User login
Repeated ANA testing after negative result of little diagnostic value
MADRID – Repeated antinuclear antibody testing after a negative result has limited use for the diagnosis of ANA-associated rheumatologic conditions, according to data from a multicenter, retrospective analysis that considered a 7-year period.
Considering more than 7,875 repeated ANA tests in 4,887 patients, “the vast majority of results didn’t change,” Ai Li Yeo, MBBS, a PhD candidate, rheumatologist, and infectious disease fellow at Monash University, Melbourne, reported at the European Congress of Rheumatology.
ANA tests were repeated between 2 and as many as 45 times in individual patients, she reported, but the results of 79% of these tests remained unchanged – 45% of tests were persistently negative and 34% persistently positive using a cutoff titer of 1:160.
“Our study showed that there was a very low yield in repeating an ANA test for the diagnosis of ANA-associated rheumatological conditions unless there was evidence of evolving multisystem clinical features,” Dr. Yeo said.
Indeed, the positive predictive value was just 0.01. “So for a hundred patients staring off with a negative ANA results that on repeat testing became positive, the probability is that one patient will have a new ANA-associated rheumatological condition diagnosis,” Dr. Yeo said.
“ANA testing is frequently performed and is part of the classification criteria for autoimmune conditions such as lupus and scleroderma,” she observed. However, the test provides no information on the severity or activity of the disease, and the value of serial monitoring for such conditions is unclear.
“Minimizing unnecessary tests is a global health economic priority,” Dr. Yeo said. She noted that there are multiple initiatives in place to try to open a dialog about using health care resources most effectively, such as ‘Choosing Wisely’ set up by the American Board of Internal Medicine (ABIM) Foundation.
The aim of the present analysis was to calculate the cost of repeated ANA testing and to see if any change in the ANA result was associated with new diagnoses of ANA-associated rheumatological conditions.
The analysis considered more than 36,700 tests that were performed on samples from more than 28,800 patients within the Monash Health tertiary health network between 2011 and 2018. Of these, 22,657 (62%) had given a negative result and 14,058 (38%) had given a positive result.
“Not surprisingly, the age of those who tested positive was significantly higher than those who tested negative,” Dr. Yeo said (52.6 vs. 48.9 years; P less than .001). There was also a higher number of women than men tested, and women more often tested positive.
Around one-fifth of tests performed were repeat tests, of which 511 (6.5%) changed from being negative to positive over a median of 1.71 years.
“A small percentage of people alternated between results,” Dr. Yeo acknowledged, with 9.4% of people going from a positive to a negative result; 10.5% moving from a negative to a positive result, and 1.9% going from positive to negative to positive.
With repeated tests, just five new diagnoses of ANA-associated rheumatologic conditions were made: two cases of systemic lupus erythematosus (SLE), one case of scleroderma, and two cases of undifferentiated connective tissue disease. There was a range of ANA titers and patterns and evolving clinical features of a multisystem disease.
Based on the direct costs of ANA testing in her health care system, not performing repeated tests could yield significant savings, Dr. Yeo said, a 21.4% reduction, in fact, based on this analysis. The cost of an ANA test in Australia ranges from 15 to 46 euros, making the cost of all tests in this analysis 564,745 euros. Taking away the cost of all the single ANA tests performed (443,209 euros) gives a potential cost saving of more than 121,000 euros, she said.
“We now have an opportunity to prevent unnecessary ANA testing, Dr. Yeo said. “Ultimately, our aim is to change behavior at the start of the ordering cycle by educating medical students and doctors about inappropriate test ordering.”
The majority of repeated tests had been ordered by nonrheumatologists (82% of cases), and Dr. Yeo said that rheumatologists ordered repeat tests in 11% of cases. However, there was little information available in this retrospective analysis as to why the tests had been repeated.
The research was picked as one of the six best clinical abstracts at the meeting, out of a total of almost 5,000 submitted abstracts.
Dr. Yeo reported having no conflicts of interest.
SOURCE: Yeo AL et al. Ann Rheum Dis. Jun 2019;78(suppl 2):76-7, Abstract OP0020. doi: 10.1136/annrheumdis-2019-eular.4517.
MADRID – Repeated antinuclear antibody testing after a negative result has limited use for the diagnosis of ANA-associated rheumatologic conditions, according to data from a multicenter, retrospective analysis that considered a 7-year period.
Considering more than 7,875 repeated ANA tests in 4,887 patients, “the vast majority of results didn’t change,” Ai Li Yeo, MBBS, a PhD candidate, rheumatologist, and infectious disease fellow at Monash University, Melbourne, reported at the European Congress of Rheumatology.
ANA tests were repeated between 2 and as many as 45 times in individual patients, she reported, but the results of 79% of these tests remained unchanged – 45% of tests were persistently negative and 34% persistently positive using a cutoff titer of 1:160.
“Our study showed that there was a very low yield in repeating an ANA test for the diagnosis of ANA-associated rheumatological conditions unless there was evidence of evolving multisystem clinical features,” Dr. Yeo said.
Indeed, the positive predictive value was just 0.01. “So for a hundred patients staring off with a negative ANA results that on repeat testing became positive, the probability is that one patient will have a new ANA-associated rheumatological condition diagnosis,” Dr. Yeo said.
“ANA testing is frequently performed and is part of the classification criteria for autoimmune conditions such as lupus and scleroderma,” she observed. However, the test provides no information on the severity or activity of the disease, and the value of serial monitoring for such conditions is unclear.
“Minimizing unnecessary tests is a global health economic priority,” Dr. Yeo said. She noted that there are multiple initiatives in place to try to open a dialog about using health care resources most effectively, such as ‘Choosing Wisely’ set up by the American Board of Internal Medicine (ABIM) Foundation.
The aim of the present analysis was to calculate the cost of repeated ANA testing and to see if any change in the ANA result was associated with new diagnoses of ANA-associated rheumatological conditions.
The analysis considered more than 36,700 tests that were performed on samples from more than 28,800 patients within the Monash Health tertiary health network between 2011 and 2018. Of these, 22,657 (62%) had given a negative result and 14,058 (38%) had given a positive result.
“Not surprisingly, the age of those who tested positive was significantly higher than those who tested negative,” Dr. Yeo said (52.6 vs. 48.9 years; P less than .001). There was also a higher number of women than men tested, and women more often tested positive.
