User login
Treat insomnia as a full-fledged disorder
CRYSTAL CITY, VA. – Insomnia is a neuropsychiatric disorder of hyperarousal that should be evaluated as a disorder and treated with any associated comorbid conditions, Karl Doghramji, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“When I was a resident, I used to say insomnia is never a disorder. It’s always a symptom; you’ve got to find the primary disorder to know what the insomnia is caused by,” said Dr. Doghramji, medical director of the Jefferson Sleep Disorders Center at Jefferson Medical College, Philadelphia. “We no longer believe that. Throw that out the window.”
According to the new definition under the DSM-5, insomnia is characterized by dissatisfaction with sleep quality or quantity in the presence of adequate opportunity for sleep that causes significant distress or impairment for more than 3 nights per week over a period of 3 months. A survey of almost 7,500 U.S. health plan subscribers conducted a few years ago found that the prevalence of insomnia was estimated at 23.2% (Sleep. 2011 Sep 1;34[9]:1161-71).
Insomnia is also not well identified in clinical practice: In results published from his own group, Dr. Doghramji and colleagues evaluated 97 patients who were administered the Insomnia Severity Index; of those patients, 79.4% met the criteria for insomnia, but there was no mention of insomnia in the discharge notes for those patients (J Nerv Ment Dis. 2018 Oct;206[10]:765-9).
Many cognitive impairments can occur as a result of insomnia, which affects performance at work; decreases enjoyment of social activities; can lead to motor vehicle accidents or falls; and can affect health in the form of diabetes, hypertension, and increased mortality. Insomnia also can predict the risk of future depression and is a risk factor for suicide, Dr. Doghramji said at the meeting presented by Global Academy for Medical Education.
Adults can have insomnia for many reasons, including genetics, stress, negative conditioning, intrapsychic conflict, and bad habits, as well as medical and psychiatric conditions. While knowledge surrounding insomnia has advanced under a hyperarousal model, “It is really a hyperarousal disturbance which defies psychological understanding,” said Dr. Doghramji, who is also professor of psychiatry, neurology, and medicine at the university.
Evaluating the type of insomnia a patient is experiencing should be the first step in managing the disorder, followed by determining whether the insomnia is contributing to daytime impairment or decreased quality of life for the patient. From there, the insomnia can be treated with behavioral or pharmacotherapy. However, if insomnia is associated with another comorbid condition, the condition should be treated alongside the insomnia.
Sleep is highly comorbid with psychiatric and medical conditions (Sleep Med Clin. 2019 Jun;14[2]:167-75). Initial insomnia is more likely to be associated with delayed sleep phase disorder and restless legs syndrome, while middle insomnia is associated with sleep apnea and depression. Patients who wake early and are unable to go back to sleep (terminal insomnia) are likely to have depression, shift work disorder, or advanced sleep phase disorder.
said Dr. Doghramji. The comorbid condition also should be considered when deciding how to treat insomnia. For example, a patient with gastroesophageal reflux disease and insomnia would be more suited to cognitive-behavioral therapy than pharmacologic agents to help with sleep, because being able to wake up during the night from acid building in the esophagus is the body’s defense mechanism for the disease, Dr. Doghramji said.
Dr. Doghramji reported serving as a consultant for Eisai, Merck, and Pfizer. He also receives research funding from and owns stock in Merck.
Global Academy for Medical Education, Current Psychiatry, and this news organization are owned by the same company.
CRYSTAL CITY, VA. – Insomnia is a neuropsychiatric disorder of hyperarousal that should be evaluated as a disorder and treated with any associated comorbid conditions, Karl Doghramji, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“When I was a resident, I used to say insomnia is never a disorder. It’s always a symptom; you’ve got to find the primary disorder to know what the insomnia is caused by,” said Dr. Doghramji, medical director of the Jefferson Sleep Disorders Center at Jefferson Medical College, Philadelphia. “We no longer believe that. Throw that out the window.”
According to the new definition under the DSM-5, insomnia is characterized by dissatisfaction with sleep quality or quantity in the presence of adequate opportunity for sleep that causes significant distress or impairment for more than 3 nights per week over a period of 3 months. A survey of almost 7,500 U.S. health plan subscribers conducted a few years ago found that the prevalence of insomnia was estimated at 23.2% (Sleep. 2011 Sep 1;34[9]:1161-71).
Insomnia is also not well identified in clinical practice: In results published from his own group, Dr. Doghramji and colleagues evaluated 97 patients who were administered the Insomnia Severity Index; of those patients, 79.4% met the criteria for insomnia, but there was no mention of insomnia in the discharge notes for those patients (J Nerv Ment Dis. 2018 Oct;206[10]:765-9).
Many cognitive impairments can occur as a result of insomnia, which affects performance at work; decreases enjoyment of social activities; can lead to motor vehicle accidents or falls; and can affect health in the form of diabetes, hypertension, and increased mortality. Insomnia also can predict the risk of future depression and is a risk factor for suicide, Dr. Doghramji said at the meeting presented by Global Academy for Medical Education.
Adults can have insomnia for many reasons, including genetics, stress, negative conditioning, intrapsychic conflict, and bad habits, as well as medical and psychiatric conditions. While knowledge surrounding insomnia has advanced under a hyperarousal model, “It is really a hyperarousal disturbance which defies psychological understanding,” said Dr. Doghramji, who is also professor of psychiatry, neurology, and medicine at the university.
Evaluating the type of insomnia a patient is experiencing should be the first step in managing the disorder, followed by determining whether the insomnia is contributing to daytime impairment or decreased quality of life for the patient. From there, the insomnia can be treated with behavioral or pharmacotherapy. However, if insomnia is associated with another comorbid condition, the condition should be treated alongside the insomnia.
