User login
Gabapentinoids associated with suicidal behavior, overdose
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
FROM BMJ
Key clinical point: Patients might be at increased risk of suicidal behavior, unintentional overdose, head and body injuries, and traffic incidents during periods of treatment with gabapentinoids. Pregabalin is associated with higher hazards of these outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years.
Major finding: During treatment periods, patients were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13).
Study details: An analysis of data from 191,973 people from the Swedish Prescribed Drug Register, which collected prescriptions for pregabalin or gabapentin between 2006 and 2013.
Disclosures: The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no relevant disclosures. One author reported grants from Shire and Evolan, and has served as a speaker for Shire.
Source: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
Neoadjuvant-adjuvant erlotinib shows promise in locally advanced NSCLC
, suggests the Chinese Thoracic Oncology Group’s EMERGING trial.
Investigators led by Wen-Zhao Zhong, MD, a professor at Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China, enrolled 72 patients with stage IIIA-N2 EGFR-mutant NSCLC in the phase 2 randomized controlled trial. The patients were randomized to receive either erlotinib, an EGFR tyrosine kinase inhibitor (TKI), or the chemotherapy doublet of gemcitabine (Gemzar) and cisplatin as both neoadjuvant and adjuvant therapy.
Results reported in the Journal of Clinical Oncology showed that the objective response rate was 54.1% with neoadjuvant erlotinib (median, 42 days of therapy) versus 34.3% with neoadjuvant chemotherapy (with most patients receiving two cycles). However, the difference was not significant (odds ratio, 2.26; P = .092).
None of the patients in either arm achieved a pathologic complete response, but 9.7% in the erlotinib arm achieved a major pathologic response (less than 10% residual viable tumor cells) versus none of those in the chemotherapy arm.
With a median follow-up of 14.1 months, median progression-free survival was 21.5 months with erlotinib, nearly double the 11.4 months seen with chemotherapy (hazard ratio for events, 0.39; P less than .001). Overall survival did not differ significantly but was immature at the time of analysis.
Adverse events were largely as expected for each therapy. Incidence of grade 3 or 4 adverse events was 0% with erlotinib versus 29.4% with chemotherapy.
“These results suggest that biomarker-guided neoadjuvant/adjuvant EGFR-TKI treatment strategies in stage IIIA-N2 NSCLC are promising,” Dr. Zhong and colleagues wrote. “Our promising findings warrant additional investigation.”
“The optimal duration of neoadjuvant TKI also warrants additional investigation to validate the role of perioperative TKI therapy in oncogene-driven NSCLC,” they concluded. “Future studies should investigate the translational value of sequential plasma and tissue samples in a neoadjuvant setting using multiomics-based assays to identify predictive characteristics of patients who would benefit from neoadjuvant targeted therapies and predict prognosis.”
Dr. Zhong disclosed receiving honoraria from AstraZeneca, Eli Lilly, Pfizer, Roche, and Sanofi. The trial was supported by the Chinese Thoracic Oncology Group and Shanghai Roche Pharmaceutical.
SOURCE: Zhong W-Z et al. J Clin Oncol. 2019 June 13. doi: 10.1200/JCO.19.00075.
, suggests the Chinese Thoracic Oncology Group’s EMERGING trial.
Investigators led by Wen-Zhao Zhong, MD, a professor at Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China, enrolled 72 patients with stage IIIA-N2 EGFR-mutant NSCLC in the phase 2 randomized controlled trial. The patients were randomized to receive either erlotinib, an EGFR tyrosine kinase inhibitor (TKI), or the chemotherapy doublet of gemcitabine (Gemzar) and cisplatin as both neoadjuvant and adjuvant therapy.
Results reported in the Journal of Clinical Oncology showed that the objective response rate was 54.1% with neoadjuvant erlotinib (median, 42 days of therapy) versus 34.3% with neoadjuvant chemotherapy (with most patients receiving two cycles). However, the difference was not significant (odds ratio, 2.26; P = .092).
None of the patients in either arm achieved a pathologic complete response, but 9.7% in the erlotinib arm achieved a major pathologic response (less than 10% residual viable tumor cells) versus none of those in the chemotherapy arm.
With a median follow-up of 14.1 months, median progression-free survival was 21.5 months with erlotinib, nearly double the 11.4 months seen with chemotherapy (hazard ratio for events, 0.39; P less than .001). Overall survival did not differ significantly but was immature at the time of analysis.
Adverse events were largely as expected for each therapy. Incidence of grade 3 or 4 adverse events was 0% with erlotinib versus 29.4% with chemotherapy.
“These results suggest that biomarker-guided neoadjuvant/adjuvant EGFR-TKI treatment strategies in stage IIIA-N2 NSCLC are promising,” Dr. Zhong and colleagues wrote. “Our promising findings warrant additional investigation.”
“The optimal duration of neoadjuvant TKI also warrants additional investigation to validate the role of perioperative TKI therapy in oncogene-driven NSCLC,” they concluded. “Future studies should investigate the translational value of sequential plasma and tissue samples in a neoadjuvant setting using multiomics-based assays to identify predictive characteristics of patients who would benefit from neoadjuvant targeted therapies and predict prognosis.”
Dr. Zhong disclosed receiving honoraria from AstraZeneca, Eli Lilly, Pfizer, Roche, and Sanofi. The trial was supported by the Chinese Thoracic Oncology Group and Shanghai Roche Pharmaceutical.
SOURCE: Zhong W-Z et al. J Clin Oncol. 2019 June 13. doi: 10.1200/JCO.19.00075.