Around one-fifth of tests performed were repeat tests, of which 511 (6.5%) changed from being negative to positive over a median of 1.71 years.
“A small percentage of people alternated between results,” Dr. Yeo acknowledged, with 9.4% of people going from a positive to a negative result; 10.5% moving from a negative to a positive result, and 1.9% going from positive to negative to positive.
With repeated tests, just five new diagnoses of ANA-associated rheumatologic conditions were made: two cases of systemic lupus erythematosus (SLE), one case of scleroderma, and two cases of undifferentiated connective tissue disease. There was a range of ANA titers and patterns and evolving clinical features of a multisystem disease.
Based on the direct costs of ANA testing in her health care system, not performing repeated tests could yield significant savings, Dr. Yeo said, a 21.4% reduction, in fact, based on this analysis. The cost of an ANA test in Australia ranges from 15 to 46 euros, making the cost of all tests in this analysis 564,745 euros. Taking away the cost of all the single ANA tests performed (443,209 euros) gives a potential cost saving of more than 121,000 euros, she said.
“We now have an opportunity to prevent unnecessary ANA testing, Dr. Yeo said. “Ultimately, our aim is to change behavior at the start of the ordering cycle by educating medical students and doctors about inappropriate test ordering.”
The majority of repeated tests had been ordered by nonrheumatologists (82% of cases), and Dr. Yeo said that rheumatologists ordered repeat tests in 11% of cases. However, there was little information available in this retrospective analysis as to why the tests had been repeated.
The research was picked as one of the six best clinical abstracts at the meeting, out of a total of almost 5,000 submitted abstracts.
Dr. Yeo reported having no conflicts of interest.
SOURCE: Yeo AL et al. Ann Rheum Dis. Jun 2019;78(suppl 2):76-7, Abstract OP0020. doi: 10.1136/annrheumdis-2019-eular.4517.
MADRID – Repeated antinuclear antibody testing after a negative result has limited use for the diagnosis of ANA-associated rheumatologic conditions, according to data from a multicenter, retrospective analysis that considered a 7-year period.
Considering more than 7,875 repeated ANA tests in 4,887 patients, “the vast majority of results didn’t change,” Ai Li Yeo, MBBS, a PhD candidate, rheumatologist, and infectious disease fellow at Monash University, Melbourne, reported at the European Congress of Rheumatology.
ANA tests were repeated between 2 and as many as 45 times in individual patients, she reported, but the results of 79% of these tests remained unchanged – 45% of tests were persistently negative and 34% persistently positive using a cutoff titer of 1:160.
“Our study showed that there was a very low yield in repeating an ANA test for the diagnosis of ANA-associated rheumatological conditions unless there was evidence of evolving multisystem clinical features,” Dr. Yeo said.
Indeed, the positive predictive value was just 0.01. “So for a hundred patients staring off with a negative ANA results that on repeat testing became positive, the probability is that one patient will have a new ANA-associated rheumatological condition diagnosis,” Dr. Yeo said.
“ANA testing is frequently performed and is part of the classification criteria for autoimmune conditions such as lupus and scleroderma,” she observed. However, the test provides no information on the severity or activity of the disease, and the value of serial monitoring for such conditions is unclear.
“Minimizing unnecessary tests is a global health economic priority,” Dr. Yeo said. She noted that there are multiple initiatives in place to try to open a dialog about using health care resources most effectively, such as ‘Choosing Wisely’ set up by the American Board of Internal Medicine (ABIM) Foundation.
The aim of the present analysis was to calculate the cost of repeated ANA testing and to see if any change in the ANA result was associated with new diagnoses of ANA-associated rheumatological conditions.
The analysis considered more than 36,700 tests that were performed on samples from more than 28,800 patients within the Monash Health tertiary health network between 2011 and 2018. Of these, 22,657 (62%) had given a negative result and 14,058 (38%) had given a positive result.
“Not surprisingly, the age of those who tested positive was significantly higher than those who tested negative,” Dr. Yeo said (52.6 vs. 48.9 years; P less than .001). There was also a higher number of women than men tested, and women more often tested positive.
Around one-fifth of tests performed were repeat tests, of which 511 (6.5%) changed from being negative to positive over a median of 1.71 years.
“A small percentage of people alternated between results,” Dr. Yeo acknowledged, with 9.4% of people going from a positive to a negative result; 10.5% moving from a negative to a positive result, and 1.9% going from positive to negative to positive.
With repeated tests, just five new diagnoses of ANA-associated rheumatologic conditions were made: two cases of systemic lupus erythematosus (SLE), one case of scleroderma, and two cases of undifferentiated connective tissue disease. There was a range of ANA titers and patterns and evolving clinical features of a multisystem disease.
Based on the direct costs of ANA testing in her health care system, not performing repeated tests could yield significant savings, Dr. Yeo said, a 21.4% reduction, in fact, based on this analysis. The cost of an ANA test in Australia ranges from 15 to 46 euros, making the cost of all tests in this analysis 564,745 euros. Taking away the cost of all the single ANA tests performed (443,209 euros) gives a potential cost saving of more than 121,000 euros, she said.
“We now have an opportunity to prevent unnecessary ANA testing, Dr. Yeo said. “Ultimately, our aim is to change behavior at the start of the ordering cycle by educating medical students and doctors about inappropriate test ordering.”
The majority of repeated tests had been ordered by nonrheumatologists (82% of cases), and Dr. Yeo said that rheumatologists ordered repeat tests in 11% of cases. However, there was little information available in this retrospective analysis as to why the tests had been repeated.
The research was picked as one of the six best clinical abstracts at the meeting, out of a total of almost 5,000 submitted abstracts.
Dr. Yeo reported having no conflicts of interest.
SOURCE: Yeo AL et al. Ann Rheum Dis. Jun 2019;78(suppl 2):76-7, Abstract OP0020. doi: 10.1136/annrheumdis-2019-eular.4517.
REPORTING FROM EULAR 2019 CONGRESS
Women express low decision regret after preimplantation testing for aneuploidy
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
FROM HUMAN REPRODUCTION
July: An important month for pediatric hospital medicine
National conferences and grassroots initiatives
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
National conferences and grassroots initiatives
National conferences and grassroots initiatives
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
Each July, the largest gathering of pediatric hospitalists occurs, and 2019 is no different! This year, hospitalists who care for children will gather at Pediatric Hospital Medicine (PHM) in Seattle from July 25 to 28, with the goal of enhancing participants’ knowledge and competence in the areas of innovation, clinical medicine, education, health services, practice management, quality improvement, and research.
