User login
Lesions on face, arms, and legs
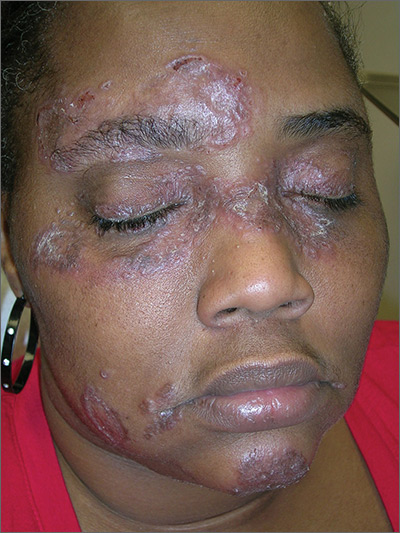
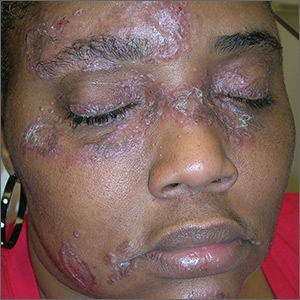
The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Can You Put Your Finger on the Diagnosis?
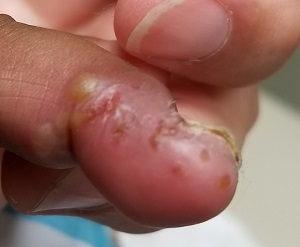
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.
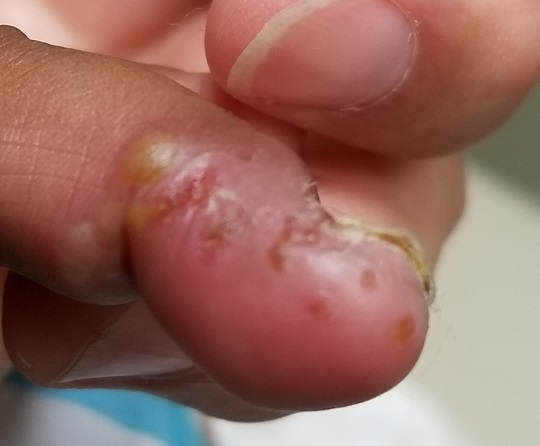
EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
Poverty, incarceration may drive deaths from drug use
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: Reduced household income and increased incarceration are significantly associated with drug-related deaths in the U.S. population.
Major finding: High incarceration rates are associated with an increase in drug-related deaths of more than 50% at the county level.
Study details: The data come from a regression analysis of data from multiple institutions, including the U.S. National Vital Statistics System and the Institute for Health Metrics and Evaluation, as well as incarceration data from the Vera Institute of Justice for 2,640 U.S. counties from 1983 to 2014.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
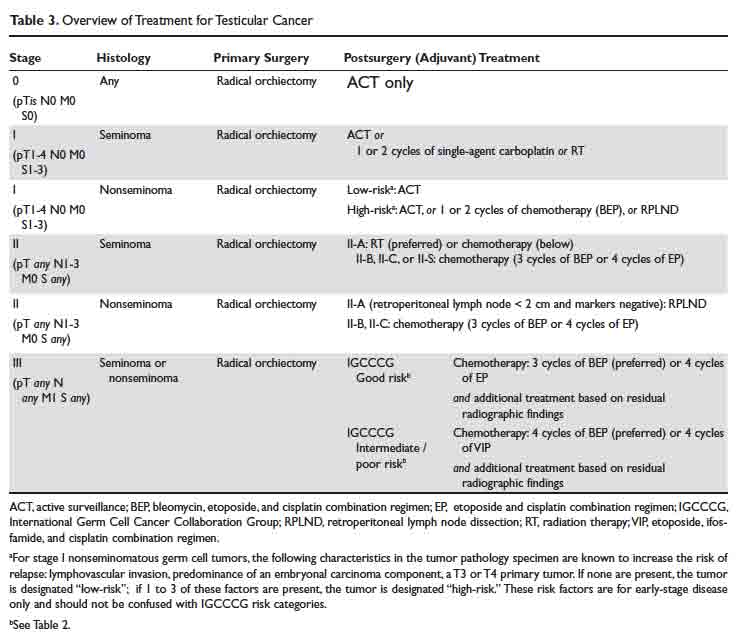
Testicular Cancer: Diagnosis and Treatment
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
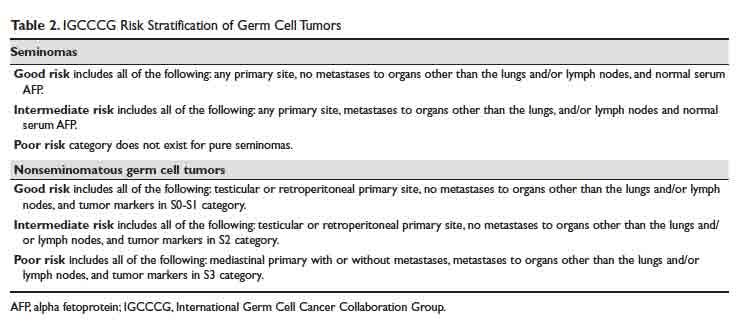
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.
The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
Can serum inflammatory markers predict concussion recovery?
Levels of interleukin-6 (IL-6) and IL-1 receptor antagonist (IL-1RA) are significantly elevated 6 hours after concussion, and higher IL-6 levels are associated with slower recovery, according to a study of 41 high school and college football players with concussion. The findings were published online ahead of print July 3 in Neurology.
“With so many people sustaining concussions and a sizeable number of them having prolonged symptoms and recovery, any tools we can develop to help determine who would be at greater risk of problems would be very beneficial,” said study author Timothy B. Meier, PhD, assistant professor of neurosurgery at the Medical College of Wisconsin in Milwaukee, in a news release. “These results are a crucial first step.”
Symptoms of sport-related concussion typically resolve within 1-2 weeks but may last longer. Although prior studies have focused on biomarkers that are specific to brain injury, nonspecific inflammatory markers also may hold promise in predicting recovery after a mild traumatic brain injury, the authors said.
To examine whether acute elevations in serum inflammatory markers predict symptom recovery following sport-related concussion, Dr. Meier and his research colleagues enrolled 857 high school and college football players into a prospective cohort study. They included in their analyses 41 concussed athletes and 43 matched control athletes with an average age of 18 years. None of the concussed athletes lost consciousness, two had posttraumatic amnesia, and one had retrograde amnesia. The concussed athletes had a mean symptom duration of 8.86 days.
The researchers measured serum levels of IL-6, IL-1RA, IL-1 beta, IL-10, tumor necrosis factor, C-reactive protein, and interferon-gamma and recorded Sport Concussion Assessment Tool, 3rd edition, symptom severity scores.
Participants with concussion underwent testing at the start of the season, within 6 hours of injury, 24-48 hours after injury, and at 8, 15, and 45 days after injury. Control athletes underwent testing at similar times.
Among athletes with concussion, IL-1RA and IL-6 were elevated at 6 hours, compared with all other postinjury visits and with controls. IL-6 and IL-1RA significantly discriminated concussed from control athletes at 6 hours postconcussion with an area under the receiver operating characteristic curve of 0.79 for IL-6 and 0.79 for IL-1RA. Furthermore, IL-6 levels at 6 hours significantly correlated with symptom duration, “with a 1-unit increase in natural log-transformed IL-6 associated with 39% lower hazard of symptom recovery,” the researchers reported.
The extent to which these results generalize to females, youth athletes, or athletes who develop postconcussion syndrome is unclear, and larger studies may be needed to adequately assess inflammatory markers as clinical biomarkers of sport-related concussion, the authors noted.
“Eventually, these results may help us better understand the relationship between injury and inflammation and potentially lead to new treatments,” Dr. Meier said.