, suggests the Chinese Thoracic Oncology Group’s EMERGING trial.
Investigators led by Wen-Zhao Zhong, MD, a professor at Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China, enrolled 72 patients with stage IIIA-N2 EGFR-mutant NSCLC in the phase 2 randomized controlled trial. The patients were randomized to receive either erlotinib, an EGFR tyrosine kinase inhibitor (TKI), or the chemotherapy doublet of gemcitabine (Gemzar) and cisplatin as both neoadjuvant and adjuvant therapy.
Results reported in the Journal of Clinical Oncology showed that the objective response rate was 54.1% with neoadjuvant erlotinib (median, 42 days of therapy) versus 34.3% with neoadjuvant chemotherapy (with most patients receiving two cycles). However, the difference was not significant (odds ratio, 2.26; P = .092).
None of the patients in either arm achieved a pathologic complete response, but 9.7% in the erlotinib arm achieved a major pathologic response (less than 10% residual viable tumor cells) versus none of those in the chemotherapy arm.
With a median follow-up of 14.1 months, median progression-free survival was 21.5 months with erlotinib, nearly double the 11.4 months seen with chemotherapy (hazard ratio for events, 0.39; P less than .001). Overall survival did not differ significantly but was immature at the time of analysis.
Adverse events were largely as expected for each therapy. Incidence of grade 3 or 4 adverse events was 0% with erlotinib versus 29.4% with chemotherapy.
“These results suggest that biomarker-guided neoadjuvant/adjuvant EGFR-TKI treatment strategies in stage IIIA-N2 NSCLC are promising,” Dr. Zhong and colleagues wrote. “Our promising findings warrant additional investigation.”
“The optimal duration of neoadjuvant TKI also warrants additional investigation to validate the role of perioperative TKI therapy in oncogene-driven NSCLC,” they concluded. “Future studies should investigate the translational value of sequential plasma and tissue samples in a neoadjuvant setting using multiomics-based assays to identify predictive characteristics of patients who would benefit from neoadjuvant targeted therapies and predict prognosis.”
Dr. Zhong disclosed receiving honoraria from AstraZeneca, Eli Lilly, Pfizer, Roche, and Sanofi. The trial was supported by the Chinese Thoracic Oncology Group and Shanghai Roche Pharmaceutical.
SOURCE: Zhong W-Z et al. J Clin Oncol. 2019 June 13. doi: 10.1200/JCO.19.00075.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Creating better performance incentives
P4P programs suffer from several flaws
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.
P4P programs suffer from several flaws
P4P programs suffer from several flaws
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.
Many performance improvement programs try to create a higher value health system by incentivizing physicians and health systems to behave in particular ways. These have often been pay-for-performance programs that offer bonuses or impose penalties depending on how providers perform on various metrics.
“In theory, this makes sense,” said Dhruv Khullar, MD, MPP, lead author of a JAMA article about the future of incentives, and assistant professor at Weill Cornell Medicine in New York. “But in practice, these programs have not been successful in consistently improving quality, and sometimes they have been counterproductive. In our article, we argued that focusing too narrowly on financial rewards is not the right strategy to improve health system performance – and is sometimes at odds with the physician professionalism and what really motivates most clinicians.”
Pay-for-performance programs suffer from several fundamental flaws: they focus too narrowly on financial incentives and use centralized accountability instead of local culture, for example, Dr. Khullar said.
“A better future state would involve capitalizing on physician professionalism through nonfinancial rewards, resources for quality improvement, team-based assessments, and emphasizing continuous learning and organizational culture,” he noted. Performance programs would take a more global view of clinical care by emphasizing culture, teams, trust, and learning. Such a system would allow hospitalists and other physicians to worry less about meeting specific metrics and focus more on providing high-quality care to their patients.
“I would hope physicians, payers, and administrators would reconsider some previously held beliefs about quality improvement, especially the idea that better quality requires giving people bonus payments or imposing financial penalties,” Dr. Khullar said. “We believe the next wave of performance improvement programs should entertain other paths to better quality, which are more in line with human motivation and physician professionalism.”
Reference
1. Khullar D, Wolfson D, Casalino LP. Professionalism, Performance, and the Future of Physician Incentives. JAMA. 2018 Nov 26 (Epub ahead of print). doi: 10.1001/jama.2018.17719. Accessed Dec. 11, 2018.
Stand Up To Cancer adds research muscle
Two researchers have joined Stand Up To Cancer’s scientific advisory committee, which directs the organization’s research initiatives, reviews grant proposals, and oversees active grants.
One new committee member is John D. Carpten, PhD, of the University of Southern California in Los Angeles. Dr. Carpten’s research encompasses several cancer types and focuses on the use of genomic technologies and bioinformatics analysis.
The other new committee member is Roderic I. Pettigrew, MD, PhD, of Texas A&M University in College Station. Dr. Pettigrew is known for pioneering four-dimensional imaging of the cardiovascular system using MRI.
In other news, Fox Chase Cancer Center in Philadelphia has appointed five new endowed chairs. The chair holders “will have new resources to strengthen [the center’s] efforts in various areas of cancer research and to implement more effective cancer treatments,” according to Fox Chase.
Hossein Borghaei, DO, now holds the Gloria and Edmund M. Dunn Chair in Thoracic Oncology. Dr. Borghaei conducts research focused on the development of new cancer treatments, particularly immunotherapies and monoclonal antibodies.