But what makes this year particularly special is the launch of the subspecialty exam for certification in pediatric hospital medicine coming later this fall, solidifying its growth and importance within hospital medicine and the entire health care landscape. The American Board of Pediatrics (ABP) has approved PHM as the newest board subspecialty with a 2-year fellowship accredited by the Accreditation Council for Graduate Medical Education (ACGME). This conference will be a great opportunity to join with others to review competencies for board review, as well as to network with those who are also navigating the road ahead.
During 2019, the Pediatric Hospitalist Special Interest Group (SIG) of SHM has been working tirelessly on several initiatives, including a revision of the Pediatric Hospital Medicine Core Competencies as well as additional work to develop Choosing Wisely 2.0 recommendations. These will help us ensure we are developing the best curricula for the next generation of pediatric hospitalists, while cutting back on unnecessary tests and procedures for those practicing today. Each of these initiatives, as well as the July conference, highlights the opportunities that we have within SHM to work with other like-minded providers who care for children. While we partner with all professionals across many organizations, like the American Academy of Pediatrics and the Academic Pediatric Association to name a few, I wanted to share my reflections on SHM and my appreciation for the “big tent” philosophy that has served us so well thus far.
Having an opportunity to sit on the board of SHM has allowed me a chance to really appreciate the efforts that this organization invests in all who care for patients in the hospital; we have an active group of advanced-practice providers, practice administrators, residents, students, academic hospitalists, and the list goes on and on. We collaborate with a number of spectacular societies dedicated to medical specialties, and we are always open to new ways of improving the methods of delivering care to patients, in hospitals, post-acute care facilities, homes – you name it! As health care delivery models continue to evolve, I believe we are well positioned to be leaders in the delivery of acute care medicine in the hospital and beyond.
I have also learned of happenings at the grassroots level by attending SHM chapter meetings across the United States. For example, the Hampton Roads Chapter led a great Point-of-Care Ultrasound (POCUS) workshop, and influenced by that, I shared an idea at home in Nashville – borrowing my son as a model to demonstrate ultrasound techniques that hospitalists can use to assist in clinical care. I hope you, as pediatric hospitalists, will see if you have a local chapter and attend a meeting; whether you are a member of SHM or not, you can mingle with those who provide acute care treatments to all your communities and share best practices. If you don’t see an SHM chapter close by, let’s get one going! SHM is here to help launch a chapter that can help bring your community together and provide education and networking closer to home.
If you can’t attend PHM in Seattle this year, I hope you will make every effort to be at PHM 2020, where our own SIG leader, Dr. Jeffrey Grill from Louisville, Ky., will be chairing the next rendition of this amazing conference. The SHM Meetings team led by Michelle Kann will be working tirelessly to make it a great event with continued growth in content and attendance.
Dr. Rehm is associate professor, pediatrics, and director, division of pediatric outreach medicine at Vanderbilt University and Monroe Carell Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn. She is also a member of the SHM board of directors.
Racial, age disparities confirmed in triple-negative breast cancer
Odds of triple-negative breast cancer (TNBC) are elevated for minority women and younger women, results of a nationwide cross-sectional cohort study of more than a million breast cancer cases confirm.
Previous studies have suggested certain sociodemographic groups are disproportionately affected by TNBC, but have been limited by the population studied, size, and characteristics assessed, note the investigators, who were led by Lia C. Scott, PhD, MPH, of the division of epidemiology and biostatistics at the School of Public Health, Georgia State University, Atlanta. “It is imperative that cancer research identify factors that drive disparities and focus on prevention,” they wrote in Cancer.
Dr. Scott and coinvestigators used the United States Cancer Statistics database to identify 1,151,724 cases of breast cancer diagnosed during 2010-2014 in 39 states having the necessary data. TNBC accounted for roughly 8.4% of all cases.
In unadjusted analyses using non-Hispanic white women as the comparator, odds of TNBC were significantly higher for non-Hispanic black women (odds ratio, 2.27), Hispanic women (OR, 1.22), and American Indian/Alaska Native women (OR, 1.26). On the other hand, odds were lower for Asian women (OR, 0.92).
By age group, compared with women 50-64 years old, women younger than 40 years were most likely to have the TNBC phenotype (OR, 1.95), while women aged 75 or older were least likely (OR, 0.75). Odds of TNBC were also significantly elevated for women whose cancer was diagnosed at stage III or higher (OR, 1.69) or at stage IV (OR, 1.47).
Findings were essentially the same in analyses simultaneously adjusted for age, race, and stage.
“The results of the current study demonstrated that there is a significant burden of disease in TNBC diagnosed among women of color, specifically non-Hispanic black women, and younger women,” Dr. Scott and coinvestigators write. “Given the large sample size and geospatial coverage of the data, these results are somewhat different from and also more generalizable, compared with data from previous studies.”
“With the advent and availability of more comprehensive cancer data, such as the United States Cancer Statistics database, it is important that we continue to explore disparities in order to better inform practice and policy around screenable cancers like breast cancer,” she further commented in a statement. “We hope that this update on the epidemiology of triple-negative breast cancer can provide a basis to further explore contributing factors in future research.”
Dr. Scott disclosed that she received a Dissertation Training Grant (F31-Diversity) from the National Institutes of Health. The study was funded by the National Institute on Minority Health and Health Disparities of the National Institutes of Health; the Centers for Disease Control and Prevention’s National Program of Cancer Registries contributed funds to cover the standard research data center fees for researchers conducting analyses under approved research projects.
SOURCE: Scott LC et al. Cancer. 2019 Jul 8. doi: 10.1002/cncr.32207.
Odds of triple-negative breast cancer (TNBC) are elevated for minority women and younger women, results of a nationwide cross-sectional cohort study of more than a million breast cancer cases confirm.
Previous studies have suggested certain sociodemographic groups are disproportionately affected by TNBC, but have been limited by the population studied, size, and characteristics assessed, note the investigators, who were led by Lia C. Scott, PhD, MPH, of the division of epidemiology and biostatistics at the School of Public Health, Georgia State University, Atlanta. “It is imperative that cancer research identify factors that drive disparities and focus on prevention,” they wrote in Cancer.