The research was supported by the U.S. Department of Defense, National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences, National Institute of Mental Health, and the National Center for Advancing Translational Sciences. The authors had no relevant disclosures.
SOURCE: Nitta ME et al. Neurology. 2019 Jul 3. doi: 10.1212/WNL.0000000000007864.
Levels of interleukin-6 (IL-6) and IL-1 receptor antagonist (IL-1RA) are significantly elevated 6 hours after concussion, and higher IL-6 levels are associated with slower recovery, according to a study of 41 high school and college football players with concussion. The findings were published online ahead of print July 3 in Neurology.
“With so many people sustaining concussions and a sizeable number of them having prolonged symptoms and recovery, any tools we can develop to help determine who would be at greater risk of problems would be very beneficial,” said study author Timothy B. Meier, PhD, assistant professor of neurosurgery at the Medical College of Wisconsin in Milwaukee, in a news release. “These results are a crucial first step.”
Symptoms of sport-related concussion typically resolve within 1-2 weeks but may last longer. Although prior studies have focused on biomarkers that are specific to brain injury, nonspecific inflammatory markers also may hold promise in predicting recovery after a mild traumatic brain injury, the authors said.
To examine whether acute elevations in serum inflammatory markers predict symptom recovery following sport-related concussion, Dr. Meier and his research colleagues enrolled 857 high school and college football players into a prospective cohort study. They included in their analyses 41 concussed athletes and 43 matched control athletes with an average age of 18 years. None of the concussed athletes lost consciousness, two had posttraumatic amnesia, and one had retrograde amnesia. The concussed athletes had a mean symptom duration of 8.86 days.
The researchers measured serum levels of IL-6, IL-1RA, IL-1 beta, IL-10, tumor necrosis factor, C-reactive protein, and interferon-gamma and recorded Sport Concussion Assessment Tool, 3rd edition, symptom severity scores.
Participants with concussion underwent testing at the start of the season, within 6 hours of injury, 24-48 hours after injury, and at 8, 15, and 45 days after injury. Control athletes underwent testing at similar times.
Among athletes with concussion, IL-1RA and IL-6 were elevated at 6 hours, compared with all other postinjury visits and with controls. IL-6 and IL-1RA significantly discriminated concussed from control athletes at 6 hours postconcussion with an area under the receiver operating characteristic curve of 0.79 for IL-6 and 0.79 for IL-1RA. Furthermore, IL-6 levels at 6 hours significantly correlated with symptom duration, “with a 1-unit increase in natural log-transformed IL-6 associated with 39% lower hazard of symptom recovery,” the researchers reported.
The extent to which these results generalize to females, youth athletes, or athletes who develop postconcussion syndrome is unclear, and larger studies may be needed to adequately assess inflammatory markers as clinical biomarkers of sport-related concussion, the authors noted.
“Eventually, these results may help us better understand the relationship between injury and inflammation and potentially lead to new treatments,” Dr. Meier said.
The research was supported by the U.S. Department of Defense, National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences, National Institute of Mental Health, and the National Center for Advancing Translational Sciences. The authors had no relevant disclosures.
SOURCE: Nitta ME et al. Neurology. 2019 Jul 3. doi: 10.1212/WNL.0000000000007864.
Levels of interleukin-6 (IL-6) and IL-1 receptor antagonist (IL-1RA) are significantly elevated 6 hours after concussion, and higher IL-6 levels are associated with slower recovery, according to a study of 41 high school and college football players with concussion. The findings were published online ahead of print July 3 in Neurology.
“With so many people sustaining concussions and a sizeable number of them having prolonged symptoms and recovery, any tools we can develop to help determine who would be at greater risk of problems would be very beneficial,” said study author Timothy B. Meier, PhD, assistant professor of neurosurgery at the Medical College of Wisconsin in Milwaukee, in a news release. “These results are a crucial first step.”
Symptoms of sport-related concussion typically resolve within 1-2 weeks but may last longer. Although prior studies have focused on biomarkers that are specific to brain injury, nonspecific inflammatory markers also may hold promise in predicting recovery after a mild traumatic brain injury, the authors said.
To examine whether acute elevations in serum inflammatory markers predict symptom recovery following sport-related concussion, Dr. Meier and his research colleagues enrolled 857 high school and college football players into a prospective cohort study. They included in their analyses 41 concussed athletes and 43 matched control athletes with an average age of 18 years. None of the concussed athletes lost consciousness, two had posttraumatic amnesia, and one had retrograde amnesia. The concussed athletes had a mean symptom duration of 8.86 days.
The researchers measured serum levels of IL-6, IL-1RA, IL-1 beta, IL-10, tumor necrosis factor, C-reactive protein, and interferon-gamma and recorded Sport Concussion Assessment Tool, 3rd edition, symptom severity scores.
Participants with concussion underwent testing at the start of the season, within 6 hours of injury, 24-48 hours after injury, and at 8, 15, and 45 days after injury. Control athletes underwent testing at similar times.
Among athletes with concussion, IL-1RA and IL-6 were elevated at 6 hours, compared with all other postinjury visits and with controls. IL-6 and IL-1RA significantly discriminated concussed from control athletes at 6 hours postconcussion with an area under the receiver operating characteristic curve of 0.79 for IL-6 and 0.79 for IL-1RA. Furthermore, IL-6 levels at 6 hours significantly correlated with symptom duration, “with a 1-unit increase in natural log-transformed IL-6 associated with 39% lower hazard of symptom recovery,” the researchers reported.
The extent to which these results generalize to females, youth athletes, or athletes who develop postconcussion syndrome is unclear, and larger studies may be needed to adequately assess inflammatory markers as clinical biomarkers of sport-related concussion, the authors noted.
“Eventually, these results may help us better understand the relationship between injury and inflammation and potentially lead to new treatments,” Dr. Meier said.
The research was supported by the U.S. Department of Defense, National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences, National Institute of Mental Health, and the National Center for Advancing Translational Sciences. The authors had no relevant disclosures.
SOURCE: Nitta ME et al. Neurology. 2019 Jul 3. doi: 10.1212/WNL.0000000000007864.
FROM NEUROLOGY
Key clinical point: Serum biomarkers of inflammation may help identify which athletes will take longer to recover after a sport-related concussion.
Major finding: IL-6 and IL-1RA significantly discriminated concussed from control athletes at 6 hours postconcussion with an area under the receiver operating characteristic curve of 0.79 for IL-6 and 0.79 for IL-1RA. Furthermore, IL-6 levels at 6 hours significantly correlated with symptom duration.
Study details: A prospective cohort study of high school and college football players. The analyses included 41 concussed athletes and 43 matched control athletes with an average age of about 18 years.
Disclosures: The research was supported by the U.S. Department of Defense, National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences, National Institute of Mental Health, and the National Center for Advancing Translational Sciences. The authors had no relevant disclosures.
Source: Nitta ME et al. Neurology. 2019 Jul 3. doi: 10.1212/WNL.0000000000007864.
FDA approves Xpovio for relapsed/refractory multiple myeloma
The oral therapy was approved for patients who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody, according to the FDA.
The approval provides a “treatment option for patients with multiple myeloma with no (other) available therapy,” said Richard Pazdur, MD, director of the FDA Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research.
The approval was based on a study that included 83 patients with RRMM who had an overall response rate of 25.3% to Xpovio in combination with dexamethasone.
“The median time to first response was 4 weeks, with a range of 1-10 weeks. The median duration of response was 3.8 months. The efficacy evaluation was supported by additional information from an ongoing, randomized trial in patients with multiple myeloma,” according to the statement.
Common side effects seen in patients taking Xpovio in combination with dexamethasone include leukopenia, neutropenia, thrombocytopenia, and anemia. Patients also reported vomiting, nausea, fatigue, diarrhea, fever, decreased appetite and weight, constipation, upper respiratory tract infections, and hyponatremia.