Sleep is highly comorbid with psychiatric and medical conditions (Sleep Med Clin. 2019 Jun;14[2]:167-75). Initial insomnia is more likely to be associated with delayed sleep phase disorder and restless legs syndrome, while middle insomnia is associated with sleep apnea and depression. Patients who wake early and are unable to go back to sleep (terminal insomnia) are likely to have depression, shift work disorder, or advanced sleep phase disorder.
said Dr. Doghramji. The comorbid condition also should be considered when deciding how to treat insomnia. For example, a patient with gastroesophageal reflux disease and insomnia would be more suited to cognitive-behavioral therapy than pharmacologic agents to help with sleep, because being able to wake up during the night from acid building in the esophagus is the body’s defense mechanism for the disease, Dr. Doghramji said.
Dr. Doghramji reported serving as a consultant for Eisai, Merck, and Pfizer. He also receives research funding from and owns stock in Merck.
Global Academy for Medical Education, Current Psychiatry, and this news organization are owned by the same company.
CRYSTAL CITY, VA. – Insomnia is a neuropsychiatric disorder of hyperarousal that should be evaluated as a disorder and treated with any associated comorbid conditions, Karl Doghramji, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“When I was a resident, I used to say insomnia is never a disorder. It’s always a symptom; you’ve got to find the primary disorder to know what the insomnia is caused by,” said Dr. Doghramji, medical director of the Jefferson Sleep Disorders Center at Jefferson Medical College, Philadelphia. “We no longer believe that. Throw that out the window.”
According to the new definition under the DSM-5, insomnia is characterized by dissatisfaction with sleep quality or quantity in the presence of adequate opportunity for sleep that causes significant distress or impairment for more than 3 nights per week over a period of 3 months. A survey of almost 7,500 U.S. health plan subscribers conducted a few years ago found that the prevalence of insomnia was estimated at 23.2% (Sleep. 2011 Sep 1;34[9]:1161-71).
Insomnia is also not well identified in clinical practice: In results published from his own group, Dr. Doghramji and colleagues evaluated 97 patients who were administered the Insomnia Severity Index; of those patients, 79.4% met the criteria for insomnia, but there was no mention of insomnia in the discharge notes for those patients (J Nerv Ment Dis. 2018 Oct;206[10]:765-9).
Many cognitive impairments can occur as a result of insomnia, which affects performance at work; decreases enjoyment of social activities; can lead to motor vehicle accidents or falls; and can affect health in the form of diabetes, hypertension, and increased mortality. Insomnia also can predict the risk of future depression and is a risk factor for suicide, Dr. Doghramji said at the meeting presented by Global Academy for Medical Education.
Adults can have insomnia for many reasons, including genetics, stress, negative conditioning, intrapsychic conflict, and bad habits, as well as medical and psychiatric conditions. While knowledge surrounding insomnia has advanced under a hyperarousal model, “It is really a hyperarousal disturbance which defies psychological understanding,” said Dr. Doghramji, who is also professor of psychiatry, neurology, and medicine at the university.
Evaluating the type of insomnia a patient is experiencing should be the first step in managing the disorder, followed by determining whether the insomnia is contributing to daytime impairment or decreased quality of life for the patient. From there, the insomnia can be treated with behavioral or pharmacotherapy. However, if insomnia is associated with another comorbid condition, the condition should be treated alongside the insomnia.
Sleep is highly comorbid with psychiatric and medical conditions (Sleep Med Clin. 2019 Jun;14[2]:167-75). Initial insomnia is more likely to be associated with delayed sleep phase disorder and restless legs syndrome, while middle insomnia is associated with sleep apnea and depression. Patients who wake early and are unable to go back to sleep (terminal insomnia) are likely to have depression, shift work disorder, or advanced sleep phase disorder.
said Dr. Doghramji. The comorbid condition also should be considered when deciding how to treat insomnia. For example, a patient with gastroesophageal reflux disease and insomnia would be more suited to cognitive-behavioral therapy than pharmacologic agents to help with sleep, because being able to wake up during the night from acid building in the esophagus is the body’s defense mechanism for the disease, Dr. Doghramji said.
Dr. Doghramji reported serving as a consultant for Eisai, Merck, and Pfizer. He also receives research funding from and owns stock in Merck.
Global Academy for Medical Education, Current Psychiatry, and this news organization are owned by the same company.
EXPERT ANALYSIS FROM FOCUS ON NEUROPSYCHIATRY 2019
Improving self-confidence
The best way to ensure that I’ll run late in clinic is to start late. I avoid such delayed starts as scabies, yet, sometimes it’s unavoidable. I walked into my 1:30 appointment at 1:35. “Can I ask you a question?” my bony, patient with the long gray beard asked. “Sure.” I replied. “Is your time important to you?” he snapped.
Oh, boy. Here we go.
“I’m sorry I’m running late and kept you waiting,” I offered, “but I had a sick patient this morning.” When he retorted that his time was important, too, I interrupted him.
“Please sit on the exam table and tell me how I can help you so we don’t waste any more of your time.” He went on to complain that the treatments for his facial seborrheic dermatitis did not resolve the problem. When he stops treatment, it flares. I explained that this was a chronic condition and that he could manage it with my help. He resisted, but with each parry, his aggressiveness weakened. We reviewed behavior, product, and medication options for him. By the end of the visit, he was (mostly) pleased and left with a plan and prescription to help.
Early in my career, this appointment might have been disastrous: It would have ruined my afternoon and possibly led to a formal patient complaint. His antagonistic comments and boorish behavior would have unsettled me. But it didn’t now.
I had the confidence to know his diagnosis and how to help him, despite his dissatisfaction. Those with strong self-confidence not only have better patient satisfaction and higher quality but also are more efficient and have high level of satisfaction with their career. When your confidence is low, medical decision making and managing patient expectations become difficult. This is particularly true when a patient comes “informed.” Often their knowledge is helpful but, as we know, sometimes it’s bogus, even detrimental. Although we ought to have come a long way from the brash doctor-knows-best days of our past, we also ought not capitulate to patients. Sometimes, you have to be the doctor. Balancing confidence with compassion is tricky yet essential to success.