Dr. Scott and coinvestigators used the United States Cancer Statistics database to identify 1,151,724 cases of breast cancer diagnosed during 2010-2014 in 39 states having the necessary data. TNBC accounted for roughly 8.4% of all cases.
In unadjusted analyses using non-Hispanic white women as the comparator, odds of TNBC were significantly higher for non-Hispanic black women (odds ratio, 2.27), Hispanic women (OR, 1.22), and American Indian/Alaska Native women (OR, 1.26). On the other hand, odds were lower for Asian women (OR, 0.92).
By age group, compared with women 50-64 years old, women younger than 40 years were most likely to have the TNBC phenotype (OR, 1.95), while women aged 75 or older were least likely (OR, 0.75). Odds of TNBC were also significantly elevated for women whose cancer was diagnosed at stage III or higher (OR, 1.69) or at stage IV (OR, 1.47).
Findings were essentially the same in analyses simultaneously adjusted for age, race, and stage.
“The results of the current study demonstrated that there is a significant burden of disease in TNBC diagnosed among women of color, specifically non-Hispanic black women, and younger women,” Dr. Scott and coinvestigators write. “Given the large sample size and geospatial coverage of the data, these results are somewhat different from and also more generalizable, compared with data from previous studies.”
“With the advent and availability of more comprehensive cancer data, such as the United States Cancer Statistics database, it is important that we continue to explore disparities in order to better inform practice and policy around screenable cancers like breast cancer,” she further commented in a statement. “We hope that this update on the epidemiology of triple-negative breast cancer can provide a basis to further explore contributing factors in future research.”
Dr. Scott disclosed that she received a Dissertation Training Grant (F31-Diversity) from the National Institutes of Health. The study was funded by the National Institute on Minority Health and Health Disparities of the National Institutes of Health; the Centers for Disease Control and Prevention’s National Program of Cancer Registries contributed funds to cover the standard research data center fees for researchers conducting analyses under approved research projects.
SOURCE: Scott LC et al. Cancer. 2019 Jul 8. doi: 10.1002/cncr.32207.
Odds of triple-negative breast cancer (TNBC) are elevated for minority women and younger women, results of a nationwide cross-sectional cohort study of more than a million breast cancer cases confirm.
Previous studies have suggested certain sociodemographic groups are disproportionately affected by TNBC, but have been limited by the population studied, size, and characteristics assessed, note the investigators, who were led by Lia C. Scott, PhD, MPH, of the division of epidemiology and biostatistics at the School of Public Health, Georgia State University, Atlanta. “It is imperative that cancer research identify factors that drive disparities and focus on prevention,” they wrote in Cancer.
Dr. Scott and coinvestigators used the United States Cancer Statistics database to identify 1,151,724 cases of breast cancer diagnosed during 2010-2014 in 39 states having the necessary data. TNBC accounted for roughly 8.4% of all cases.
In unadjusted analyses using non-Hispanic white women as the comparator, odds of TNBC were significantly higher for non-Hispanic black women (odds ratio, 2.27), Hispanic women (OR, 1.22), and American Indian/Alaska Native women (OR, 1.26). On the other hand, odds were lower for Asian women (OR, 0.92).
By age group, compared with women 50-64 years old, women younger than 40 years were most likely to have the TNBC phenotype (OR, 1.95), while women aged 75 or older were least likely (OR, 0.75). Odds of TNBC were also significantly elevated for women whose cancer was diagnosed at stage III or higher (OR, 1.69) or at stage IV (OR, 1.47).
Findings were essentially the same in analyses simultaneously adjusted for age, race, and stage.
“The results of the current study demonstrated that there is a significant burden of disease in TNBC diagnosed among women of color, specifically non-Hispanic black women, and younger women,” Dr. Scott and coinvestigators write. “Given the large sample size and geospatial coverage of the data, these results are somewhat different from and also more generalizable, compared with data from previous studies.”
“With the advent and availability of more comprehensive cancer data, such as the United States Cancer Statistics database, it is important that we continue to explore disparities in order to better inform practice and policy around screenable cancers like breast cancer,” she further commented in a statement. “We hope that this update on the epidemiology of triple-negative breast cancer can provide a basis to further explore contributing factors in future research.”
Dr. Scott disclosed that she received a Dissertation Training Grant (F31-Diversity) from the National Institutes of Health. The study was funded by the National Institute on Minority Health and Health Disparities of the National Institutes of Health; the Centers for Disease Control and Prevention’s National Program of Cancer Registries contributed funds to cover the standard research data center fees for researchers conducting analyses under approved research projects.
SOURCE: Scott LC et al. Cancer. 2019 Jul 8. doi: 10.1002/cncr.32207.
FROM CANCER
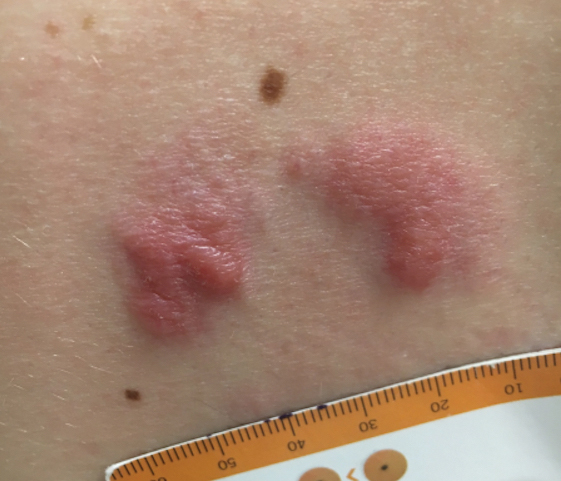
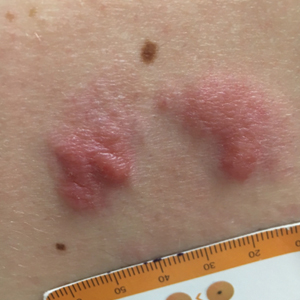
Grouped Erythematous Papules and Plaques on the Trunk
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).