Patients taking Xpovio should be monitored for low blood counts, platelets, and sodium levels, and should avoid other medications that may cause dizziness or confusion. Patients’ hydration status, blood counts, and other medications should be optimized to avoid dizziness or confusion. Females of reproductive age and males with a female partner of reproductive potential must use effective contraception during treatment with Xpovio. Women who are pregnant or breastfeeding should not take Xpovio.
Xpovio must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Xpovio in combination with dexamethasone was granted accelerated approval, and further clinical trials are required to verify and describe the drug’s clinical benefit.
The FDA granted the approval of Xpovio to Karyopharm Therapeutics.
The oral therapy was approved for patients who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody, according to the FDA.
The approval provides a “treatment option for patients with multiple myeloma with no (other) available therapy,” said Richard Pazdur, MD, director of the FDA Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research.
The approval was based on a study that included 83 patients with RRMM who had an overall response rate of 25.3% to Xpovio in combination with dexamethasone.
“The median time to first response was 4 weeks, with a range of 1-10 weeks. The median duration of response was 3.8 months. The efficacy evaluation was supported by additional information from an ongoing, randomized trial in patients with multiple myeloma,” according to the statement.
Common side effects seen in patients taking Xpovio in combination with dexamethasone include leukopenia, neutropenia, thrombocytopenia, and anemia. Patients also reported vomiting, nausea, fatigue, diarrhea, fever, decreased appetite and weight, constipation, upper respiratory tract infections, and hyponatremia.
Patients taking Xpovio should be monitored for low blood counts, platelets, and sodium levels, and should avoid other medications that may cause dizziness or confusion. Patients’ hydration status, blood counts, and other medications should be optimized to avoid dizziness or confusion. Females of reproductive age and males with a female partner of reproductive potential must use effective contraception during treatment with Xpovio. Women who are pregnant or breastfeeding should not take Xpovio.
Xpovio must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Xpovio in combination with dexamethasone was granted accelerated approval, and further clinical trials are required to verify and describe the drug’s clinical benefit.
The FDA granted the approval of Xpovio to Karyopharm Therapeutics.
The oral therapy was approved for patients who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody, according to the FDA.
The approval provides a “treatment option for patients with multiple myeloma with no (other) available therapy,” said Richard Pazdur, MD, director of the FDA Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research.
The approval was based on a study that included 83 patients with RRMM who had an overall response rate of 25.3% to Xpovio in combination with dexamethasone.
“The median time to first response was 4 weeks, with a range of 1-10 weeks. The median duration of response was 3.8 months. The efficacy evaluation was supported by additional information from an ongoing, randomized trial in patients with multiple myeloma,” according to the statement.
Common side effects seen in patients taking Xpovio in combination with dexamethasone include leukopenia, neutropenia, thrombocytopenia, and anemia. Patients also reported vomiting, nausea, fatigue, diarrhea, fever, decreased appetite and weight, constipation, upper respiratory tract infections, and hyponatremia.
Patients taking Xpovio should be monitored for low blood counts, platelets, and sodium levels, and should avoid other medications that may cause dizziness or confusion. Patients’ hydration status, blood counts, and other medications should be optimized to avoid dizziness or confusion. Females of reproductive age and males with a female partner of reproductive potential must use effective contraception during treatment with Xpovio. Women who are pregnant or breastfeeding should not take Xpovio.
Xpovio must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks.
Xpovio in combination with dexamethasone was granted accelerated approval, and further clinical trials are required to verify and describe the drug’s clinical benefit.
The FDA granted the approval of Xpovio to Karyopharm Therapeutics.
Feasibility—and safety—of reducing the traditional 14 prenatal visits to 8 or 10
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.
Comment & Controversy
WHAT IS YOUR APPROACH TO THE PERSISTENT OCCIPUT POSTERIOR MALPOSITION?
ROBERT L. BARBIERI, MD
(EDITORIAL; MARCH 2019)
A classic approach for managing fetal malposition
For those of us who trained and practiced obstetrics in the days of the 6% primary cesarean delivery (CD) rate, we never considered the management of the persistent occiput
- The cervix must be fully dilated.
- Dense regional anesthesia must be achieved.
- The vertex must have reached +1 station.
- The position must be clearly established, and this does not require anything other than the ability to palpate an ear, as it can be pointed only in one direction. If you feel ultrasonography is needed, be my guest.
- Use an obstetric lubricant to reduce resistance and minimize lacerations.
- While a trial of manual rotation is reasonable, it commonly will not succeed and requires that an operator’s hand be inserted rather than a slender and less traumatic device (forceps).
- Next, palpate the sagittal suture to determine whether the position is straight OP versus left OP or right OP. This should not be difficult unless the poor woman has gone through 2 or 3 hours of unproductive pushing, thereby creating caput.
- After proper forceps application is confirmed, gently apply upward pressure. This will make rotation easier.
- Dr. Irving’s recommendations notwithstanding, the forceps handles are not carried in a wide sweep. One should use Kielland’s forceps, which do not have a pelvic curve and were invented for this precise indication. The forceps are simply rotated.
- Try to avoid delivery as an OP, as this pulls a much larger diameter deflexed head through the pelvis and usually results in significant lacerations.
- Episiotomy is not always required if rotation has succeeded.
- Once descent to the outlet has been achieved, it is probably best to switch to a forceps with a pelvic curve to achieve easier extension.
- This should complete the delivery, but as a general rule, if more than minimal resistance is met in any of the above steps, abandon the procedure and move to CD.
- This process should result in at least a 70% success rate.
As is most likely understood by the current generation of obstetricians who appear to be satisfied with a 30% to 40% primary CD rate, the above reflects the views of a long-retired ObGyn (whose CD rate never exceeded 10%) and may be inappropriate for those who are not adequately trained in or comfortable with vaginal obstetrics.
David M. Priver, MD
San Diego, California
Continue to: HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Membrane stripping can be problematic
The recent discussion on stripping membranes to facilitate the initiation of labor and delivery was intriguing. This practice was reviewed extensively during my training in the 1960s and abandoned when the results were disappointing or contradictory. Although the practice has been revitalized recently, I am concerned that potential risks and the absence of a recommended protocol of safeguards may allow new problems to develop.
In a metropolitan community where I provide consultative services, the only patients I see for evaluation of pregnancies beyond 40 to 41 weeks come from providers who are non-physicians. Apparently, they are concerned that they may have to turn their patients over to physician providers for interventions that they are not capable of doing. My advice to them is simply that nothing good happens after 40 to 41 weeks.
Well-grown babies may continue to grow if they are healthy, and they may incur greater risks of dystotic labor and delivery resulting in injury or the need for physician-administered surgical assistance. If, on the other hand, growth markedly diminishes or ceases, fetal harm or neonatal complications may occur through asphyxia, meconium aspiration, or trauma. In either event, physician-based assistance is strongly encouraged, as long as due diligence in determining gestational age has been done.
Promoting membrane stripping without having a protocol for ascertainment of risk factors is worrisome to me. In my opinion, large population studies that fail to demonstrate increased risks of infection may fail to demonstrate that membrane stripping may induce a degree of perinatal infection comparable to that of prolonged labor with multiple internal examinations with or without ultimate cesarean birth. Prior to considering membrane stripping as a strategy, one should recognize certain important considerations, namely:
- Patients most in need of active intervention may have the least favorable cervical findings, and as a result they are potentially at risk for the greatest discomfort.
- The frequency of group B streptococcal colonization of the vagina at term should be recognized, and a culture should be obtained immediately prior to intervention. When a culture is a positive, membrane stripping should be avoided, or at least a sober consideration of its use and appropriate antibiotic coverage should occur.
- Consider performing transvaginal ultrasonography prior to membrane stripping to exclude the possibility of a placental edge close enough to be encountered and compromised, with resultant hemorrhage in an outpatient venue ill equipped to provide adequate emergency support.