Margie L. Clapper, PhD, holds the Samuel M.V. Hamilton Chair in Cancer Prevention. Dr. Clapper is known for creating one of the first programs in the United States to focus on reducing the risk of cancer in high-risk patients and supporting the early detection and prevention of cancers.
Erica Golemis, PhD, holds the William Wikoff Smith Chair in Cancer Research. Dr. Golemis studies changes in cell signaling associated with tumor development and progression, as well as cancer treatment resistance.
Mariusz A. Wasik, MD, holds the Donald E. and Shirley C. Morel, Stanley and Stella Bayster Chair in Molecular Diagnostics. Dr. Wasik’s research is focused on aberrant cell signaling and oncogenic mutations in lymphomas, as well as cell transformation driven by ALK.
Johnathan R. Whetstine, PhD, holds the Jack Schultz Chair in Basic Science. Dr. Whetstine studies epigenetic mechanisms that regulate cell cycle progression, impact cancer therapy, and drive copy gain, amplifications, and drug resistance.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Two researchers have joined Stand Up To Cancer’s scientific advisory committee, which directs the organization’s research initiatives, reviews grant proposals, and oversees active grants.
One new committee member is John D. Carpten, PhD, of the University of Southern California in Los Angeles. Dr. Carpten’s research encompasses several cancer types and focuses on the use of genomic technologies and bioinformatics analysis.
The other new committee member is Roderic I. Pettigrew, MD, PhD, of Texas A&M University in College Station. Dr. Pettigrew is known for pioneering four-dimensional imaging of the cardiovascular system using MRI.
In other news, Fox Chase Cancer Center in Philadelphia has appointed five new endowed chairs. The chair holders “will have new resources to strengthen [the center’s] efforts in various areas of cancer research and to implement more effective cancer treatments,” according to Fox Chase.
Hossein Borghaei, DO, now holds the Gloria and Edmund M. Dunn Chair in Thoracic Oncology. Dr. Borghaei conducts research focused on the development of new cancer treatments, particularly immunotherapies and monoclonal antibodies.
Margie L. Clapper, PhD, holds the Samuel M.V. Hamilton Chair in Cancer Prevention. Dr. Clapper is known for creating one of the first programs in the United States to focus on reducing the risk of cancer in high-risk patients and supporting the early detection and prevention of cancers.
Erica Golemis, PhD, holds the William Wikoff Smith Chair in Cancer Research. Dr. Golemis studies changes in cell signaling associated with tumor development and progression, as well as cancer treatment resistance.
Mariusz A. Wasik, MD, holds the Donald E. and Shirley C. Morel, Stanley and Stella Bayster Chair in Molecular Diagnostics. Dr. Wasik’s research is focused on aberrant cell signaling and oncogenic mutations in lymphomas, as well as cell transformation driven by ALK.
Johnathan R. Whetstine, PhD, holds the Jack Schultz Chair in Basic Science. Dr. Whetstine studies epigenetic mechanisms that regulate cell cycle progression, impact cancer therapy, and drive copy gain, amplifications, and drug resistance.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Two researchers have joined Stand Up To Cancer’s scientific advisory committee, which directs the organization’s research initiatives, reviews grant proposals, and oversees active grants.
One new committee member is John D. Carpten, PhD, of the University of Southern California in Los Angeles. Dr. Carpten’s research encompasses several cancer types and focuses on the use of genomic technologies and bioinformatics analysis.
The other new committee member is Roderic I. Pettigrew, MD, PhD, of Texas A&M University in College Station. Dr. Pettigrew is known for pioneering four-dimensional imaging of the cardiovascular system using MRI.
In other news, Fox Chase Cancer Center in Philadelphia has appointed five new endowed chairs. The chair holders “will have new resources to strengthen [the center’s] efforts in various areas of cancer research and to implement more effective cancer treatments,” according to Fox Chase.
Hossein Borghaei, DO, now holds the Gloria and Edmund M. Dunn Chair in Thoracic Oncology. Dr. Borghaei conducts research focused on the development of new cancer treatments, particularly immunotherapies and monoclonal antibodies.
Margie L. Clapper, PhD, holds the Samuel M.V. Hamilton Chair in Cancer Prevention. Dr. Clapper is known for creating one of the first programs in the United States to focus on reducing the risk of cancer in high-risk patients and supporting the early detection and prevention of cancers.
Erica Golemis, PhD, holds the William Wikoff Smith Chair in Cancer Research. Dr. Golemis studies changes in cell signaling associated with tumor development and progression, as well as cancer treatment resistance.
Mariusz A. Wasik, MD, holds the Donald E. and Shirley C. Morel, Stanley and Stella Bayster Chair in Molecular Diagnostics. Dr. Wasik’s research is focused on aberrant cell signaling and oncogenic mutations in lymphomas, as well as cell transformation driven by ALK.