When I meet with our young doctors, I try to provide feedback not only on their medical acumen but also on their confidence to deploy that expertise. Like a skill, self-confidence can be improved. The best way is to recognize difficult conversations and do not avoid them. When you feel your face flush and heart race, take a good belly breath and step into it. You don’t have to confront or argue with your patient, you do have to assert and negotiate. Helping a difficult patient can feel like you’ve done something wrong, but chances are, you haven’t. Reframe the situation, think of it as you doing the hard work to help them. Being confident is as important as getting the diagnosis right. Even when you don’t know the diagnosis, you can be most helpful when you are direct and say so. “I’m not sure what you have, but here is how I’m going to help you.”
To improve self-confidence you’ll have to practice. When you have a difficult visit that ultimately ended well, make a note of it. Reflect on it. The next time you have a challenging patient, remember your previous success and how you felt. Then breathe and do it again. After all, you are the doctor.
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
The best way to ensure that I’ll run late in clinic is to start late. I avoid such delayed starts as scabies, yet, sometimes it’s unavoidable. I walked into my 1:30 appointment at 1:35. “Can I ask you a question?” my bony, patient with the long gray beard asked. “Sure.” I replied. “Is your time important to you?” he snapped.
Oh, boy. Here we go.
“I’m sorry I’m running late and kept you waiting,” I offered, “but I had a sick patient this morning.” When he retorted that his time was important, too, I interrupted him.
“Please sit on the exam table and tell me how I can help you so we don’t waste any more of your time.” He went on to complain that the treatments for his facial seborrheic dermatitis did not resolve the problem. When he stops treatment, it flares. I explained that this was a chronic condition and that he could manage it with my help. He resisted, but with each parry, his aggressiveness weakened. We reviewed behavior, product, and medication options for him. By the end of the visit, he was (mostly) pleased and left with a plan and prescription to help.
Early in my career, this appointment might have been disastrous: It would have ruined my afternoon and possibly led to a formal patient complaint. His antagonistic comments and boorish behavior would have unsettled me. But it didn’t now.
I had the confidence to know his diagnosis and how to help him, despite his dissatisfaction. Those with strong self-confidence not only have better patient satisfaction and higher quality but also are more efficient and have high level of satisfaction with their career. When your confidence is low, medical decision making and managing patient expectations become difficult. This is particularly true when a patient comes “informed.” Often their knowledge is helpful but, as we know, sometimes it’s bogus, even detrimental. Although we ought to have come a long way from the brash doctor-knows-best days of our past, we also ought not capitulate to patients. Sometimes, you have to be the doctor. Balancing confidence with compassion is tricky yet essential to success.
When I meet with our young doctors, I try to provide feedback not only on their medical acumen but also on their confidence to deploy that expertise. Like a skill, self-confidence can be improved. The best way is to recognize difficult conversations and do not avoid them. When you feel your face flush and heart race, take a good belly breath and step into it. You don’t have to confront or argue with your patient, you do have to assert and negotiate. Helping a difficult patient can feel like you’ve done something wrong, but chances are, you haven’t. Reframe the situation, think of it as you doing the hard work to help them. Being confident is as important as getting the diagnosis right. Even when you don’t know the diagnosis, you can be most helpful when you are direct and say so. “I’m not sure what you have, but here is how I’m going to help you.”
To improve self-confidence you’ll have to practice. When you have a difficult visit that ultimately ended well, make a note of it. Reflect on it. The next time you have a challenging patient, remember your previous success and how you felt. Then breathe and do it again. After all, you are the doctor.
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
The best way to ensure that I’ll run late in clinic is to start late. I avoid such delayed starts as scabies, yet, sometimes it’s unavoidable. I walked into my 1:30 appointment at 1:35. “Can I ask you a question?” my bony, patient with the long gray beard asked. “Sure.” I replied. “Is your time important to you?” he snapped.
Oh, boy. Here we go.
“I’m sorry I’m running late and kept you waiting,” I offered, “but I had a sick patient this morning.” When he retorted that his time was important, too, I interrupted him.
“Please sit on the exam table and tell me how I can help you so we don’t waste any more of your time.” He went on to complain that the treatments for his facial seborrheic dermatitis did not resolve the problem. When he stops treatment, it flares. I explained that this was a chronic condition and that he could manage it with my help. He resisted, but with each parry, his aggressiveness weakened. We reviewed behavior, product, and medication options for him. By the end of the visit, he was (mostly) pleased and left with a plan and prescription to help.
Early in my career, this appointment might have been disastrous: It would have ruined my afternoon and possibly led to a formal patient complaint. His antagonistic comments and boorish behavior would have unsettled me. But it didn’t now.
I had the confidence to know his diagnosis and how to help him, despite his dissatisfaction. Those with strong self-confidence not only have better patient satisfaction and higher quality but also are more efficient and have high level of satisfaction with their career. When your confidence is low, medical decision making and managing patient expectations become difficult. This is particularly true when a patient comes “informed.” Often their knowledge is helpful but, as we know, sometimes it’s bogus, even detrimental. Although we ought to have come a long way from the brash doctor-knows-best days of our past, we also ought not capitulate to patients. Sometimes, you have to be the doctor. Balancing confidence with compassion is tricky yet essential to success.
When I meet with our young doctors, I try to provide feedback not only on their medical acumen but also on their confidence to deploy that expertise. Like a skill, self-confidence can be improved. The best way is to recognize difficult conversations and do not avoid them. When you feel your face flush and heart race, take a good belly breath and step into it. You don’t have to confront or argue with your patient, you do have to assert and negotiate. Helping a difficult patient can feel like you’ve done something wrong, but chances are, you haven’t. Reframe the situation, think of it as you doing the hard work to help them. Being confident is as important as getting the diagnosis right. Even when you don’t know the diagnosis, you can be most helpful when you are direct and say so. “I’m not sure what you have, but here is how I’m going to help you.”
To improve self-confidence you’ll have to practice. When you have a difficult visit that ultimately ended well, make a note of it. Reflect on it. The next time you have a challenging patient, remember your previous success and how you felt. Then breathe and do it again. After all, you are the doctor.