- The comparative effectiveness of other direct cervical conditioning therapies, including use of a Foley catheter or regional prostaglandin medication, has been well explored and found effective. Also, if one takes seriously the need for any intervention, admission to the hospital for overnight cervical conditioning allows for surveillance and avoids the patient experience of being sent home cramping, bleeding, brooding infection, and questioning her trust in the provider.
Continue to: I am concerned that the promotion...
I am concerned that the promotion of this potentially rather brutish practice by highly reputable advisors can result in its growing utilization by providers some of whom may be least qualified to apply proper judgment and sensitivity to its selection. In the most primitive of circumstances, it may have utility. Personally, however, I feel that medically based strategies initiated and monitored by professionals capable of dealing with any untoward departures from the expected results must be considered in the best traditions of what we do. The appeal of simplicity must not encourage the adoption of interventions that lack the proper application of thought and plan and whose only appeal is that of simplicity.
Richard P. Perkins, MD
Fort Myers, Florida; Stockton, California
Dr. Barbieri responds
I thank Dr. Priver for his excellent description of how to use forceps to resolve a persistent occiput posterior position. I also thank Dr. Perkins for his valuable comments and agree with him that in the United States among the options available for outpatient cervical ripening, misoprostol or a balloon are more commonly used than membrane stripping. Membrane stripping is an outpatient cervical ripening technique that is commonly used in the United Kingdom.
WHAT IS YOUR APPROACH TO THE PERSISTENT OCCIPUT POSTERIOR MALPOSITION?
ROBERT L. BARBIERI, MD
(EDITORIAL; MARCH 2019)
A classic approach for managing fetal malposition
For those of us who trained and practiced obstetrics in the days of the 6% primary cesarean delivery (CD) rate, we never considered the management of the persistent occiput
- The cervix must be fully dilated.
- Dense regional anesthesia must be achieved.
- The vertex must have reached +1 station.
- The position must be clearly established, and this does not require anything other than the ability to palpate an ear, as it can be pointed only in one direction. If you feel ultrasonography is needed, be my guest.
- Use an obstetric lubricant to reduce resistance and minimize lacerations.
- While a trial of manual rotation is reasonable, it commonly will not succeed and requires that an operator’s hand be inserted rather than a slender and less traumatic device (forceps).
- Next, palpate the sagittal suture to determine whether the position is straight OP versus left OP or right OP. This should not be difficult unless the poor woman has gone through 2 or 3 hours of unproductive pushing, thereby creating caput.
- After proper forceps application is confirmed, gently apply upward pressure. This will make rotation easier.
- Dr. Irving’s recommendations notwithstanding, the forceps handles are not carried in a wide sweep. One should use Kielland’s forceps, which do not have a pelvic curve and were invented for this precise indication. The forceps are simply rotated.
- Try to avoid delivery as an OP, as this pulls a much larger diameter deflexed head through the pelvis and usually results in significant lacerations.
- Episiotomy is not always required if rotation has succeeded.
- Once descent to the outlet has been achieved, it is probably best to switch to a forceps with a pelvic curve to achieve easier extension.
- This should complete the delivery, but as a general rule, if more than minimal resistance is met in any of the above steps, abandon the procedure and move to CD.
- This process should result in at least a 70% success rate.
As is most likely understood by the current generation of obstetricians who appear to be satisfied with a 30% to 40% primary CD rate, the above reflects the views of a long-retired ObGyn (whose CD rate never exceeded 10%) and may be inappropriate for those who are not adequately trained in or comfortable with vaginal obstetrics.
David M. Priver, MD
San Diego, California
Continue to: HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Membrane stripping can be problematic
The recent discussion on stripping membranes to facilitate the initiation of labor and delivery was intriguing. This practice was reviewed extensively during my training in the 1960s and abandoned when the results were disappointing or contradictory. Although the practice has been revitalized recently, I am concerned that potential risks and the absence of a recommended protocol of safeguards may allow new problems to develop.
In a metropolitan community where I provide consultative services, the only patients I see for evaluation of pregnancies beyond 40 to 41 weeks come from providers who are non-physicians. Apparently, they are concerned that they may have to turn their patients over to physician providers for interventions that they are not capable of doing. My advice to them is simply that nothing good happens after 40 to 41 weeks.
Well-grown babies may continue to grow if they are healthy, and they may incur greater risks of dystotic labor and delivery resulting in injury or the need for physician-administered surgical assistance. If, on the other hand, growth markedly diminishes or ceases, fetal harm or neonatal complications may occur through asphyxia, meconium aspiration, or trauma. In either event, physician-based assistance is strongly encouraged, as long as due diligence in determining gestational age has been done.
Promoting membrane stripping without having a protocol for ascertainment of risk factors is worrisome to me. In my opinion, large population studies that fail to demonstrate increased risks of infection may fail to demonstrate that membrane stripping may induce a degree of perinatal infection comparable to that of prolonged labor with multiple internal examinations with or without ultimate cesarean birth. Prior to considering membrane stripping as a strategy, one should recognize certain important considerations, namely:
- Patients most in need of active intervention may have the least favorable cervical findings, and as a result they are potentially at risk for the greatest discomfort.
- The frequency of group B streptococcal colonization of the vagina at term should be recognized, and a culture should be obtained immediately prior to intervention. When a culture is a positive, membrane stripping should be avoided, or at least a sober consideration of its use and appropriate antibiotic coverage should occur.
- Consider performing transvaginal ultrasonography prior to membrane stripping to exclude the possibility of a placental edge close enough to be encountered and compromised, with resultant hemorrhage in an outpatient venue ill equipped to provide adequate emergency support.
- The comparative effectiveness of other direct cervical conditioning therapies, including use of a Foley catheter or regional prostaglandin medication, has been well explored and found effective. Also, if one takes seriously the need for any intervention, admission to the hospital for overnight cervical conditioning allows for surveillance and avoids the patient experience of being sent home cramping, bleeding, brooding infection, and questioning her trust in the provider.
Continue to: I am concerned that the promotion...
I am concerned that the promotion of this potentially rather brutish practice by highly reputable advisors can result in its growing utilization by providers some of whom may be least qualified to apply proper judgment and sensitivity to its selection. In the most primitive of circumstances, it may have utility. Personally, however, I feel that medically based strategies initiated and monitored by professionals capable of dealing with any untoward departures from the expected results must be considered in the best traditions of what we do. The appeal of simplicity must not encourage the adoption of interventions that lack the proper application of thought and plan and whose only appeal is that of simplicity.
Richard P. Perkins, MD
Fort Myers, Florida; Stockton, California
Dr. Barbieri responds
I thank Dr. Priver for his excellent description of how to use forceps to resolve a persistent occiput posterior position. I also thank Dr. Perkins for his valuable comments and agree with him that in the United States among the options available for outpatient cervical ripening, misoprostol or a balloon are more commonly used than membrane stripping. Membrane stripping is an outpatient cervical ripening technique that is commonly used in the United Kingdom.
WHAT IS YOUR APPROACH TO THE PERSISTENT OCCIPUT POSTERIOR MALPOSITION?
ROBERT L. BARBIERI, MD
(EDITORIAL; MARCH 2019)
A classic approach for managing fetal malposition
For those of us who trained and practiced obstetrics in the days of the 6% primary cesarean delivery (CD) rate, we never considered the management of the persistent occiput
- The cervix must be fully dilated.
- Dense regional anesthesia must be achieved.
- The vertex must have reached +1 station.
- The position must be clearly established, and this does not require anything other than the ability to palpate an ear, as it can be pointed only in one direction. If you feel ultrasonography is needed, be my guest.
- Use an obstetric lubricant to reduce resistance and minimize lacerations.
- While a trial of manual rotation is reasonable, it commonly will not succeed and requires that an operator’s hand be inserted rather than a slender and less traumatic device (forceps).