Johnathan R. Whetstine, PhD, holds the Jack Schultz Chair in Basic Science. Dr. Whetstine studies epigenetic mechanisms that regulate cell cycle progression, impact cancer therapy, and drive copy gain, amplifications, and drug resistance.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Wolf in sheep’s clothing: metatarsal osteosarcoma
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
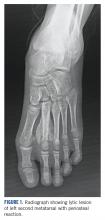
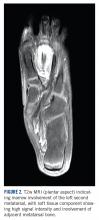
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
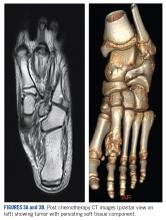
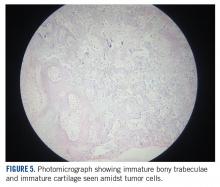
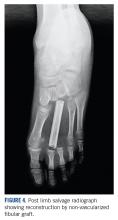
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
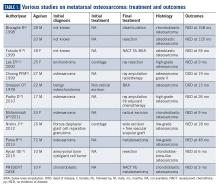
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
Metatarsal bones are an unusual subsite for small bone involvement in osteosarcomas. This subgroup is often misdiagnosed and hence associated with significant treatment delays. The standard treatment of metatarsal osteosarcomas remains the same as for those treated at other sites, namely neoadjuvant chemotherapy followed by surgery and adjuvant chemotherapy. Limb salvage surgery or metatarsectomy in the foot is often a challenge owing to the poor compartmentalization of the disease. We hereby describe the case of a young girl with a metatarsal osteosarcoma who was managed with neoadjuvant chemotherapy and limb salvage surgery.
Introduction
Osteosarcomas are the most common primary malignant bone tumor in children and adolescents. Although predominantly occurring in pediatric and adolescent age groups, bimodal distribution (with a second incidence peak occurring in the sixth and seventh decades) is not uncommon.1 Osteosarcomas of the foot and small bones represent a rare and distinct clinical entity. This must have been a well-known observation for years that led to Watson-Jones stating, “Sarcoma of this [metatarsal] bone has not yet been reported in thousands of years in any country.”2 The incidence of osteosarcomas of the foot is estimated to be from 0.2% to 2%.3
These tumors, owing to their rarity, often lead to diagnostic dilemmas and hence treatment delays.4 They are usually mistaken for inflammatory conditions and often treated with—but not limited to—curettages and drainage procedures.5 The following case of osteosarcoma of the metatarsal bone in a young girl highlights the importance of having a high index of clinical suspicion prior to treatment.
Case Presentation and Summary
A 10-year-old girl visited our outpatient clinic with a painful progressive swelling on the dorsum of the left foot of 2 months’ duration. There was no history of antecedent trauma or fever. Physical examination revealed a bony hard swelling measuring around 5 x 6 cm on the dorsum of the left foot around the region of the second metatarsal. There was no regional lymphadenopathy or distal neurovascular deficit. She was evaluated with a plain radiograph that demonstrated a lytic lesion in the left second metatarsal associated with cortical destruction and periosteal reaction (Figure 1). A subsequent magnetic resonance image (MRI) revealed a bony lesion destroying part of the left second metatarsal with cortical destruction and marrow involvement and affecting the soft tissue around the adjacent third metatarsal (Figure 2). Needle biopsy showed chondroblastic osteosarcoma. Computed tomography (CT) of the thorax and bone scan were both negative for distant metastases.
She received 3 cycles of a MAP (highdose methotrexate, doxorubicin, and cisplatin) regimen as neoadjuvant chemotherapy. Response assessment scans showed partial response (Figures 3A and B). We performed a wide excision of the second and third metatarsal with reconstruction using a segment of non-vascularized fibular graft as rigid fixation (Figure 4). The postoperative period was uneventful. She was able to begin partial weight bearing on the fourth postoperative day and her sutures were removed on the twelfth postoperative day. She received adjuvant chemotherapy following surgery. The final histopathology report showed residual disease with Huvos grade III response (>90% necrosis) with all margins negative for malignancy (Figure 5). At present, the child is disease-free at 5 months of treatment completion and is undergoing regular follow-up visits.
Discussion
Metatarsal involvement amongst smallbone osteosarcomas is uncommon.3 There are about 32 cases of osteosarcomas reported in the literature from 1940 to 2018 involving the metatarsal bones (Table 1). According to a review article from the Mayo Clinic, the most common bone of the foot involved is the calcaneum.6 While the incidence of osteosarcomas of the foot as a whole is around 0.2% to 2%,3 metatarsal involvement is documented in 0.5% of these patients.7 However, a recent study depicted metatarsal involvement in 33% of all osteosarcomas of the foot.8
Osteosarcomas at conventional sites tend to have a bimodal age distribution with respect to disease affliction.9 Metatarsal osteosarcomas, however, are more common in an older age group.4,10 Our patient is probably the second youngest reported case of metatarsal osteosarcoma in the literature.11
Biscaglia et al propounded that osteosarcomas of the metatarsal were a distinct subgroup due to the rarity of occurrence, anatomical location, and prognosis.4 This often led to misdiagnosis and subsequent inadequate or inappropriate surgery. In six out of the ten cases (60%) described in Table 1, an incorrect pretreatment diagnosis was made that led to treatment delay. None, except one patient, received neoadjuvant chemotherapy, which is currently the standard of care. The average duration from symptom onset to diagnosis was found to be 2 years.4 However, in our case, the duration of symptoms was approximately 2 months.
Surgery for metatarsal osteosarcomas can be challenging, as the compartments of the foot are narrow spaces with poor demarcation. Limb salvage surgery in the form of metatarsectomy needs proper preoperative planning and execution. Neoadjuvant chemotherapy will serve to downstage the tumor within the fascial barriers of the metatarsal compartment.It has also been postulated that osteosarcoma of the foot may have a better prognosis and survival compared to other osteosarcoma subsites.10 This can be extrapolated from the fact that the majority are found to be low grade, and despite a long delay in treatment, there was no rapid increase in size and/or metastatic spread. However, tumor grade remains an important factor affecting survival— patients with higher grade tumors have worse survival.8
A number of differentials, including benign tumors, are to be kept in mind when diagnosing and treating such patients (Table 2). The most common benign tumors affecting the metatarsal are giant cell tumors (GCT) followed by chondromyxoid fibroma. Osteosarcomas and Ewing sarcomas constitute the malignant tumors.12 Occasionally, infections like osteomyelitis of the small bones may mimic malignancy. The absence of an extensive soft tissue component and/or calcifications with the presence of bony changes (like sequestrum) favors a diagnosis of infection/osteomyelitis. In addition, clinical findings like fever, skin redness, and presence of a painful swelling (especially after onset of fever) point to an inflammatory pathology rather than malignancy. Stress fractures rarely simulate tumors. MRI showing marrow and soft tissue edema with a visible fracture line points to the diagnosis.