Dr. Benabio is director of health care transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Illinois law expands abortion rights for women
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
A/T/N system predicts cognitive decline
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
FROM JAMA
CMS seeks answers on prior authorization, other hassles to eliminate
Got an idea on how to reduce administrative burden to help reduce the cost of delivering health care? The Centers for Medicare & Medicaid Services wants to hear from you.
In a request for information published June 6, the agency seeks parties across the health care spectrum “to recommend further changes to rules, policies, and procedures that would shift more of clinicians’ time and our health care system’s resources from needless paperwork to high-quality care that improves patient health,” CMS officials said in a statement.
The request for information, part of the agency’s Patients Over Paperwork initiative, seeks suggestions on how to reduce hassles associated with reporting and documentation, coding, prior authorization, rural issues, dual eligible patients, enrollment/eligibility determination and the agency’s own process for issuing regulations and policies.
“Patients over Paperwork has made great inroads in clearing away needlessly complex, outdated, or duplicative requirements that drain clinicians’ time but contribute little to quality of care or patient health,” CMS Administrator Seema Verma said in a statement. “Our goal is to ensure that doctors are spending more time with their patients and less time in administrative tasks.”
The request for information is scheduled to published in the Federal Register on June 11. Comments are due to the agency on Aug. 12. Comments can be made at www.regulations.gov and should refer to file code CMS-6082-NC.
AGA will submit comments to CMS on this issue given the huge burden that prior authorization plays in practices and the time that it takes away from providing care to patients. In the meantime, ask your legislator to support Improving Seniors Access to Timely Care Act of 2019, which was recently introduced in Congress to streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Learn more at http://ow.ly/tJfX30oW5l7.
SOURCE: Federal Register, CMS-6082-NC, https://federalregister.gov/d/2019-12215.
Got an idea on how to reduce administrative burden to help reduce the cost of delivering health care? The Centers for Medicare & Medicaid Services wants to hear from you.
In a request for information published June 6, the agency seeks parties across the health care spectrum “to recommend further changes to rules, policies, and procedures that would shift more of clinicians’ time and our health care system’s resources from needless paperwork to high-quality care that improves patient health,” CMS officials said in a statement.
The request for information, part of the agency’s Patients Over Paperwork initiative, seeks suggestions on how to reduce hassles associated with reporting and documentation, coding, prior authorization, rural issues, dual eligible patients, enrollment/eligibility determination and the agency’s own process for issuing regulations and policies.
“Patients over Paperwork has made great inroads in clearing away needlessly complex, outdated, or duplicative requirements that drain clinicians’ time but contribute little to quality of care or patient health,” CMS Administrator Seema Verma said in a statement. “Our goal is to ensure that doctors are spending more time with their patients and less time in administrative tasks.”
The request for information is scheduled to published in the Federal Register on June 11. Comments are due to the agency on Aug. 12. Comments can be made at www.regulations.gov and should refer to file code CMS-6082-NC.
AGA will submit comments to CMS on this issue given the huge burden that prior authorization plays in practices and the time that it takes away from providing care to patients. In the meantime, ask your legislator to support Improving Seniors Access to Timely Care Act of 2019, which was recently introduced in Congress to streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Learn more at http://ow.ly/tJfX30oW5l7.
SOURCE: Federal Register, CMS-6082-NC, https://federalregister.gov/d/2019-12215.
Got an idea on how to reduce administrative burden to help reduce the cost of delivering health care? The Centers for Medicare & Medicaid Services wants to hear from you.
In a request for information published June 6, the agency seeks parties across the health care spectrum “to recommend further changes to rules, policies, and procedures that would shift more of clinicians’ time and our health care system’s resources from needless paperwork to high-quality care that improves patient health,” CMS officials said in a statement.
The request for information, part of the agency’s Patients Over Paperwork initiative, seeks suggestions on how to reduce hassles associated with reporting and documentation, coding, prior authorization, rural issues, dual eligible patients, enrollment/eligibility determination and the agency’s own process for issuing regulations and policies.
“Patients over Paperwork has made great inroads in clearing away needlessly complex, outdated, or duplicative requirements that drain clinicians’ time but contribute little to quality of care or patient health,” CMS Administrator Seema Verma said in a statement. “Our goal is to ensure that doctors are spending more time with their patients and less time in administrative tasks.”
The request for information is scheduled to published in the Federal Register on June 11. Comments are due to the agency on Aug. 12. Comments can be made at www.regulations.gov and should refer to file code CMS-6082-NC.
AGA will submit comments to CMS on this issue given the huge burden that prior authorization plays in practices and the time that it takes away from providing care to patients. In the meantime, ask your legislator to support Improving Seniors Access to Timely Care Act of 2019, which was recently introduced in Congress to streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Learn more at http://ow.ly/tJfX30oW5l7.
SOURCE: Federal Register, CMS-6082-NC, https://federalregister.gov/d/2019-12215.
Key clinical point: CMS is looking to expand Patients Over Paperwork initiative.
Major finding: The agency issued a request for information to solicit ideas to reduce administrative burdens.
Study details: Ideas are sought in the following areas: reporting and documentation, coding, prior authorization, rural issues, dual eligible patients, enrollment/eligibility determination and the agency’s own process for issuing regulations and policies. The request for information is scheduled to published in the Federal Register on June 11. Comments are due to the agency on Aug. 12. Comments can be made at www.regulations.gov and should refer to file code CMS-6082-NC.Disclosures: None .
Source: Federal Register, CMS-6082-NC, https://federalregister.gov/d/2019-12215.
Malnutrition leads to worse outcomes in frail elderly patients treated for PAD
Frailty increasingly has been seen as a factor in procedural outcomes, including vascular surgery. Nutrition factors among older adults have also become an issue of concern, and older adults undergoing interventions for peripheral arterial disease (PAD) may be at risk for malnutrition. At the Vascular Annual Meeting, Laura Drudi, MD, of McGill University, Montreal, reported on a study that she and her colleagues performed to determine the association between preprocedural nutritional status and all-cause mortality in patients being treated for PAD.