The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
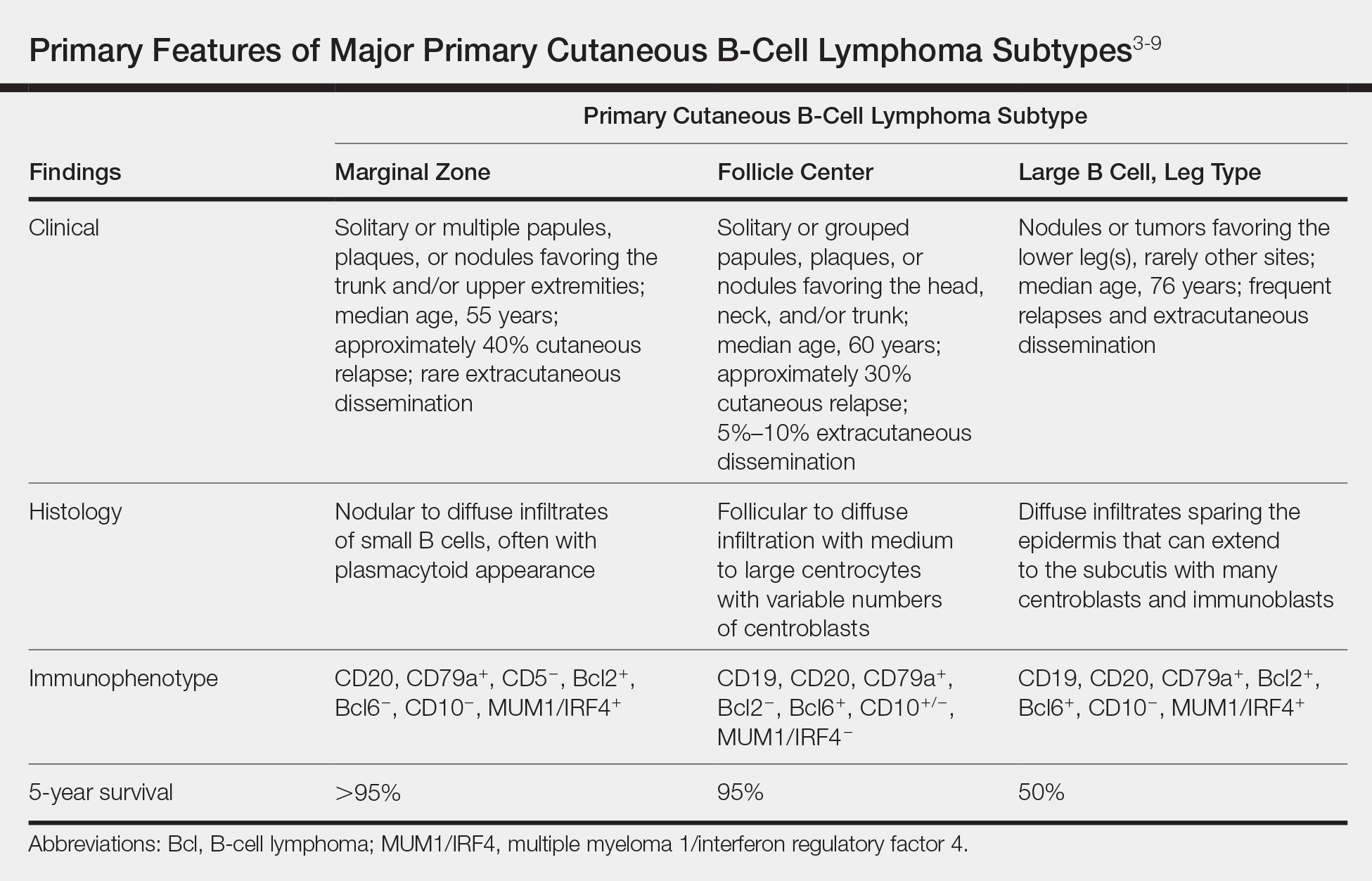
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.
Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).
The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.
Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).
The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.
Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
A 34-year-old man presented to the outpatient dermatology clinic with 3 groups of mildly pruritic, erythematous papules and plaques. The most prominent group appeared on the right posterior shoulder and had been slowly enlarging in size over the last 12 months (quiz image). A similar thinner group appeared on the left mid-back 6 months prior, and a third smaller group appeared over the left serratus anterior muscle 2 months prior. The patient reported having similar episodes dating back to his early 20s. In those instances, the lesions presented without an inciting incident, became more pronounced, and persisted for months to years before resolving. Previously affected areas included the upper and lateral back, flanks, and posterior upper arms. The patient used triamcinolone cream 0.1% up to 3 times daily on active lesions, which improved the pruritus and seemed to make the lesions resolve more quickly. He denied fever, chills, night sweats, anorexia, weight loss, fatigue, cough, and shortness of breath. His only medication was ranitidine 150 mg twice daily for gastroesophageal reflux disease. Physical examination revealed no palpable lymphadenopathy.
Predicting outcomes in acute leukemia, NSCLC
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Mindfulness-based relapse prevention tied to lower anxiety, depression
SAN ANTONIO – A mindfulness-based relapse prevention program resulted in significantly greater declines in anxiety and depressive symptoms among participants in an opioid addiction treatment program than those seen in patients who received treatment as usual, suggest results of a small nonrandomized controlled trial. Relapse rates trended downward with mindfulness but were not significantly different from the treatment-as-usual (TAU) group.
“Mindfulness-based relapse prevention (MBRP) can be successfully implemented in an outpatient setting with as good as or better results as treatment as usual,” Keith J. Zullig, PhD, MSPH, chair and professor in the department of social and behavioral sciences at the West Virginia University School of Public Health in Morgantown, said at the annual meeting of the College on Problems of Drug Dependence.
Though relapse rates did not show a statistically significant drop with mindfulness treatment compared with treatment as usual, the downward trend suggests that it is worthwhile to conduct a larger scale study, Dr. Zullig said.
, he added.
The researchers recruited 60 participants from a Comprehensive Opioid Addiction Treatment program who had been substance free for at least 90 consecutive days. Participants chose whether to enter the MBRP group or the treatment-as-usual group.
The treatment-as-usual group attended biweekly 60-minute sessions with a cognitive-based therapy process group led by a licensed therapist for 36 weeks. The MBRP group involved 24 weeks of biweekly attendance at 60-minute sessions, also led by a licensed therapist, followed by 12 weeks in the treatment-as-usual group.