A plane radiograph showing cortical bone destruction with a soft tissue component and calcification should be considered suspicious and must be thoroughly evaluated prior to surgical treatment.13 In a young patient such as ours, the important differentials that need to be considered include Ewing sarcoma, chronic osteomyelitis, and eosinophilic granuloma, which can radiologically mimic osteosarcoma at this location.
Conclusions
Osteosarcoma of the metatarsal is rare. Our case remains unique as it reports the second youngest patient in the literature. Erroneous or delayed diagnosis resulting in inadequate tumor excision and limb loss (amputation) often occurs in a majority of the cases. Proper pretreatment radiological imaging becomes imperative, and when clinical suspicion is high, a needle biopsy must follow in those cases. Early diagnosis with administration of neoadjuvant chemotherapy may allow us to perform limb salvage surgery or wide excision in these cases.
Acknowledgement
We would like to thank Dr. Sithara Aravind, Associate Professor, Department of Pathology, Malabar Cancer Center, for the photomicrographs.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
2. Watson-Jones R. Fractures and Joint Injuries. Vol. I, 4th ed. Edinburgh and London: E & S Livingstone Ltd.1960:347.
3. Wu KK. Osteogenic sarcoma of the tarsal navicular bone. J Foot Surg. 1989;28(4):363-369.
4. Biscaglia R, Gasbarrini A, Böhling T, Bacchini P, Bertoni F, Picci P. Osteosarcoma of the bones of the foot: an easily misdiagnosed malignant tumour. Mayo Clin Proc. 1998;73(9):842-847.
5. Kundu ZS, Gupta V, Sangwan SS, Rana P. Curettage of benign bone tumors and tumor like lesions: A retrospective analysis. Indian J Orthop. 2013;47(3):295-301.
6. Choong PFM, Qureshil AA, Sim FH, Unni KK. Osteosarcoma of the foot. A review of 52 patients at the Mayo Clinic. Acta Orthop Scand. 1999;70(4):361-364.
7. Sneppen O, Dissing I, Heerfordt J, Schiödt T. Osteosarcoma of the metatarsal bones: Review of the literature and report of a case. Acta Orthop Scand. 1978;49(2):220-223.
8. Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462(1):109-120.
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology and End Results Program. Cancer.
2009;115(7):1531-1543.
10. Wang CW, Chen CY, Yang RS. Talar osteosarcoma treated with limb sparing surgery. J Bone Joint Surg Am. 2011;93:e22.
11. Aycan OE, Vanel D, Righi A, Arikan Y, Manfrini M. Chondroblastoma-like osteosarcoma:
a case report and review. Skeletal Radiol. 2015;44(6):869-873.
12. Jarkiewicz-Kochman E, Gołebiowski M, Swiatkowski J, Pacholec E, Rajewski R. Tumours of the metatarsus. Ortop Traumatol Rehabil. 2007;9(3):319-330.
13. Schatz J, Soper J, McCormack S, Healy M, Deady L, Brown W. Imaging of tumours in the ankle and foot. Top Magn Reson Imaging. 2010;21(1):37-50.
14. Fukuda K, Ushigome S, Nikaidou T, Asanuma K, Masui F. Osteosarcoma of the metatarsal. Skeletal Radiol. 1999;28(5):294-297.
15. Parsa R, Marcus M, Orlando R, Parsa C. Low-grade central osteosarcoma of the second metatarsal in a 72 year old male. Internet J Orthop Surg. 2013;21(2): 1-8.
16. Lee EY, Seeger LL, Nelson SD, Eckardt JJ. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000;29(8):474-476.
17. Padhy D, Madhuri V, Pulimood SA, Danda S, Walter NM, Wang LL. Metatarsal osteosarcoma in Rothmund-Thomson syndrome: a case report. J Bone Joint
Surg Am. 2010;92(3):726-730.
18. Mohammadi A, Porghasem J, Noroozinia F, Ilkhanizadeh B, Ghasemi-Rad M, Khenari S. Periosteal osteosarcoma of the fifth metatarsal: A rare pedal tumor. J Foot Ankle Surg. 2011;50(5):620-622.
19. Nishio J, Iwasaki H, Takagi S, et al. Low-grade central osteosarcoma of the metatarsal bone: A clinicopathological, immunohistochemical, cytogenetic and molecular cytogenetic analysis. Anticancer Res. 2012;32(12):5429-5435.
Pediatric-onset MS may slow information processing in adulthood
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
FROM JAMA NEUROLOGY
Consider drug treatment in late-life women with osteoporosis
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Medical treatment to prevent osteoporotic hip fracture in women over age 80 likely is worthwhile.
Major finding: Five-year hip fracture probability was 13% among women with osteoporosis and 4% among women without osteoporosis but at high fracture risk.
Study details: A prospective cohort study of 1,528 women 80 years and older who were potential candidates for osteoporosis drug treatment.