Dr. Drudi detailed their post hoc analysis of the FRAILED (Frailty Assessment in Lower Extremity arterial Disease) prospective cohort, which comprised two centers recruiting patients during July 1, 2015–Oct.1, 2016. Individuals who underwent vascular interventions for Rutherford class 3 or higher PAD were enrolled. Trained observers used the Mini Nutritional Assessment (MNA)–Short Form to assess the patients before their procedures. Scores less than or equal to 7 on a 14-point scale were considered malnourished, with scores of 8-11 indicated that patients were at risk for malnutrition.
The modified Essential Frailty Toolset (mEFT) was simultaneously used to measure frailty, with scores of 3 or less on a 5-point scale considered frail. The primary endpoint of the study was all-cause mortality at 12 months after the procedure. Results were available for a cohort of 148 patients (39.2% women) with a mean age of 70 years, and a mean body mass index of 26.7 kg/m2. Among these patients, 59 (40%) had claudication and 89 (60%) had chronic limb-threatening ischemia. A total of 98 (66%) patients underwent endovascular revascularization and 50 (34%) underwent open or hybrid revascularization.
Overall, 3% of subjects were classified as malnourished and 33% were at risk for malnutrition. There were 9 (6%) deaths at 12 months. Mini Nutritional Assessment–Short Form scores were modestly but significantly correlated with the mEFT scores (Pearson’s R = –0.48; P less than .001).
”We found that patients with malnourishment or at risk of malnourishment had a 2.5-fold higher crude 1-year mortality, compared with those with normal nutritional status,” said Dr. Drudi.
In the 41% of patients deemed frail, malnutrition was associated with all-cause mortality (adjusted odds ratio, 2.08 per point decrease in MNA scores); whereas in the nonfrail patients, MNA scores had little or no effect on mortality (adjusted OR, 1.05).
“Preprocedural nutritional status is associated with mortality in frail older adults undergoing interventions for PAD. Clinical trials are needed to determine whether pre- and postprocedural nutritional interventions can improve clinical outcomes in these vulnerable individuals,” Dr. Drudi concluded.
Frailty increasingly has been seen as a factor in procedural outcomes, including vascular surgery. Nutrition factors among older adults have also become an issue of concern, and older adults undergoing interventions for peripheral arterial disease (PAD) may be at risk for malnutrition. At the Vascular Annual Meeting, Laura Drudi, MD, of McGill University, Montreal, reported on a study that she and her colleagues performed to determine the association between preprocedural nutritional status and all-cause mortality in patients being treated for PAD.
Dr. Drudi detailed their post hoc analysis of the FRAILED (Frailty Assessment in Lower Extremity arterial Disease) prospective cohort, which comprised two centers recruiting patients during July 1, 2015–Oct.1, 2016. Individuals who underwent vascular interventions for Rutherford class 3 or higher PAD were enrolled. Trained observers used the Mini Nutritional Assessment (MNA)–Short Form to assess the patients before their procedures. Scores less than or equal to 7 on a 14-point scale were considered malnourished, with scores of 8-11 indicated that patients were at risk for malnutrition.
The modified Essential Frailty Toolset (mEFT) was simultaneously used to measure frailty, with scores of 3 or less on a 5-point scale considered frail. The primary endpoint of the study was all-cause mortality at 12 months after the procedure. Results were available for a cohort of 148 patients (39.2% women) with a mean age of 70 years, and a mean body mass index of 26.7 kg/m2. Among these patients, 59 (40%) had claudication and 89 (60%) had chronic limb-threatening ischemia. A total of 98 (66%) patients underwent endovascular revascularization and 50 (34%) underwent open or hybrid revascularization.
Overall, 3% of subjects were classified as malnourished and 33% were at risk for malnutrition. There were 9 (6%) deaths at 12 months. Mini Nutritional Assessment–Short Form scores were modestly but significantly correlated with the mEFT scores (Pearson’s R = –0.48; P less than .001).
”We found that patients with malnourishment or at risk of malnourishment had a 2.5-fold higher crude 1-year mortality, compared with those with normal nutritional status,” said Dr. Drudi.
In the 41% of patients deemed frail, malnutrition was associated with all-cause mortality (adjusted odds ratio, 2.08 per point decrease in MNA scores); whereas in the nonfrail patients, MNA scores had little or no effect on mortality (adjusted OR, 1.05).
“Preprocedural nutritional status is associated with mortality in frail older adults undergoing interventions for PAD. Clinical trials are needed to determine whether pre- and postprocedural nutritional interventions can improve clinical outcomes in these vulnerable individuals,” Dr. Drudi concluded.
Frailty increasingly has been seen as a factor in procedural outcomes, including vascular surgery. Nutrition factors among older adults have also become an issue of concern, and older adults undergoing interventions for peripheral arterial disease (PAD) may be at risk for malnutrition. At the Vascular Annual Meeting, Laura Drudi, MD, of McGill University, Montreal, reported on a study that she and her colleagues performed to determine the association between preprocedural nutritional status and all-cause mortality in patients being treated for PAD.
Dr. Drudi detailed their post hoc analysis of the FRAILED (Frailty Assessment in Lower Extremity arterial Disease) prospective cohort, which comprised two centers recruiting patients during July 1, 2015–Oct.1, 2016. Individuals who underwent vascular interventions for Rutherford class 3 or higher PAD were enrolled. Trained observers used the Mini Nutritional Assessment (MNA)–Short Form to assess the patients before their procedures. Scores less than or equal to 7 on a 14-point scale were considered malnourished, with scores of 8-11 indicated that patients were at risk for malnutrition.
The modified Essential Frailty Toolset (mEFT) was simultaneously used to measure frailty, with scores of 3 or less on a 5-point scale considered frail. The primary endpoint of the study was all-cause mortality at 12 months after the procedure. Results were available for a cohort of 148 patients (39.2% women) with a mean age of 70 years, and a mean body mass index of 26.7 kg/m2. Among these patients, 59 (40%) had claudication and 89 (60%) had chronic limb-threatening ischemia. A total of 98 (66%) patients underwent endovascular revascularization and 50 (34%) underwent open or hybrid revascularization.