- Next, palpate the sagittal suture to determine whether the position is straight OP versus left OP or right OP. This should not be difficult unless the poor woman has gone through 2 or 3 hours of unproductive pushing, thereby creating caput.
- After proper forceps application is confirmed, gently apply upward pressure. This will make rotation easier.
- Dr. Irving’s recommendations notwithstanding, the forceps handles are not carried in a wide sweep. One should use Kielland’s forceps, which do not have a pelvic curve and were invented for this precise indication. The forceps are simply rotated.
- Try to avoid delivery as an OP, as this pulls a much larger diameter deflexed head through the pelvis and usually results in significant lacerations.
- Episiotomy is not always required if rotation has succeeded.
- Once descent to the outlet has been achieved, it is probably best to switch to a forceps with a pelvic curve to achieve easier extension.
- This should complete the delivery, but as a general rule, if more than minimal resistance is met in any of the above steps, abandon the procedure and move to CD.
- This process should result in at least a 70% success rate.
As is most likely understood by the current generation of obstetricians who appear to be satisfied with a 30% to 40% primary CD rate, the above reflects the views of a long-retired ObGyn (whose CD rate never exceeded 10%) and may be inappropriate for those who are not adequately trained in or comfortable with vaginal obstetrics.
David M. Priver, MD
San Diego, California
Continue to: HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
HOW DO YOU FEEL ABOUT EXPECTANTLY MANAGING A WELL-DATED PREGNANCY PAST 41 WEEKS’ GESTATION?
ROBERT L. BARBIERI, MD
(EDITORIAL; FEBRUARY 2019)
Membrane stripping can be problematic
The recent discussion on stripping membranes to facilitate the initiation of labor and delivery was intriguing. This practice was reviewed extensively during my training in the 1960s and abandoned when the results were disappointing or contradictory. Although the practice has been revitalized recently, I am concerned that potential risks and the absence of a recommended protocol of safeguards may allow new problems to develop.
In a metropolitan community where I provide consultative services, the only patients I see for evaluation of pregnancies beyond 40 to 41 weeks come from providers who are non-physicians. Apparently, they are concerned that they may have to turn their patients over to physician providers for interventions that they are not capable of doing. My advice to them is simply that nothing good happens after 40 to 41 weeks.
Well-grown babies may continue to grow if they are healthy, and they may incur greater risks of dystotic labor and delivery resulting in injury or the need for physician-administered surgical assistance. If, on the other hand, growth markedly diminishes or ceases, fetal harm or neonatal complications may occur through asphyxia, meconium aspiration, or trauma. In either event, physician-based assistance is strongly encouraged, as long as due diligence in determining gestational age has been done.
Promoting membrane stripping without having a protocol for ascertainment of risk factors is worrisome to me. In my opinion, large population studies that fail to demonstrate increased risks of infection may fail to demonstrate that membrane stripping may induce a degree of perinatal infection comparable to that of prolonged labor with multiple internal examinations with or without ultimate cesarean birth. Prior to considering membrane stripping as a strategy, one should recognize certain important considerations, namely:
- Patients most in need of active intervention may have the least favorable cervical findings, and as a result they are potentially at risk for the greatest discomfort.
- The frequency of group B streptococcal colonization of the vagina at term should be recognized, and a culture should be obtained immediately prior to intervention. When a culture is a positive, membrane stripping should be avoided, or at least a sober consideration of its use and appropriate antibiotic coverage should occur.
- Consider performing transvaginal ultrasonography prior to membrane stripping to exclude the possibility of a placental edge close enough to be encountered and compromised, with resultant hemorrhage in an outpatient venue ill equipped to provide adequate emergency support.
- The comparative effectiveness of other direct cervical conditioning therapies, including use of a Foley catheter or regional prostaglandin medication, has been well explored and found effective. Also, if one takes seriously the need for any intervention, admission to the hospital for overnight cervical conditioning allows for surveillance and avoids the patient experience of being sent home cramping, bleeding, brooding infection, and questioning her trust in the provider.
Continue to: I am concerned that the promotion...
I am concerned that the promotion of this potentially rather brutish practice by highly reputable advisors can result in its growing utilization by providers some of whom may be least qualified to apply proper judgment and sensitivity to its selection. In the most primitive of circumstances, it may have utility. Personally, however, I feel that medically based strategies initiated and monitored by professionals capable of dealing with any untoward departures from the expected results must be considered in the best traditions of what we do. The appeal of simplicity must not encourage the adoption of interventions that lack the proper application of thought and plan and whose only appeal is that of simplicity.
Richard P. Perkins, MD
Fort Myers, Florida; Stockton, California
Dr. Barbieri responds
I thank Dr. Priver for his excellent description of how to use forceps to resolve a persistent occiput posterior position. I also thank Dr. Perkins for his valuable comments and agree with him that in the United States among the options available for outpatient cervical ripening, misoprostol or a balloon are more commonly used than membrane stripping. Membrane stripping is an outpatient cervical ripening technique that is commonly used in the United Kingdom.
Flying acid zombies, poster face-lifts, and feces of champions
The flying dead
Just when you thought cicada infestations couldn’t get worse – the cicadas are now zombies on an acid trip.
West Virginia University researchers recently discovered that a fungus called Massopora, which has compounds similar to those found in psychedelic mushrooms, can infect cicadas and cause seriously weird behavior.
How weird, you ask? Well, male cicadas try to mate with everything they encounter, even though the fungus has eaten away their limbs … and their genitals. Talk about a bad trip.
It gets worse. These zombie-like cicadas are flying around and exposing their healthy brethren to the fungus. So, now we have to worry about potentially billions of these buggers becoming rotting, flying, hypersexual machines.
Makes a person really want to stay inside for the whole summer, perhaps in a fortified bunker. Just in case.
Also, for the curious: One of the study’s authors concedes that if a person were “motivated enough,” they might be able to get high off the cicada fungus. Not interested in finding out exactly how many cicadas you’d have to crunch on before you start tasting colors?
Poster do-over
It’s time to get ready for a revolution – and just in time for 4th of July. No, we’re not redeclaring independence from Britain. It’s something much, much more radical, at least to the scientific community.
Get ready for … the redesign of the science poster.
Mike Morrison, a doctoral student in psychology, has proposed a new way to present information at meetings that breathes new life into the tired poster design. While fascinating insights could be held on a poster, the reality of meetings and conferences is that people often skim over the posters they see, barely registering the information.
Morrison is taking advantage of the ever-present smartphone to ramp up the classic science poster to a new level. He proposes a format where the key research finding is smack in the middle, in big, readable language. Accompanying this is a QR code you can scan, which would take you to a page with the full details of the study.
Morrison tweeted out his idea, and it spread quickly through the scientific community, gaining traction from younger scientists and students who love the idea.
We’re in the age of remakes now – in which classics are reinvented for a new audience. It’s time the science poster got a face-lift, too.
Running a marathon, one Veillonella at a time
What’s the difference between an elite athlete and a person who’s out of juice after running for 30 seconds?
Well, many things, we’re sure. A proper diet, rigorous training, not spending 12 hours a day sitting behind a computer screen. But just to add insult to injury, according to a study published in Nature Medicine, athletes even poop better than us normal people.
That may require a bit of explanation. A group of researchers from Harvard University, Boston, analyzed stool samples from elite marathon runners before and just after they participated in the 2015 Boston Marathon. They found that, following the race, the athletes had significantly higher amounts of Veillonella in their microbiota. This bacteria breaks down lactic acid, which is made during intense exercise and causes muscle fatigue and stress.
Naturally, the next step was to take that bacteria, feed it to mice, then get them running on a treadmill.
While not every mouse fed Veillonella saw increased performance, on average, mice that received the bacteria saw a 13% improvement over their non–Veillonella enhanced friends.