Disclosures: Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
Source: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
Study raises questions about PPS exemption for cancer centers
It’s time to revisit legislation that exempts certain U.S. specialized cancer centers from the Medicare Prospective Payment System and from fully reporting measures of care and outcomes to the Centers for Medicare & Medicaid Services, a retrospective cohort study suggests.
The Prospective Payment System (PPS) exemption dates back to 1983 and allows exempted centers to be reimbursed on a reasonable cost basis rather than on a diagnosis-related group basis. Unlike centers that are a part of PPS, exempted centers are not required to report all process-of-care, outcome, and patient experience measures to CMS through its pay-for-performance programs.
In the new study, investigators working under senior author Karl Y. Bilimoria, MD, MS, director of the Surgical Outcomes and Quality Improvement Center and John B. Murphy Professor of Surgery at Northwestern University, Chicago, compared a variety of measures across hospital types. Data came from the American Hospital Association Annual Survey, U.S. News Best Hospitals rankings, and a sample of Medicare beneficiaries who underwent nine cancer operations.
Analyses were based on 15 hospitals affiliated with PPS-exempt cancer centers, 54 hospitals affiliated with National Cancer Institute–designated cancer centers, and 3,578 other U.S. hospitals providing cancer care. The results reported in JAMA Internal Medicine showed that hospitals affiliated with PPS-exempt cancer centers and hospitals affiliated with NCI cancer centers were similar on characteristics other than bed size and overall volume, which were larger for the latter, and on basic cancer-related services such as full-field digital mammography, genetic testing/counseling, chemotherapy, image-guided radiation, and hospice/palliative services.
U.S. News reputation scores averaged 17.5 for the PPS-exempt cancer center hospitals versus just 2.6 for the NCI cancer center hospitals (P less than .001). However, the two types of centers were statistically indistinguishable on oncology patient volume, patient safety ratings, comorbidity burden, nurse staffing, and U.S. News survival scores.
When it came to cancer operations, hospitals affiliated with PPS-exempt cancer centers and with NCI cancer centers had essentially the same adjusted postoperative outcomes for 15 of 18 measures, including mortality, readmission, and surgical site infections. Patients treated at the latter more often developed postoperative sepsis (3.1% vs. 1.7%; P = .002), acute renal failure (6.2% vs. 3.9%; P = .01), and urinary tract infection (6.4% vs. 4.0%; P = .002).
PPS-exempt cancer center hospitals had better outcomes than the group of other U.S. hospitals providing cancer care for 7 of the 18 oncology surgery measures, including mortality, sepsis, acute renal failure, pulmonary failure, and failure to rescue.
“Although PPS-exempt cancer centers may serve an important purpose by advancing the science and quality of cancer care, limited information is available on the selection of PPS-exempt cancer centers, their reimbursement, and how these centers compare with other hospitals with regard to quality of care,” Dr. Bilimoria and colleagues wrote.
“Our findings suggest the need for additional transparency, periodic reviews of the program by CMS, and consideration of whether the classification of PPS exemption should continue. Moreover, the requirement to publicly report cancer care–quality metrics should be uniformly applied across all types of hospitals, not just PPS-exempt cancer centers,” they concluded.
Dr. Bilimoria reported receiving support from the National Institutes of Health; Agency for Healthcare Research and Quality; American Board of Surgery; American College of Surgeons; and the Accreditation Council for Graduate Medical Education, Health Care. The study was supported by the Northwestern Institute for Comparative Effectiveness Research in Oncology of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
SOURCE: Bilimoria KY et al. JAMA Intern Med. 2019 June 17. doi: 10.1001/jamainternmed.2019.0914.
Similarity of the hospitals affiliated with Prospective Payment System (PPS)–exempt cancer centers and the hospitals affiliated with National Cancer Institute–designated cancer centers hospitals likely results from the fact that most of the former group of centers also belong to the latter group of centers, even though the study considered these categories to be mutually exclusive, Robert Steinbrook, MD, proposed in an Editor’s Note.
“Surprisingly, the Centers for Medicare & Medicaid Services collects no comparative cancer-specific quality data for all the PPS-exempt and NCI cancer centers; the PPS-exempt cancer centers report some cancer-specific quality measures but the PPS itself uses no cancer-specific quality measures,” he pointed out.
Failure to uniformly collect and publicly report cancer-specific quality measures for all institutions “makes little sense,” Dr. Steinbrook contended, especially given that most PPS-exempt cancer centers are also NCI-designated cancer centers.
“In 1983, Congress may have believed that the care provided at certain cancer hospitals was not suited to reimbursement under the newly introduced DRG [diagnosis-related group] methodology,” he concluded. “In 2019, however, that belief no longer is tenable. [This study] is a call for greater transparency about the quality of cancer care in the United States, the establishment of one set of rules for how Medicare pays for cancer care, and an end to the PPS-exempt cancer center program.”
Dr. Steinbrook is editor at large and online editor at the JAMA Internal Medicine editorial office at the University of California, San Francisco, and is an adjunct professor at Yale University, New Haven, Conn.
Similarity of the hospitals affiliated with Prospective Payment System (PPS)–exempt cancer centers and the hospitals affiliated with National Cancer Institute–designated cancer centers hospitals likely results from the fact that most of the former group of centers also belong to the latter group of centers, even though the study considered these categories to be mutually exclusive, Robert Steinbrook, MD, proposed in an Editor’s Note.
“Surprisingly, the Centers for Medicare & Medicaid Services collects no comparative cancer-specific quality data for all the PPS-exempt and NCI cancer centers; the PPS-exempt cancer centers report some cancer-specific quality measures but the PPS itself uses no cancer-specific quality measures,” he pointed out.