Overall, 3% of subjects were classified as malnourished and 33% were at risk for malnutrition. There were 9 (6%) deaths at 12 months. Mini Nutritional Assessment–Short Form scores were modestly but significantly correlated with the mEFT scores (Pearson’s R = –0.48; P less than .001).
”We found that patients with malnourishment or at risk of malnourishment had a 2.5-fold higher crude 1-year mortality, compared with those with normal nutritional status,” said Dr. Drudi.
In the 41% of patients deemed frail, malnutrition was associated with all-cause mortality (adjusted odds ratio, 2.08 per point decrease in MNA scores); whereas in the nonfrail patients, MNA scores had little or no effect on mortality (adjusted OR, 1.05).
“Preprocedural nutritional status is associated with mortality in frail older adults undergoing interventions for PAD. Clinical trials are needed to determine whether pre- and postprocedural nutritional interventions can improve clinical outcomes in these vulnerable individuals,” Dr. Drudi concluded.
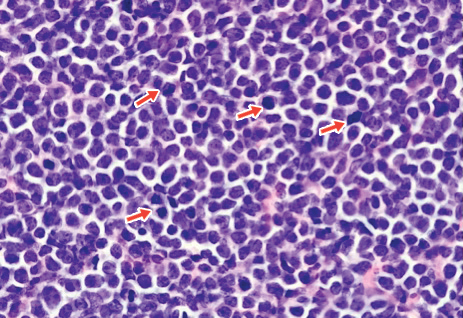
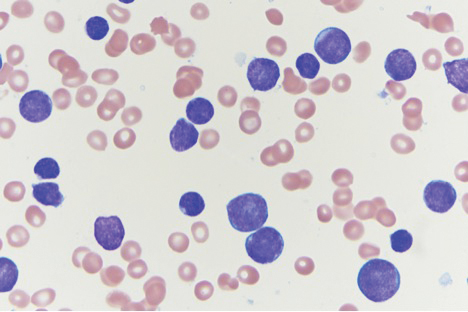
5F9 plus rituximab take a bite out of drug-resistant NHL
AMSTERDAM – The novel monoclonal antibody , thereby thwarting a mechanism that non-Hodgkin lymphomas (NHL) use to evade immune surveillance, investigators report.
Among 97 patients with relapsed or refractory aggressive or indolent lymphomas in a phase 1b/2 trial who were treated with Hu5F9 (5F9) plus rituximab, the objective response rate (ORR) was 45%, reported Mark Roschewski, MD, of the National Cancer Institute’s Center for Cancer Research in Bethesda, Md.
5F9 is an immune checkpoint inhibitor that targets CD47, the “don’t eat me” signal that inhibits macrophages from carrying out their crucial phagocytosis role.
“Rituximab, through its activity on the Fc receptor, places an extrinsic ‘eat me’ signal, so when you give these two things together you’re blocking the ‘don’t eat me’ signal and you’re placing the ‘eat me’ signal, and then the cell becomes susceptible to phagocytosis,” he said in a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
5F9 is the first agent in its class and the most advanced in clinical trials, but several similar agents are also in development. As previously reported, a similar molecule labeled TTI-621 has shown early activity in the treatment of T-cell lymphomas.
In an interview, Dr. Roschewski explained that the therapeutic approach shows promise for the treatment of NHL and nonmalignant diseases.
“This target isn’t even specific to cancer. There is rationale for using this to treat infections or other conditions. Basically, anything that your innate immune system should normally chew up, if that cell has always been evading it using that signal, this removes that [evasion] mechanism,” he said.
In preclinical studies, 5F9 showed the most activity against NHL and acute myeloid leukemia, he noted.
Results of the phase 1b portion of the study were reported in the New England Journal of Medicine (2018;379:1711-21). At the EHA Congress, Dr. Roschewski reported on extended follow-up of the phase 1b cohort and preliminary phase 2 data.
In phase 2, the investigators enrolled patients with diffuse large B-cell lymphoma (DLBCL) that was either primary refractory to standard therapy or relapsed/refractory after two or more prior lines of therapy and who were not eligible for chimeric antigen receptor T-cell therapy. They also enrolled a smaller cohort of patients with the indolent lymphoma histologies follicular lymphoma and marginal zone lymphoma (MZL) whose disease was relapsed or refractory to at least two prior lines.
Dr. Roschewski reported on pooled data for 115 patients enrolled in phases 1b and 2: 70 with DLBCL, 41 with FL, and 4 with MZL.
The patients were heavily pretreated with a median of three prior lines of therapy. Of the patients with DLBCL, 59% had primary refractory disease, and 89% of the patients with DLBCL in phase 2 were not eligible for CAR T-cell therapy.
Among all patients in this analysis, 85% had disease that was refractory to a prior rituximab-containing regimen, and the majority had disease that was refractory to the last rituximab-containing regimen.
Among 97 patients evaluable for response (59 with DLBCL, 35 with FL, and 3 with MZL), the ORR was 45%, including 19% CR and 27% partial responses (PR). An additional 17% of patients had stable disease, and 38% experienced disease progression.
The ORR for DLBCL patients was 35%, consisting of 15% CR and 20% PR. An additional 12% of patients with DLBCL had stable disease, and 53% experienced progression.
Of the patients with indolent lymphomas, the ORR was 61%, including 24% CR and 37% PR. Of this group, 24% had stable disease, and 16% had disease progression.
For all patients, the median time to response was 1.8 months (range 1.6-7.3 months).
Efficacy among patients with DLBCL was similar across subtypes and for patients with primary refractory vs. acquired refractory disease. The responses also were similar irrespective of prior lines of therapy.
Patients tolerated the combination well, with no maximum tolerated dose at up to 45 mg/kg of 5F9, and no significant dose-related toxicities.