The researchers noted that this sort of probiotic treatment could be useful to patients with metabolic diseases, such as diabetes. Plus, there’s the obvious benefit to athletes. We look forward to hearing advertisements swearing by Veillonella-infused Wheaties, the true breakfast of champions.
Put a little boredom in your life
Is your job an endless, soul-sucking vortex of dreariness? Do you stare at the wall for hours at a time while you’re at work, wishing you had something better to do? Do you look forward to leaving early to go to the dentist? [Editor query: What does this have to do with health care? LOTME: You’ll see. We’re building dramatic tension.]
Did you answer yes to any of these questions? Way to go! You’ve taken the first step on the road to creativity. [Still waiting. We’re almost there.]
A recent study examined the effects of boredom by assigning people to either a really boring task – sorting a bowl of red and green beans by color for 30 minutes using only one hand – or a nonboring art project using paper, glue, and, of course, beans. [What’s with the beans? It’s not our fault they used beans.]
The next task was the same for both groups: Come up with some reasons for being late for work and think of ideas for a new product by a hypothetical company. The answers were graded on their uniqueness, and the bean-sorting group had more creative ideas than did the art-project group.
The point is, ladies and gentlemen, that boredom is good. Boredom is right. Boredom works. [A Gordon Gekko reference? Couldn’t you find something from this century? No.]
By now you’re probably wondering: “But how can I get one of those really boring jobs? After all, I’m a doctor. I heal the sick and care for the needy. My work is way too interesting to inspire true creativity.” Have you ever considered writing a medical humor column?
[Note to readers: We’ve pulled the staff away from their building blocks and crayons and given them a timeout. Hopefully things will be a little better by next week.]

The flying dead
Just when you thought cicada infestations couldn’t get worse – the cicadas are now zombies on an acid trip.
West Virginia University researchers recently discovered that a fungus called Massopora, which has compounds similar to those found in psychedelic mushrooms, can infect cicadas and cause seriously weird behavior.
How weird, you ask? Well, male cicadas try to mate with everything they encounter, even though the fungus has eaten away their limbs … and their genitals. Talk about a bad trip.
It gets worse. These zombie-like cicadas are flying around and exposing their healthy brethren to the fungus. So, now we have to worry about potentially billions of these buggers becoming rotting, flying, hypersexual machines.
Makes a person really want to stay inside for the whole summer, perhaps in a fortified bunker. Just in case.
Also, for the curious: One of the study’s authors concedes that if a person were “motivated enough,” they might be able to get high off the cicada fungus. Not interested in finding out exactly how many cicadas you’d have to crunch on before you start tasting colors?
Poster do-over
It’s time to get ready for a revolution – and just in time for 4th of July. No, we’re not redeclaring independence from Britain. It’s something much, much more radical, at least to the scientific community.
Get ready for … the redesign of the science poster.
Mike Morrison, a doctoral student in psychology, has proposed a new way to present information at meetings that breathes new life into the tired poster design. While fascinating insights could be held on a poster, the reality of meetings and conferences is that people often skim over the posters they see, barely registering the information.
Morrison is taking advantage of the ever-present smartphone to ramp up the classic science poster to a new level. He proposes a format where the key research finding is smack in the middle, in big, readable language. Accompanying this is a QR code you can scan, which would take you to a page with the full details of the study.
Morrison tweeted out his idea, and it spread quickly through the scientific community, gaining traction from younger scientists and students who love the idea.
We’re in the age of remakes now – in which classics are reinvented for a new audience. It’s time the science poster got a face-lift, too.
Running a marathon, one Veillonella at a time
What’s the difference between an elite athlete and a person who’s out of juice after running for 30 seconds?
Well, many things, we’re sure. A proper diet, rigorous training, not spending 12 hours a day sitting behind a computer screen. But just to add insult to injury, according to a study published in Nature Medicine, athletes even poop better than us normal people.
That may require a bit of explanation. A group of researchers from Harvard University, Boston, analyzed stool samples from elite marathon runners before and just after they participated in the 2015 Boston Marathon. They found that, following the race, the athletes had significantly higher amounts of Veillonella in their microbiota. This bacteria breaks down lactic acid, which is made during intense exercise and causes muscle fatigue and stress.
Naturally, the next step was to take that bacteria, feed it to mice, then get them running on a treadmill.
While not every mouse fed Veillonella saw increased performance, on average, mice that received the bacteria saw a 13% improvement over their non–Veillonella enhanced friends.
The researchers noted that this sort of probiotic treatment could be useful to patients with metabolic diseases, such as diabetes. Plus, there’s the obvious benefit to athletes. We look forward to hearing advertisements swearing by Veillonella-infused Wheaties, the true breakfast of champions.
Put a little boredom in your life
Is your job an endless, soul-sucking vortex of dreariness? Do you stare at the wall for hours at a time while you’re at work, wishing you had something better to do? Do you look forward to leaving early to go to the dentist? [Editor query: What does this have to do with health care? LOTME: You’ll see. We’re building dramatic tension.]
Did you answer yes to any of these questions? Way to go! You’ve taken the first step on the road to creativity. [Still waiting. We’re almost there.]
A recent study examined the effects of boredom by assigning people to either a really boring task – sorting a bowl of red and green beans by color for 30 minutes using only one hand – or a nonboring art project using paper, glue, and, of course, beans. [What’s with the beans? It’s not our fault they used beans.]
The next task was the same for both groups: Come up with some reasons for being late for work and think of ideas for a new product by a hypothetical company. The answers were graded on their uniqueness, and the bean-sorting group had more creative ideas than did the art-project group.
The point is, ladies and gentlemen, that boredom is good. Boredom is right. Boredom works. [A Gordon Gekko reference? Couldn’t you find something from this century? No.]
By now you’re probably wondering: “But how can I get one of those really boring jobs? After all, I’m a doctor. I heal the sick and care for the needy. My work is way too interesting to inspire true creativity.” Have you ever considered writing a medical humor column?
[Note to readers: We’ve pulled the staff away from their building blocks and crayons and given them a timeout. Hopefully things will be a little better by next week.]

The flying dead
Just when you thought cicada infestations couldn’t get worse – the cicadas are now zombies on an acid trip.
West Virginia University researchers recently discovered that a fungus called Massopora, which has compounds similar to those found in psychedelic mushrooms, can infect cicadas and cause seriously weird behavior.
How weird, you ask? Well, male cicadas try to mate with everything they encounter, even though the fungus has eaten away their limbs … and their genitals. Talk about a bad trip.
It gets worse. These zombie-like cicadas are flying around and exposing their healthy brethren to the fungus. So, now we have to worry about potentially billions of these buggers becoming rotting, flying, hypersexual machines.
Makes a person really want to stay inside for the whole summer, perhaps in a fortified bunker. Just in case.
Also, for the curious: One of the study’s authors concedes that if a person were “motivated enough,” they might be able to get high off the cicada fungus. Not interested in finding out exactly how many cicadas you’d have to crunch on before you start tasting colors?
Poster do-over
It’s time to get ready for a revolution – and just in time for 4th of July. No, we’re not redeclaring independence from Britain. It’s something much, much more radical, at least to the scientific community.
Get ready for … the redesign of the science poster.
Mike Morrison, a doctoral student in psychology, has proposed a new way to present information at meetings that breathes new life into the tired poster design. While fascinating insights could be held on a poster, the reality of meetings and conferences is that people often skim over the posters they see, barely registering the information.
Morrison is taking advantage of the ever-present smartphone to ramp up the classic science poster to a new level. He proposes a format where the key research finding is smack in the middle, in big, readable language. Accompanying this is a QR code you can scan, which would take you to a page with the full details of the study.
Morrison tweeted out his idea, and it spread quickly through the scientific community, gaining traction from younger scientists and students who love the idea.