The MBRP instruction involved the following:
- Mindful skill building
- Breathing
- Meditation
- Mindful movement (“gentle yoga practiced with mindful awareness of the body”)
- Using all the senses
- Increasing awareness of breath, body sensations, thoughts, and emotional energy
- Mindfulness in everyday life
- Daily home practice of formal mindfulness meditation for 30 minutes per day, 5-6 days a week
- Discussing practice/exercises both in and outside class
Researchers tracked retention rates, any prohibited substance relapse, and four self-reported measures at 12, 24, and 36 weeks’ follow-up. The self-reported measures looked at craving, with the Desire for Drug Questionnaire; anxiety, with the Overall Anxiety Severity and Impairment Scale, range 0-20); depression, with the Overall Depression Severity and Impairment Scale, range 0-20; and mindfulness, with the Five Facet Mindfulness Questionnaire.
Participants in both groups were statistically similar in gender, employment, education, insurance, and marital status at baseline.
Of the 24 patients who entered the MBRP program, 14 completed the full 24 weeks of intervention and 12 subsequent weeks. Among the 36 participants who entered the treatment-as-usual group, 20 completed the 36 weeks.
Retention was 75% in both groups at 24 weeks, but retention from 24 to 36 weeks was nonsignificantly greater in the mindfulness group (93% vs. 91% treatment as usual).
Relapse at both 24 and 36 weeks was lower among those using mindfulness but without a statistically significant difference. At 24 weeks, 44% of the treatment-as-usual participants had relapsed at least once, compared with 33% of the MBRP participants (intent to treat).
At 36 weeks (n = 37), 45% of the 22 remaining in the treatment-as-usual group had relapsed, compared with 40% of the 15 in the MBRP group. However, 20% of those in MBRP (3 of 15) relapsed between the 24 and 36 week follow-ups, compared with 5% (1 of 22) in the treatment-as-usual group, still a nonsignificant difference.
Anxiety scores were higher at baseline in the MBRP group (11 MBRP vs. 7.25 TAU) but were similar in both groups at 36 weeks (5.79 MBRP vs. 5.6 TAU). Depression scores also were higher at baseline in the MBRP (8 vs. 6.3) but ended slightly lower than the treatment-as-usual group at 36 weeks (3.71 MBRP vs. 4.35 TAU). The reductions in depression and anxiety scores for the MBRP group were significantly greater than in the treatment-as-usual group.
Mindfulness scores were not significantly different at baseline between the groups but were significantly higher at 36 weeks in the mindfulness groups (3.47 vs. 3.3, range 1-5).
“Relapse rates were trending lower in the MBRP group although not statistically significant,” Dr. Zullig said. “Significant decreases occurred in craving in both MBRP and treatment-as-usual groups.”
The Centers for Disease Control and Prevention funded the research. The authors had no disclosures.
SAN ANTONIO – A mindfulness-based relapse prevention program resulted in significantly greater declines in anxiety and depressive symptoms among participants in an opioid addiction treatment program than those seen in patients who received treatment as usual, suggest results of a small nonrandomized controlled trial. Relapse rates trended downward with mindfulness but were not significantly different from the treatment-as-usual (TAU) group.
“Mindfulness-based relapse prevention (MBRP) can be successfully implemented in an outpatient setting with as good as or better results as treatment as usual,” Keith J. Zullig, PhD, MSPH, chair and professor in the department of social and behavioral sciences at the West Virginia University School of Public Health in Morgantown, said at the annual meeting of the College on Problems of Drug Dependence.
Though relapse rates did not show a statistically significant drop with mindfulness treatment compared with treatment as usual, the downward trend suggests that it is worthwhile to conduct a larger scale study, Dr. Zullig said.
, he added.
The researchers recruited 60 participants from a Comprehensive Opioid Addiction Treatment program who had been substance free for at least 90 consecutive days. Participants chose whether to enter the MBRP group or the treatment-as-usual group.
The treatment-as-usual group attended biweekly 60-minute sessions with a cognitive-based therapy process group led by a licensed therapist for 36 weeks. The MBRP group involved 24 weeks of biweekly attendance at 60-minute sessions, also led by a licensed therapist, followed by 12 weeks in the treatment-as-usual group.
The MBRP instruction involved the following:
- Mindful skill building
- Breathing
- Meditation
- Mindful movement (“gentle yoga practiced with mindful awareness of the body”)
- Using all the senses
- Increasing awareness of breath, body sensations, thoughts, and emotional energy
- Mindfulness in everyday life
- Daily home practice of formal mindfulness meditation for 30 minutes per day, 5-6 days a week
- Discussing practice/exercises both in and outside class
Researchers tracked retention rates, any prohibited substance relapse, and four self-reported measures at 12, 24, and 36 weeks’ follow-up. The self-reported measures looked at craving, with the Desire for Drug Questionnaire; anxiety, with the Overall Anxiety Severity and Impairment Scale, range 0-20); depression, with the Overall Depression Severity and Impairment Scale, range 0-20; and mindfulness, with the Five Facet Mindfulness Questionnaire.
Participants in both groups were statistically similar in gender, employment, education, insurance, and marital status at baseline.
Of the 24 patients who entered the MBRP program, 14 completed the full 24 weeks of intervention and 12 subsequent weeks. Among the 36 participants who entered the treatment-as-usual group, 20 completed the 36 weeks.
Retention was 75% in both groups at 24 weeks, but retention from 24 to 36 weeks was nonsignificantly greater in the mindfulness group (93% vs. 91% treatment as usual).
Relapse at both 24 and 36 weeks was lower among those using mindfulness but without a statistically significant difference. At 24 weeks, 44% of the treatment-as-usual participants had relapsed at least once, compared with 33% of the MBRP participants (intent to treat).
At 36 weeks (n = 37), 45% of the 22 remaining in the treatment-as-usual group had relapsed, compared with 40% of the 15 in the MBRP group. However, 20% of those in MBRP (3 of 15) relapsed between the 24 and 36 week follow-ups, compared with 5% (1 of 22) in the treatment-as-usual group, still a nonsignificant difference.