Most adverse events were grade 1 or 2. The most common adverse events included expected on-target anemia, caused by clearance of aging red blood cells, which are cleared by the CD47-blocking effects of 5F9. This anemia can be mitigated with an initial priming dose of 1 mg/kg 5FP that causes a transient mild decline in hemoglobin and a temporary reticulocytosis that soon resolves. Hemoglobin levels return to baseline even with continued 5F9 at doses much higher than the priming dose, Dr. Roschewski said.
Other adverse events were infusion reactions and related symptoms. There were no autoimmune adverse events, and just 8 of the 115 patients available for the safety analysis (7%) had to discontinue therapy.
Enrollment in the phase 2 trial is continuing, and a 30-mg/kg maintenance dose of 5F9 has been selected for a trial in patients with DLBCL who are either ineligible for CAR T-cell therapy or have disease that progressed on three or more prior lines of therapy.
The study is funded by Forty Seven and the Leukemia and Lymphoma Society. Dr. Roschewski reported having no financial disclosures.
SOURCE: Roschewski M et al. EHA Congress, Abstract S867.
AMSTERDAM – The novel monoclonal antibody , thereby thwarting a mechanism that non-Hodgkin lymphomas (NHL) use to evade immune surveillance, investigators report.
Among 97 patients with relapsed or refractory aggressive or indolent lymphomas in a phase 1b/2 trial who were treated with Hu5F9 (5F9) plus rituximab, the objective response rate (ORR) was 45%, reported Mark Roschewski, MD, of the National Cancer Institute’s Center for Cancer Research in Bethesda, Md.
5F9 is an immune checkpoint inhibitor that targets CD47, the “don’t eat me” signal that inhibits macrophages from carrying out their crucial phagocytosis role.
“Rituximab, through its activity on the Fc receptor, places an extrinsic ‘eat me’ signal, so when you give these two things together you’re blocking the ‘don’t eat me’ signal and you’re placing the ‘eat me’ signal, and then the cell becomes susceptible to phagocytosis,” he said in a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
5F9 is the first agent in its class and the most advanced in clinical trials, but several similar agents are also in development. As previously reported, a similar molecule labeled TTI-621 has shown early activity in the treatment of T-cell lymphomas.
In an interview, Dr. Roschewski explained that the therapeutic approach shows promise for the treatment of NHL and nonmalignant diseases.
“This target isn’t even specific to cancer. There is rationale for using this to treat infections or other conditions. Basically, anything that your innate immune system should normally chew up, if that cell has always been evading it using that signal, this removes that [evasion] mechanism,” he said.
In preclinical studies, 5F9 showed the most activity against NHL and acute myeloid leukemia, he noted.
Results of the phase 1b portion of the study were reported in the New England Journal of Medicine (2018;379:1711-21). At the EHA Congress, Dr. Roschewski reported on extended follow-up of the phase 1b cohort and preliminary phase 2 data.
In phase 2, the investigators enrolled patients with diffuse large B-cell lymphoma (DLBCL) that was either primary refractory to standard therapy or relapsed/refractory after two or more prior lines of therapy and who were not eligible for chimeric antigen receptor T-cell therapy. They also enrolled a smaller cohort of patients with the indolent lymphoma histologies follicular lymphoma and marginal zone lymphoma (MZL) whose disease was relapsed or refractory to at least two prior lines.
Dr. Roschewski reported on pooled data for 115 patients enrolled in phases 1b and 2: 70 with DLBCL, 41 with FL, and 4 with MZL.
The patients were heavily pretreated with a median of three prior lines of therapy. Of the patients with DLBCL, 59% had primary refractory disease, and 89% of the patients with DLBCL in phase 2 were not eligible for CAR T-cell therapy.
Among all patients in this analysis, 85% had disease that was refractory to a prior rituximab-containing regimen, and the majority had disease that was refractory to the last rituximab-containing regimen.
Among 97 patients evaluable for response (59 with DLBCL, 35 with FL, and 3 with MZL), the ORR was 45%, including 19% CR and 27% partial responses (PR). An additional 17% of patients had stable disease, and 38% experienced disease progression.
The ORR for DLBCL patients was 35%, consisting of 15% CR and 20% PR. An additional 12% of patients with DLBCL had stable disease, and 53% experienced progression.
Of the patients with indolent lymphomas, the ORR was 61%, including 24% CR and 37% PR. Of this group, 24% had stable disease, and 16% had disease progression.
For all patients, the median time to response was 1.8 months (range 1.6-7.3 months).
Efficacy among patients with DLBCL was similar across subtypes and for patients with primary refractory vs. acquired refractory disease. The responses also were similar irrespective of prior lines of therapy.
Patients tolerated the combination well, with no maximum tolerated dose at up to 45 mg/kg of 5F9, and no significant dose-related toxicities.
Most adverse events were grade 1 or 2. The most common adverse events included expected on-target anemia, caused by clearance of aging red blood cells, which are cleared by the CD47-blocking effects of 5F9. This anemia can be mitigated with an initial priming dose of 1 mg/kg 5FP that causes a transient mild decline in hemoglobin and a temporary reticulocytosis that soon resolves. Hemoglobin levels return to baseline even with continued 5F9 at doses much higher than the priming dose, Dr. Roschewski said.
Other adverse events were infusion reactions and related symptoms. There were no autoimmune adverse events, and just 8 of the 115 patients available for the safety analysis (7%) had to discontinue therapy.
Enrollment in the phase 2 trial is continuing, and a 30-mg/kg maintenance dose of 5F9 has been selected for a trial in patients with DLBCL who are either ineligible for CAR T-cell therapy or have disease that progressed on three or more prior lines of therapy.
The study is funded by Forty Seven and the Leukemia and Lymphoma Society. Dr. Roschewski reported having no financial disclosures.
SOURCE: Roschewski M et al. EHA Congress, Abstract S867.
AMSTERDAM – The novel monoclonal antibody , thereby thwarting a mechanism that non-Hodgkin lymphomas (NHL) use to evade immune surveillance, investigators report.
Among 97 patients with relapsed or refractory aggressive or indolent lymphomas in a phase 1b/2 trial who were treated with Hu5F9 (5F9) plus rituximab, the objective response rate (ORR) was 45%, reported Mark Roschewski, MD, of the National Cancer Institute’s Center for Cancer Research in Bethesda, Md.