We’re in the age of remakes now – in which classics are reinvented for a new audience. It’s time the science poster got a face-lift, too.
Running a marathon, one Veillonella at a time
What’s the difference between an elite athlete and a person who’s out of juice after running for 30 seconds?
Well, many things, we’re sure. A proper diet, rigorous training, not spending 12 hours a day sitting behind a computer screen. But just to add insult to injury, according to a study published in Nature Medicine, athletes even poop better than us normal people.
That may require a bit of explanation. A group of researchers from Harvard University, Boston, analyzed stool samples from elite marathon runners before and just after they participated in the 2015 Boston Marathon. They found that, following the race, the athletes had significantly higher amounts of Veillonella in their microbiota. This bacteria breaks down lactic acid, which is made during intense exercise and causes muscle fatigue and stress.
Naturally, the next step was to take that bacteria, feed it to mice, then get them running on a treadmill.
While not every mouse fed Veillonella saw increased performance, on average, mice that received the bacteria saw a 13% improvement over their non–Veillonella enhanced friends.
The researchers noted that this sort of probiotic treatment could be useful to patients with metabolic diseases, such as diabetes. Plus, there’s the obvious benefit to athletes. We look forward to hearing advertisements swearing by Veillonella-infused Wheaties, the true breakfast of champions.
Put a little boredom in your life
Is your job an endless, soul-sucking vortex of dreariness? Do you stare at the wall for hours at a time while you’re at work, wishing you had something better to do? Do you look forward to leaving early to go to the dentist? [Editor query: What does this have to do with health care? LOTME: You’ll see. We’re building dramatic tension.]
Did you answer yes to any of these questions? Way to go! You’ve taken the first step on the road to creativity. [Still waiting. We’re almost there.]
A recent study examined the effects of boredom by assigning people to either a really boring task – sorting a bowl of red and green beans by color for 30 minutes using only one hand – or a nonboring art project using paper, glue, and, of course, beans. [What’s with the beans? It’s not our fault they used beans.]
The next task was the same for both groups: Come up with some reasons for being late for work and think of ideas for a new product by a hypothetical company. The answers were graded on their uniqueness, and the bean-sorting group had more creative ideas than did the art-project group.
The point is, ladies and gentlemen, that boredom is good. Boredom is right. Boredom works. [A Gordon Gekko reference? Couldn’t you find something from this century? No.]
By now you’re probably wondering: “But how can I get one of those really boring jobs? After all, I’m a doctor. I heal the sick and care for the needy. My work is way too interesting to inspire true creativity.” Have you ever considered writing a medical humor column?
[Note to readers: We’ve pulled the staff away from their building blocks and crayons and given them a timeout. Hopefully things will be a little better by next week.]

RNase drug shows promise for Sjögren’s fatigue
MADRID – RSLV-132, a novel drug that eliminates circulating nucleic acids, improved the symptoms of mental fatigue in patients with primary Sjögren’s syndrome (pSS) in a phase 2, double-blind, randomized, placebo-controlled “proof-of-concept” study.

The mental component of fatigue on the Profile of Fatigue (PRO-F) scale improved by 1.53 points among the patients given RSLV-132, while there was a worsening of 0.06 points in the placebo group (P = .046). Scores range from 0 to 7 on the PRO-F.
There were also improvements in some patient-reported outcomes, namely the EULAR pSS Patient Reported Index (ESSPRI) and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue (FACIT-F), and improvement of neuropsychological measures of cognitive function, but none were statistically significant. All of these outcomes, including fatigue, were secondary outcomes of the study.
“Fatigue is a major issue for patients with Sjögren’s syndrome,” Wan-Fai Ng, MBChB, PhD, said in an interview at the European Congress of Rheumatology. Indeed, fatigue can be disabling in the majority of individuals, he added.
Currently, the best way to manage fatigue is to first ask about it, said Dr. Ng, who is professor of rheumatology at the Institute of Cellular Medicine at Newcastle University, Newcastle-upon-Tyne, England. He then checks for any underlying problem that could be better managed – sleep problems or anemia, for example – and optimizes treatment for any underlying disease. “I think many people would at least feel satisfied that people take the symptoms seriously.”
Dr. Ng and his coauthors investigated the effects of RSLV-132, a first-in-class human RNase fused to the human Fc domain of human immunoglobulin (Ig) G1, in the RESOLVE 132-04 study. This novel drug been designed to increase serum RNase activity to digest RNA-associated immune complexes, Dr. Ng and associates observed in their abstract. As a consequence of this, they say, activation of toll-like receptors and the production of interferon (IFN) is affected, as is B-cell proliferation and the production of autoantibodies – all mechanisms that are “key to pSS pathogenesis.”
The IFN pathway has been implicated in fatigue, Dr. Ng observed when he presented the RESOLVE 132-04 study’s findings, which involved 30 patients with pSS who had been treated for 3 months. Inclusion criteria were pSS as defined by the American-European Consensus Group 2002 criteria, anti-Ro antibody positivity, and increased expression of three IFN-regulated genes: HERC5, EPSTI1, and CMPK2. Exclusion criteria were the use of hydroxychloroquine, prednisolone at daily doses above 10 mg, and the use of biologic disease-modifying antirheumatic drugs.
Patients were randomized in a 3:1 ratio to receive either 10 mg/kg of RSLV-132 (n = 20) or placebo (n = 8) at weeks 0, 1, 2, and then every fortnight until week 12. Dr. Ng noted that although 30 patients were randomized, two patients had dropped out in the placebo group before they could be “treated.”
The primary endpoint was the change in the blood cell gene expression or serum protein levels indicative of reduced inflammation. The results indicated reductions in noncoding RNA molecules in patients who received RSLV-132 versus placebo, “consistent with the mode of action of the molecule.” In addition, “the majority of inflammatory markers were reduced,” Dr. Ng said.
Another finding showed a nonsignificant trend for improvement of 0.8 units in the physical component in the RSLV-132 group, compared against 0.06 units with placebo (P = .142).
Patients who received RSLV-132 reduced the time needed to complete the Digital Symbol Substitution Test by 16.4 s, compared with an increase of 2.8 s for placebo (P = .024).
Similar trends were observed for ESSPRI and FACIT-F scores.
These are very early data and clearly a bigger study would be needed before any conclusions could be drawn, Dr. Ng said in an interview. What these data suggest is that “maybe there is some way that we could manage fatigue, and we just need to go and explore that.”
RSLV-132 has also been studied in patients with systemic lupus erythematosus (Lupus. 2017;26:825-34).
The trial was sponsored by Resolve Therapeutics. Dr. Ng was an investigator in the trial and disclosed other research collaborations with electroCore, GlaxoSmithKline, and AbbVie. He also disclosed acting as a consultant for Novartis, GlaxoSmithKline, AbbVie, MedImmune, Pfizer, and Bristol-Myers Squibb.
Source: Fisher B et al. Ann Rheum Dis. 2019 Jun;78(suppl 2):177, Abstract OP0202. doi: 10.1136/annrheumdis-2019-eular.3098.
MADRID – RSLV-132, a novel drug that eliminates circulating nucleic acids, improved the symptoms of mental fatigue in patients with primary Sjögren’s syndrome (pSS) in a phase 2, double-blind, randomized, placebo-controlled “proof-of-concept” study.

The mental component of fatigue on the Profile of Fatigue (PRO-F) scale improved by 1.53 points among the patients given RSLV-132, while there was a worsening of 0.06 points in the placebo group (P = .046). Scores range from 0 to 7 on the PRO-F.
There were also improvements in some patient-reported outcomes, namely the EULAR pSS Patient Reported Index (ESSPRI) and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue (FACIT-F), and improvement of neuropsychological measures of cognitive function, but none were statistically significant. All of these outcomes, including fatigue, were secondary outcomes of the study.