Anxiety scores were higher at baseline in the MBRP group (11 MBRP vs. 7.25 TAU) but were similar in both groups at 36 weeks (5.79 MBRP vs. 5.6 TAU). Depression scores also were higher at baseline in the MBRP (8 vs. 6.3) but ended slightly lower than the treatment-as-usual group at 36 weeks (3.71 MBRP vs. 4.35 TAU). The reductions in depression and anxiety scores for the MBRP group were significantly greater than in the treatment-as-usual group.
Mindfulness scores were not significantly different at baseline between the groups but were significantly higher at 36 weeks in the mindfulness groups (3.47 vs. 3.3, range 1-5).
“Relapse rates were trending lower in the MBRP group although not statistically significant,” Dr. Zullig said. “Significant decreases occurred in craving in both MBRP and treatment-as-usual groups.”
The Centers for Disease Control and Prevention funded the research. The authors had no disclosures.
SAN ANTONIO – A mindfulness-based relapse prevention program resulted in significantly greater declines in anxiety and depressive symptoms among participants in an opioid addiction treatment program than those seen in patients who received treatment as usual, suggest results of a small nonrandomized controlled trial. Relapse rates trended downward with mindfulness but were not significantly different from the treatment-as-usual (TAU) group.
“Mindfulness-based relapse prevention (MBRP) can be successfully implemented in an outpatient setting with as good as or better results as treatment as usual,” Keith J. Zullig, PhD, MSPH, chair and professor in the department of social and behavioral sciences at the West Virginia University School of Public Health in Morgantown, said at the annual meeting of the College on Problems of Drug Dependence.
Though relapse rates did not show a statistically significant drop with mindfulness treatment compared with treatment as usual, the downward trend suggests that it is worthwhile to conduct a larger scale study, Dr. Zullig said.
, he added.
The researchers recruited 60 participants from a Comprehensive Opioid Addiction Treatment program who had been substance free for at least 90 consecutive days. Participants chose whether to enter the MBRP group or the treatment-as-usual group.
The treatment-as-usual group attended biweekly 60-minute sessions with a cognitive-based therapy process group led by a licensed therapist for 36 weeks. The MBRP group involved 24 weeks of biweekly attendance at 60-minute sessions, also led by a licensed therapist, followed by 12 weeks in the treatment-as-usual group.
The MBRP instruction involved the following:
- Mindful skill building
- Breathing
- Meditation
- Mindful movement (“gentle yoga practiced with mindful awareness of the body”)
- Using all the senses
- Increasing awareness of breath, body sensations, thoughts, and emotional energy
- Mindfulness in everyday life
- Daily home practice of formal mindfulness meditation for 30 minutes per day, 5-6 days a week
- Discussing practice/exercises both in and outside class
Researchers tracked retention rates, any prohibited substance relapse, and four self-reported measures at 12, 24, and 36 weeks’ follow-up. The self-reported measures looked at craving, with the Desire for Drug Questionnaire; anxiety, with the Overall Anxiety Severity and Impairment Scale, range 0-20); depression, with the Overall Depression Severity and Impairment Scale, range 0-20; and mindfulness, with the Five Facet Mindfulness Questionnaire.
Participants in both groups were statistically similar in gender, employment, education, insurance, and marital status at baseline.
Of the 24 patients who entered the MBRP program, 14 completed the full 24 weeks of intervention and 12 subsequent weeks. Among the 36 participants who entered the treatment-as-usual group, 20 completed the 36 weeks.
Retention was 75% in both groups at 24 weeks, but retention from 24 to 36 weeks was nonsignificantly greater in the mindfulness group (93% vs. 91% treatment as usual).
Relapse at both 24 and 36 weeks was lower among those using mindfulness but without a statistically significant difference. At 24 weeks, 44% of the treatment-as-usual participants had relapsed at least once, compared with 33% of the MBRP participants (intent to treat).
At 36 weeks (n = 37), 45% of the 22 remaining in the treatment-as-usual group had relapsed, compared with 40% of the 15 in the MBRP group. However, 20% of those in MBRP (3 of 15) relapsed between the 24 and 36 week follow-ups, compared with 5% (1 of 22) in the treatment-as-usual group, still a nonsignificant difference.
Anxiety scores were higher at baseline in the MBRP group (11 MBRP vs. 7.25 TAU) but were similar in both groups at 36 weeks (5.79 MBRP vs. 5.6 TAU). Depression scores also were higher at baseline in the MBRP (8 vs. 6.3) but ended slightly lower than the treatment-as-usual group at 36 weeks (3.71 MBRP vs. 4.35 TAU). The reductions in depression and anxiety scores for the MBRP group were significantly greater than in the treatment-as-usual group.
Mindfulness scores were not significantly different at baseline between the groups but were significantly higher at 36 weeks in the mindfulness groups (3.47 vs. 3.3, range 1-5).
“Relapse rates were trending lower in the MBRP group although not statistically significant,” Dr. Zullig said. “Significant decreases occurred in craving in both MBRP and treatment-as-usual groups.”
The Centers for Disease Control and Prevention funded the research. The authors had no disclosures.
REPORTING FROM CPDD 2019
HCC surveillance after anti-HCV therapy cost effective only for patients with cirrhosis
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Follow Where She Leads
ANSWER
The correct interpretation of this ECG includes normal sinus rhythm with a right bundle branch block (RBBB) and a left anterior fascicular block (LAFB). The patient also has T-wave inversions in the inferior leads.
Normal sinus rhythm is evidenced by a ventricular rate ≥ 60 beats/min and < 100 beats/min, as well as a P wave for every QRS complex, a QRS complex for every P wave, and a consistent PR interval.
Criteria for a RBBB include a QRS > 120 ms with a biphasic RSR’ seen in leads V1–V3 and slurred S waves in leads I, aVL, V5, and V6. An LAFB is defined by left-axis deviation (–48° on this ECG), small Q waves with tall R waves in leads I and aVL, and small R waves and deep S waves in leads II, III, and aVF.
An RBBB plus an LAFB constitutes bifascicular block. In such cases, the ventricles are depolarized from one remaining fascicle: the left posterior. Affected patients are susceptible to complete heart block, particularly in the presence of congestive heart failure.
For those inexperienced with ECG interpretation, fascicular blocks may be confusing. Recall that evidence of Q waves in leads II, III, and aVF is associated with inferior myocardial infarction; by substituting the Q waves with S waves in the same leads, you won’t miss an LAFB.