5F9 is an immune checkpoint inhibitor that targets CD47, the “don’t eat me” signal that inhibits macrophages from carrying out their crucial phagocytosis role.
“Rituximab, through its activity on the Fc receptor, places an extrinsic ‘eat me’ signal, so when you give these two things together you’re blocking the ‘don’t eat me’ signal and you’re placing the ‘eat me’ signal, and then the cell becomes susceptible to phagocytosis,” he said in a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
5F9 is the first agent in its class and the most advanced in clinical trials, but several similar agents are also in development. As previously reported, a similar molecule labeled TTI-621 has shown early activity in the treatment of T-cell lymphomas.
In an interview, Dr. Roschewski explained that the therapeutic approach shows promise for the treatment of NHL and nonmalignant diseases.
“This target isn’t even specific to cancer. There is rationale for using this to treat infections or other conditions. Basically, anything that your innate immune system should normally chew up, if that cell has always been evading it using that signal, this removes that [evasion] mechanism,” he said.
In preclinical studies, 5F9 showed the most activity against NHL and acute myeloid leukemia, he noted.
Results of the phase 1b portion of the study were reported in the New England Journal of Medicine (2018;379:1711-21). At the EHA Congress, Dr. Roschewski reported on extended follow-up of the phase 1b cohort and preliminary phase 2 data.
In phase 2, the investigators enrolled patients with diffuse large B-cell lymphoma (DLBCL) that was either primary refractory to standard therapy or relapsed/refractory after two or more prior lines of therapy and who were not eligible for chimeric antigen receptor T-cell therapy. They also enrolled a smaller cohort of patients with the indolent lymphoma histologies follicular lymphoma and marginal zone lymphoma (MZL) whose disease was relapsed or refractory to at least two prior lines.
Dr. Roschewski reported on pooled data for 115 patients enrolled in phases 1b and 2: 70 with DLBCL, 41 with FL, and 4 with MZL.
The patients were heavily pretreated with a median of three prior lines of therapy. Of the patients with DLBCL, 59% had primary refractory disease, and 89% of the patients with DLBCL in phase 2 were not eligible for CAR T-cell therapy.
Among all patients in this analysis, 85% had disease that was refractory to a prior rituximab-containing regimen, and the majority had disease that was refractory to the last rituximab-containing regimen.
Among 97 patients evaluable for response (59 with DLBCL, 35 with FL, and 3 with MZL), the ORR was 45%, including 19% CR and 27% partial responses (PR). An additional 17% of patients had stable disease, and 38% experienced disease progression.
The ORR for DLBCL patients was 35%, consisting of 15% CR and 20% PR. An additional 12% of patients with DLBCL had stable disease, and 53% experienced progression.
Of the patients with indolent lymphomas, the ORR was 61%, including 24% CR and 37% PR. Of this group, 24% had stable disease, and 16% had disease progression.
For all patients, the median time to response was 1.8 months (range 1.6-7.3 months).
Efficacy among patients with DLBCL was similar across subtypes and for patients with primary refractory vs. acquired refractory disease. The responses also were similar irrespective of prior lines of therapy.
Patients tolerated the combination well, with no maximum tolerated dose at up to 45 mg/kg of 5F9, and no significant dose-related toxicities.
Most adverse events were grade 1 or 2. The most common adverse events included expected on-target anemia, caused by clearance of aging red blood cells, which are cleared by the CD47-blocking effects of 5F9. This anemia can be mitigated with an initial priming dose of 1 mg/kg 5FP that causes a transient mild decline in hemoglobin and a temporary reticulocytosis that soon resolves. Hemoglobin levels return to baseline even with continued 5F9 at doses much higher than the priming dose, Dr. Roschewski said.
Other adverse events were infusion reactions and related symptoms. There were no autoimmune adverse events, and just 8 of the 115 patients available for the safety analysis (7%) had to discontinue therapy.
Enrollment in the phase 2 trial is continuing, and a 30-mg/kg maintenance dose of 5F9 has been selected for a trial in patients with DLBCL who are either ineligible for CAR T-cell therapy or have disease that progressed on three or more prior lines of therapy.
The study is funded by Forty Seven and the Leukemia and Lymphoma Society. Dr. Roschewski reported having no financial disclosures.
SOURCE: Roschewski M et al. EHA Congress, Abstract S867.
REPORTING FROM EHA CONGRESS
Key clinical point: The combination of Hu5F9 and rituximab shows activity in heavily pretreated, relapsed/refractory lymphomas.
Major finding: Among all evaluable patients, the objective response rate was 45%. The objective response rate for patients with diffuse large B-cell lymphoma was 35%.
Study details: A pooled analysis of data from phase 1b/2 studies in patients with aggressive and indolent lymphomas. Among 97 patients evaluable for response, there were 59 patients with diffuse large B-cell lymphoma, 35 with follicular lymphoma, and 3 with marginal zone lymphoma.
Disclosures: The study is funded by Forty Seven and the Leukemia and Lymphoma Society. Dr. Roschewski reported having no financial disclosures.
Source: Roschewski M et al. EHA Congress, Abstract S867.
Are nutritional supplements important in the treatment of female pattern hair loss?
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Rapidly Growing Cutaneous Nodules on the Scalp
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
An 8-month-old infant girl presented with rapidly growing cutaneous nodules on the scalp of 1 month's duration. Her parents reported that she disliked lying flat but was otherwise growing and developing normally. Nondiagnostic ultrasonography of the head and brain had been performed as well as a skull radiograph, which found no evidence of lytic lesions. On physical examination, 3 erythematous to violaceous, subcutaneous, firm, fixed nodules were observed on the scalp. Notable cervical lymphadenopathy with several distinct, fixed, firm, subcutaneous nodules in the postauricular lymph chains also were noted. The patient had no pertinent medical history and was born via normal spontaneous vaginal delivery to healthy parents. The remainder of the physical examination and review of systems was negative.
Liraglutide indication expanded to include children
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.