Failure to uniformly collect and publicly report cancer-specific quality measures for all institutions “makes little sense,” Dr. Steinbrook contended, especially given that most PPS-exempt cancer centers are also NCI-designated cancer centers.
“In 1983, Congress may have believed that the care provided at certain cancer hospitals was not suited to reimbursement under the newly introduced DRG [diagnosis-related group] methodology,” he concluded. “In 2019, however, that belief no longer is tenable. [This study] is a call for greater transparency about the quality of cancer care in the United States, the establishment of one set of rules for how Medicare pays for cancer care, and an end to the PPS-exempt cancer center program.”
Dr. Steinbrook is editor at large and online editor at the JAMA Internal Medicine editorial office at the University of California, San Francisco, and is an adjunct professor at Yale University, New Haven, Conn.
Similarity of the hospitals affiliated with Prospective Payment System (PPS)–exempt cancer centers and the hospitals affiliated with National Cancer Institute–designated cancer centers hospitals likely results from the fact that most of the former group of centers also belong to the latter group of centers, even though the study considered these categories to be mutually exclusive, Robert Steinbrook, MD, proposed in an Editor’s Note.
“Surprisingly, the Centers for Medicare & Medicaid Services collects no comparative cancer-specific quality data for all the PPS-exempt and NCI cancer centers; the PPS-exempt cancer centers report some cancer-specific quality measures but the PPS itself uses no cancer-specific quality measures,” he pointed out.
Failure to uniformly collect and publicly report cancer-specific quality measures for all institutions “makes little sense,” Dr. Steinbrook contended, especially given that most PPS-exempt cancer centers are also NCI-designated cancer centers.
“In 1983, Congress may have believed that the care provided at certain cancer hospitals was not suited to reimbursement under the newly introduced DRG [diagnosis-related group] methodology,” he concluded. “In 2019, however, that belief no longer is tenable. [This study] is a call for greater transparency about the quality of cancer care in the United States, the establishment of one set of rules for how Medicare pays for cancer care, and an end to the PPS-exempt cancer center program.”
Dr. Steinbrook is editor at large and online editor at the JAMA Internal Medicine editorial office at the University of California, San Francisco, and is an adjunct professor at Yale University, New Haven, Conn.
It’s time to revisit legislation that exempts certain U.S. specialized cancer centers from the Medicare Prospective Payment System and from fully reporting measures of care and outcomes to the Centers for Medicare & Medicaid Services, a retrospective cohort study suggests.
The Prospective Payment System (PPS) exemption dates back to 1983 and allows exempted centers to be reimbursed on a reasonable cost basis rather than on a diagnosis-related group basis. Unlike centers that are a part of PPS, exempted centers are not required to report all process-of-care, outcome, and patient experience measures to CMS through its pay-for-performance programs.
In the new study, investigators working under senior author Karl Y. Bilimoria, MD, MS, director of the Surgical Outcomes and Quality Improvement Center and John B. Murphy Professor of Surgery at Northwestern University, Chicago, compared a variety of measures across hospital types. Data came from the American Hospital Association Annual Survey, U.S. News Best Hospitals rankings, and a sample of Medicare beneficiaries who underwent nine cancer operations.
Analyses were based on 15 hospitals affiliated with PPS-exempt cancer centers, 54 hospitals affiliated with National Cancer Institute–designated cancer centers, and 3,578 other U.S. hospitals providing cancer care. The results reported in JAMA Internal Medicine showed that hospitals affiliated with PPS-exempt cancer centers and hospitals affiliated with NCI cancer centers were similar on characteristics other than bed size and overall volume, which were larger for the latter, and on basic cancer-related services such as full-field digital mammography, genetic testing/counseling, chemotherapy, image-guided radiation, and hospice/palliative services.
U.S. News reputation scores averaged 17.5 for the PPS-exempt cancer center hospitals versus just 2.6 for the NCI cancer center hospitals (P less than .001). However, the two types of centers were statistically indistinguishable on oncology patient volume, patient safety ratings, comorbidity burden, nurse staffing, and U.S. News survival scores.
When it came to cancer operations, hospitals affiliated with PPS-exempt cancer centers and with NCI cancer centers had essentially the same adjusted postoperative outcomes for 15 of 18 measures, including mortality, readmission, and surgical site infections. Patients treated at the latter more often developed postoperative sepsis (3.1% vs. 1.7%; P = .002), acute renal failure (6.2% vs. 3.9%; P = .01), and urinary tract infection (6.4% vs. 4.0%; P = .002).
PPS-exempt cancer center hospitals had better outcomes than the group of other U.S. hospitals providing cancer care for 7 of the 18 oncology surgery measures, including mortality, sepsis, acute renal failure, pulmonary failure, and failure to rescue.
“Although PPS-exempt cancer centers may serve an important purpose by advancing the science and quality of cancer care, limited information is available on the selection of PPS-exempt cancer centers, their reimbursement, and how these centers compare with other hospitals with regard to quality of care,” Dr. Bilimoria and colleagues wrote.
“Our findings suggest the need for additional transparency, periodic reviews of the program by CMS, and consideration of whether the classification of PPS exemption should continue. Moreover, the requirement to publicly report cancer care–quality metrics should be uniformly applied across all types of hospitals, not just PPS-exempt cancer centers,” they concluded.
