric interview, techniques, and how to screen and treat for psychiatric disorders.
“It’s not just about medicine options,” said Jaesu Han, MD, a fellowship faculty member who is board certified in psychiatry and family medicine. “For example, we try to focus on basic principles of cognitive-behavioral therapy, the role of psychotherapy, and the role of motivational interviewing. We try to help the providers recognize that there are other tools available [besides medication]. Those principles are going to help them engage with their patients more and have a larger impact.”
During one of the intensive weekend sessions, fellows are required to give a PowerPoint lecture on a topic of their choosing, with the idea of getting them comfortable with training their primary care colleagues on principles they learn.
“Some fellows are training their staff on their lunch breaks, or they’re implementing different tools they learned in our program to their organization,” said Wendy Cant, MBA, who is TNT PCP’s administrative director.
During a PowerPoint presentation a few years ago, one fellow chose to lecture about how to administer long-term antipsychotic medication in the primary care setting, a topic that the TNT PCP faculty had not thought to recommend. “We thought that was something a primary care provider would punt on,” said Dr. Suo, who is codirector of the fellowship program. “The fellow said, ‘No. This makes absolute sense. Our offices are equipped to do IM injections; our medical staff are trained to do that. It’s something that improves adherence and outcomes. It stabilizes people, and it makes us better able to take care of that population and not have to punt.’ That was unexpected and gratifying to know that we increased his comfort level to that point.”
Fellows also participate in two interactive learning sessions per month with faculty members, and each is paired with a faculty mentor, with whom they meet monthly in person or by video call or phone call.
“One of my fellows is feeling overwhelmed because she’s been tasked with taking care of the general psychiatric population, to do initial triage before she refers off to one of very few psychiatrists,” said Matthew Reed, MD, MSPH, one of the faculty mentors who serves as director of education for TNT PCP. “Her biggest concern is, ‘How do I not hate what I’m doing with my job because it’s so stressful and all patients want are opioid analgesics, or all they want are some benzodiazepines? They always want something, and I can’t get them to do what I want them to do.’ ” Together, they discuss various tools that might help her situation, such as using motivational interviewing to foster a sense of collaboration with patients.
Dr. Reed used the analogy of skeet shooting to illustrate the point that, without inviting patients to share in decision making, they are more likely to “shoot down” interventions that clinicians suggest to them.
Currently, about 80% of the program’s 118 fellows practice in California, there is a similar proportion of men and women, and their experience levels range from trainees to seasoned clinicians.
“They have 10, 15, and in some cases 25 years of experience, and they’re saying, ‘I‘ve been practicing for years and have been struggling when it comes to psychiatric issues. I want to do better,’ ” Dr. Suo noted.
Tuition is $15,500, and it usually is covered by outside funding sources. Last year, the program received a $1 million grant from California’s Office of Statewide Health Planning and Development, which was enough to fund tuition for 62 fellows. TNT PCP administrators and faculty also formed sponsor partnerships with several health plans and counties, including Inland Empire Health Plan, LA Care, Alameda Health Consortium, and Fresno County.
Employers also have stepped up. Cedars-Sinai Medical Center recently supplied a grant to fund tuition for 30 affiliated clinicians from different service planning areas. Other organizations that support funding for the fellowship include the American Academy of Pediatrics, the California Academy of Family Physicians, the California Behavioral Health Directors Association, and the California Psychiatric Association.
Meanwhile, word about the TNT PCP program is spreading to clinicians in other states. “By far, the hardest part is helping fellows from out of state find funding in their area,” Ms. Cant said. “That’s new territory we are starting to look into, but typically it’s not out of pocket. We only had three people pay out of pocket last year.”
Program faculty hope to not only improve the fellows’ knowledge in psychiatry and better treatment for depression, anxiety, and substance abuse, but also decrease prescription of opioids and decrease the stigma that some clinicians might perceive in mental illness. “People come to our fellowship feeling like there’s a void in their training,” Dr. Suo said. “We’re able to fill that void. In the first year, we had a fellow who said that, after completing the program, she felt physically less tired at the end of the day, because she had been so anxious about what to do for her patients who came in with mental health complaints.
“Now she knows what to do, so she can relax and listen to them – and be more connected.”
In the spirit of paying his TNT PCP education forward, last year Dr. Eschweiler secured a Song Brown grant to implement a 2-day psychiatric course for family medicine NP students at Western University of Health Sciences in Pomona, Calif., where he holds a faculty post. After they completed the course, students spent 80 hours with Dr. Eschweiler or with a designated clinician in the clinic, where they applied what they learned.
“By the time we shared information with students who I received in the TNT PCP fellowship, their comfort level went up by 80%-90%,” he said.
Such stories are heartening to TNT PCP faculty. “We simply do not have enough psychiatrists in the country to provide mental health care for everybody who needs it,” Dr. Suo said. “Unfortunately, that means primary care will have to take up the slack. We want to prepare people to be able to do that.”
For information about the 2020 TNT PCP Fellowship, visit www.psychiatry.uci.edu/tnt.
dbrunk@mdedge.com