“Fatigue is a major issue for patients with Sjögren’s syndrome,” Wan-Fai Ng, MBChB, PhD, said in an interview at the European Congress of Rheumatology. Indeed, fatigue can be disabling in the majority of individuals, he added.
Currently, the best way to manage fatigue is to first ask about it, said Dr. Ng, who is professor of rheumatology at the Institute of Cellular Medicine at Newcastle University, Newcastle-upon-Tyne, England. He then checks for any underlying problem that could be better managed – sleep problems or anemia, for example – and optimizes treatment for any underlying disease. “I think many people would at least feel satisfied that people take the symptoms seriously.”
Dr. Ng and his coauthors investigated the effects of RSLV-132, a first-in-class human RNase fused to the human Fc domain of human immunoglobulin (Ig) G1, in the RESOLVE 132-04 study. This novel drug been designed to increase serum RNase activity to digest RNA-associated immune complexes, Dr. Ng and associates observed in their abstract. As a consequence of this, they say, activation of toll-like receptors and the production of interferon (IFN) is affected, as is B-cell proliferation and the production of autoantibodies – all mechanisms that are “key to pSS pathogenesis.”
The IFN pathway has been implicated in fatigue, Dr. Ng observed when he presented the RESOLVE 132-04 study’s findings, which involved 30 patients with pSS who had been treated for 3 months. Inclusion criteria were pSS as defined by the American-European Consensus Group 2002 criteria, anti-Ro antibody positivity, and increased expression of three IFN-regulated genes: HERC5, EPSTI1, and CMPK2. Exclusion criteria were the use of hydroxychloroquine, prednisolone at daily doses above 10 mg, and the use of biologic disease-modifying antirheumatic drugs.
Patients were randomized in a 3:1 ratio to receive either 10 mg/kg of RSLV-132 (n = 20) or placebo (n = 8) at weeks 0, 1, 2, and then every fortnight until week 12. Dr. Ng noted that although 30 patients were randomized, two patients had dropped out in the placebo group before they could be “treated.”
The primary endpoint was the change in the blood cell gene expression or serum protein levels indicative of reduced inflammation. The results indicated reductions in noncoding RNA molecules in patients who received RSLV-132 versus placebo, “consistent with the mode of action of the molecule.” In addition, “the majority of inflammatory markers were reduced,” Dr. Ng said.
Another finding showed a nonsignificant trend for improvement of 0.8 units in the physical component in the RSLV-132 group, compared against 0.06 units with placebo (P = .142).
Patients who received RSLV-132 reduced the time needed to complete the Digital Symbol Substitution Test by 16.4 s, compared with an increase of 2.8 s for placebo (P = .024).
Similar trends were observed for ESSPRI and FACIT-F scores.
These are very early data and clearly a bigger study would be needed before any conclusions could be drawn, Dr. Ng said in an interview. What these data suggest is that “maybe there is some way that we could manage fatigue, and we just need to go and explore that.”
RSLV-132 has also been studied in patients with systemic lupus erythematosus (Lupus. 2017;26:825-34).
The trial was sponsored by Resolve Therapeutics. Dr. Ng was an investigator in the trial and disclosed other research collaborations with electroCore, GlaxoSmithKline, and AbbVie. He also disclosed acting as a consultant for Novartis, GlaxoSmithKline, AbbVie, MedImmune, Pfizer, and Bristol-Myers Squibb.
Source: Fisher B et al. Ann Rheum Dis. 2019 Jun;78(suppl 2):177, Abstract OP0202. doi: 10.1136/annrheumdis-2019-eular.3098.
MADRID – RSLV-132, a novel drug that eliminates circulating nucleic acids, improved the symptoms of mental fatigue in patients with primary Sjögren’s syndrome (pSS) in a phase 2, double-blind, randomized, placebo-controlled “proof-of-concept” study.

The mental component of fatigue on the Profile of Fatigue (PRO-F) scale improved by 1.53 points among the patients given RSLV-132, while there was a worsening of 0.06 points in the placebo group (P = .046). Scores range from 0 to 7 on the PRO-F.
There were also improvements in some patient-reported outcomes, namely the EULAR pSS Patient Reported Index (ESSPRI) and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue (FACIT-F), and improvement of neuropsychological measures of cognitive function, but none were statistically significant. All of these outcomes, including fatigue, were secondary outcomes of the study.
“Fatigue is a major issue for patients with Sjögren’s syndrome,” Wan-Fai Ng, MBChB, PhD, said in an interview at the European Congress of Rheumatology. Indeed, fatigue can be disabling in the majority of individuals, he added.
Currently, the best way to manage fatigue is to first ask about it, said Dr. Ng, who is professor of rheumatology at the Institute of Cellular Medicine at Newcastle University, Newcastle-upon-Tyne, England. He then checks for any underlying problem that could be better managed – sleep problems or anemia, for example – and optimizes treatment for any underlying disease. “I think many people would at least feel satisfied that people take the symptoms seriously.”
Dr. Ng and his coauthors investigated the effects of RSLV-132, a first-in-class human RNase fused to the human Fc domain of human immunoglobulin (Ig) G1, in the RESOLVE 132-04 study. This novel drug been designed to increase serum RNase activity to digest RNA-associated immune complexes, Dr. Ng and associates observed in their abstract. As a consequence of this, they say, activation of toll-like receptors and the production of interferon (IFN) is affected, as is B-cell proliferation and the production of autoantibodies – all mechanisms that are “key to pSS pathogenesis.”
The IFN pathway has been implicated in fatigue, Dr. Ng observed when he presented the RESOLVE 132-04 study’s findings, which involved 30 patients with pSS who had been treated for 3 months. Inclusion criteria were pSS as defined by the American-European Consensus Group 2002 criteria, anti-Ro antibody positivity, and increased expression of three IFN-regulated genes: HERC5, EPSTI1, and CMPK2. Exclusion criteria were the use of hydroxychloroquine, prednisolone at daily doses above 10 mg, and the use of biologic disease-modifying antirheumatic drugs.
Patients were randomized in a 3:1 ratio to receive either 10 mg/kg of RSLV-132 (n = 20) or placebo (n = 8) at weeks 0, 1, 2, and then every fortnight until week 12. Dr. Ng noted that although 30 patients were randomized, two patients had dropped out in the placebo group before they could be “treated.”
The primary endpoint was the change in the blood cell gene expression or serum protein levels indicative of reduced inflammation. The results indicated reductions in noncoding RNA molecules in patients who received RSLV-132 versus placebo, “consistent with the mode of action of the molecule.” In addition, “the majority of inflammatory markers were reduced,” Dr. Ng said.
Another finding showed a nonsignificant trend for improvement of 0.8 units in the physical component in the RSLV-132 group, compared against 0.06 units with placebo (P = .142).
Patients who received RSLV-132 reduced the time needed to complete the Digital Symbol Substitution Test by 16.4 s, compared with an increase of 2.8 s for placebo (P = .024).
Similar trends were observed for ESSPRI and FACIT-F scores.
These are very early data and clearly a bigger study would be needed before any conclusions could be drawn, Dr. Ng said in an interview. What these data suggest is that “maybe there is some way that we could manage fatigue, and we just need to go and explore that.”
RSLV-132 has also been studied in patients with systemic lupus erythematosus (Lupus. 2017;26:825-34).
The trial was sponsored by Resolve Therapeutics. Dr. Ng was an investigator in the trial and disclosed other research collaborations with electroCore, GlaxoSmithKline, and AbbVie. He also disclosed acting as a consultant for Novartis, GlaxoSmithKline, AbbVie, MedImmune, Pfizer, and Bristol-Myers Squibb.
Source: Fisher B et al. Ann Rheum Dis. 2019 Jun;78(suppl 2):177, Abstract OP0202. doi: 10.1136/annrheumdis-2019-eular.3098.
REPORTING FROM EULAR 2019 CONGRESS