ANSWER
The correct interpretation of this ECG includes normal sinus rhythm with a right bundle branch block (RBBB) and a left anterior fascicular block (LAFB). The patient also has T-wave inversions in the inferior leads.
Normal sinus rhythm is evidenced by a ventricular rate ≥ 60 beats/min and < 100 beats/min, as well as a P wave for every QRS complex, a QRS complex for every P wave, and a consistent PR interval.
Criteria for a RBBB include a QRS > 120 ms with a biphasic RSR’ seen in leads V1–V3 and slurred S waves in leads I, aVL, V5, and V6. An LAFB is defined by left-axis deviation (–48° on this ECG), small Q waves with tall R waves in leads I and aVL, and small R waves and deep S waves in leads II, III, and aVF.
An RBBB plus an LAFB constitutes bifascicular block. In such cases, the ventricles are depolarized from one remaining fascicle: the left posterior. Affected patients are susceptible to complete heart block, particularly in the presence of congestive heart failure.
For those inexperienced with ECG interpretation, fascicular blocks may be confusing. Recall that evidence of Q waves in leads II, III, and aVF is associated with inferior myocardial infarction; by substituting the Q waves with S waves in the same leads, you won’t miss an LAFB.
ANSWER
The correct interpretation of this ECG includes normal sinus rhythm with a right bundle branch block (RBBB) and a left anterior fascicular block (LAFB). The patient also has T-wave inversions in the inferior leads.
Normal sinus rhythm is evidenced by a ventricular rate ≥ 60 beats/min and < 100 beats/min, as well as a P wave for every QRS complex, a QRS complex for every P wave, and a consistent PR interval.
Criteria for a RBBB include a QRS > 120 ms with a biphasic RSR’ seen in leads V1–V3 and slurred S waves in leads I, aVL, V5, and V6. An LAFB is defined by left-axis deviation (–48° on this ECG), small Q waves with tall R waves in leads I and aVL, and small R waves and deep S waves in leads II, III, and aVF.
An RBBB plus an LAFB constitutes bifascicular block. In such cases, the ventricles are depolarized from one remaining fascicle: the left posterior. Affected patients are susceptible to complete heart block, particularly in the presence of congestive heart failure.
For those inexperienced with ECG interpretation, fascicular blocks may be confusing. Recall that evidence of Q waves in leads II, III, and aVF is associated with inferior myocardial infarction; by substituting the Q waves with S waves in the same leads, you won’t miss an LAFB.
For several years, a 75-year-old woman has been followed for aortic stenosis. Historically, she has done well—but about a year ago, she began experiencing shortness of breath. Within the past 2 months, she has had significantly increasing dyspnea with minimal activity and has experienced 2 episodes of near-syncope. This morning, she complains of chest tightness.
Her medical history is remarkable for hypertension, rheumatoid arthritis, and coronary artery disease. Two years ago, catheterization for chest pain revealed stenoses of her left anterior descending artery (LAD), a diagonal branch of the LAD, and an occluded right coronary artery. A drug-eluding stent was placed, and she has had no symptoms since. She also has a history of paroxysmal atrial fibrillation but has had no episodes in the past year.
An echocardiogram performed 1 week ago revealed severe aortic valve stenosis with a trileaflet valve with heavily calcified leaflets; an aortic valve gradient of 0.4 cm2, with a peak velocity of 5 m/s and a mean gradient of 61 mm Hg; and mild aortic regurgitation. It also showed an enlarged left atrium and mild-to-moderate mitral regurgitation.
Her current medications include senna glycoside, prednisone, metoprolol, atorvastatin, and apixaban. She has a true anaphylactic allergy to penicillin.
The patient, a retired administrative assistant, was widowed 4 years ago, when her husband died as a result of an automobile accident. They had no children. She has never smoked but reports having 1 to 2 glasses of wine each night.
Review of symptoms is positive for joint pain, particularly in her hands, hips, and knees. It is worse in the morning and improves as the day progresses. She tried NSAIDs but experienced bleeding, so she was recently started on prednisone. She also has chronic constipation, for which she takes senna with reasonable results. She denies any significant constitutional, respiratory, urologic, or neurologic symptoms.
Vital signs include a blood pressure of 146/86 mm Hg; pulse, 70 beats/min; respiratory rate, 14 breaths/min-1; temperature, 97.6°F; and O2 saturation, 96% on room air. Her weight is 139 lb and her height, 5 ft 5 in; her BMI is 23.1.
Physical exam reveals corrective lenses and bilateral hearing aids. The patient has multiple bruises of varying stages on both upper extremities. Her teeth are in good repair. There is no significant jugular venous distention or thyromegaly. The lungs are clear in all fields.
The cardiac exam is remarkable for a regular rate and rhythm, with a grade III/VI crescendo-decrescendo systolic murmur best heard at the right upper sternal border. She also has a grade II/VI late systolic murmur at the apex in the left lateral decubitus position. The A2 component of the second heart sound is absent, and there are no gallops or rubs.
The abdominal exam is normal, without palpable masses. Her peripheral pulses are strong and equal bilaterally. She has arthritic changes in her hands bilaterally and has no focal neurologic symptoms.
Her ECG reveals a ventricular rate of 71 beats/min; PR interval, 152 ms; QRS duration, 142 ms; QT/QTc interval, 476/517 ms; P axis, 76°; R axis, –48°; and T axis, 161°. What is your interpretation?
Appropriateness of performing in-office uterine aspiration
In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina
In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina

In their article, "Uterine aspiration: From OR to office" (February 2019), Lauren Thaxton, MD, MBA, and Bri Tristan, MD, made the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard surgical management of early pregnancy failure. Their reasons included an equivalent safety profile, reduced costs, and patient-centered characteristics.
OBG Management posed this query to readers in a website poll: "Should the standard location for uterine apiration be in the office?" See how readers responded, below.
Poll results
A total of 73 readers cast their vote:
- 86.3% (63 readers) said yes, in appropriate clinical situations
- 13.7% (10 readers) said no
Reader comments
"Yes, in appropriate clinical situations."
-Yardlie Toussaint-Foster, DO, Downingtown, Pennsylvania
"I have been doing it this way (in the office) for years, up to 11 to 12 weeks without complication."
-John Lane, MD, Raleigh, North Carolina