Dr. Bilimoria reported receiving support from the National Institutes of Health; Agency for Healthcare Research and Quality; American Board of Surgery; American College of Surgeons; and the Accreditation Council for Graduate Medical Education, Health Care. The study was supported by the Northwestern Institute for Comparative Effectiveness Research in Oncology of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
SOURCE: Bilimoria KY et al. JAMA Intern Med. 2019 June 17. doi: 10.1001/jamainternmed.2019.0914.
It’s time to revisit legislation that exempts certain U.S. specialized cancer centers from the Medicare Prospective Payment System and from fully reporting measures of care and outcomes to the Centers for Medicare & Medicaid Services, a retrospective cohort study suggests.
The Prospective Payment System (PPS) exemption dates back to 1983 and allows exempted centers to be reimbursed on a reasonable cost basis rather than on a diagnosis-related group basis. Unlike centers that are a part of PPS, exempted centers are not required to report all process-of-care, outcome, and patient experience measures to CMS through its pay-for-performance programs.
In the new study, investigators working under senior author Karl Y. Bilimoria, MD, MS, director of the Surgical Outcomes and Quality Improvement Center and John B. Murphy Professor of Surgery at Northwestern University, Chicago, compared a variety of measures across hospital types. Data came from the American Hospital Association Annual Survey, U.S. News Best Hospitals rankings, and a sample of Medicare beneficiaries who underwent nine cancer operations.
Analyses were based on 15 hospitals affiliated with PPS-exempt cancer centers, 54 hospitals affiliated with National Cancer Institute–designated cancer centers, and 3,578 other U.S. hospitals providing cancer care. The results reported in JAMA Internal Medicine showed that hospitals affiliated with PPS-exempt cancer centers and hospitals affiliated with NCI cancer centers were similar on characteristics other than bed size and overall volume, which were larger for the latter, and on basic cancer-related services such as full-field digital mammography, genetic testing/counseling, chemotherapy, image-guided radiation, and hospice/palliative services.
U.S. News reputation scores averaged 17.5 for the PPS-exempt cancer center hospitals versus just 2.6 for the NCI cancer center hospitals (P less than .001). However, the two types of centers were statistically indistinguishable on oncology patient volume, patient safety ratings, comorbidity burden, nurse staffing, and U.S. News survival scores.
When it came to cancer operations, hospitals affiliated with PPS-exempt cancer centers and with NCI cancer centers had essentially the same adjusted postoperative outcomes for 15 of 18 measures, including mortality, readmission, and surgical site infections. Patients treated at the latter more often developed postoperative sepsis (3.1% vs. 1.7%; P = .002), acute renal failure (6.2% vs. 3.9%; P = .01), and urinary tract infection (6.4% vs. 4.0%; P = .002).
PPS-exempt cancer center hospitals had better outcomes than the group of other U.S. hospitals providing cancer care for 7 of the 18 oncology surgery measures, including mortality, sepsis, acute renal failure, pulmonary failure, and failure to rescue.
“Although PPS-exempt cancer centers may serve an important purpose by advancing the science and quality of cancer care, limited information is available on the selection of PPS-exempt cancer centers, their reimbursement, and how these centers compare with other hospitals with regard to quality of care,” Dr. Bilimoria and colleagues wrote.
“Our findings suggest the need for additional transparency, periodic reviews of the program by CMS, and consideration of whether the classification of PPS exemption should continue. Moreover, the requirement to publicly report cancer care–quality metrics should be uniformly applied across all types of hospitals, not just PPS-exempt cancer centers,” they concluded.
Dr. Bilimoria reported receiving support from the National Institutes of Health; Agency for Healthcare Research and Quality; American Board of Surgery; American College of Surgeons; and the Accreditation Council for Graduate Medical Education, Health Care. The study was supported by the Northwestern Institute for Comparative Effectiveness Research in Oncology of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
SOURCE: Bilimoria KY et al. JAMA Intern Med. 2019 June 17. doi: 10.1001/jamainternmed.2019.0914.
JAMA INTERNAL MEDICINE
Many cardiac catheterization, electrophysiology lab directors receive big industry payments
according to a new study of payments to lab directors at top hospitals.
“With continued concerns about rising health care costs, it is important to ensure that physician decisions regarding choice of devices and other pharmaceutical therapies be driven by clinical and cost effectiveness, not industry influence,” Amarnath Annapureddy, MD, of the Yale School of Medicine, New Haven, Conn. and coauthors wrote in their study published in JAMA Internal Medicine.
Dr. Annapureddy and fellow researchers analyzed Open Payments Program data from 2017 to determine payments made to cardiac catheterization and electrophysiology laboratory directors the top 100 hospitals based on US News and World Report’s ranking. They compared those payments to payments made to local interventional cardiologists and electrophysiologists, along with payments to interventional cardiologists and electrophysiologists from across the country.
In 2017, the directors of the top cardiac catheterization and electrophysiology labs received $1,416,232 and $2,307,504 from industry, respectively. The median payments to cardiac catheterization lab directors were significantly higher ($3,203 [$388-$14,156]), compared with interventional cardiologists ($1,064 [$206-$4,104]). The results were similar comparing electrophysiology lab directors with electrophysiology physicians practicing in the same zip codes ($10,521 [$1,159-$35,076] versus $2,900 [$549-$13,101]).
Overall, nearly one-third of cardiac catheterization lab directors and nearly half of electrophysiology lab directors received payments of $10,000 or more, an amount the National Academy of Medicine noted as constituting “a significant conflict of interest.”
The coauthors acknowledged their study’s limitations, including the possibility that they misclassified some lab directors and the fact that Open Payments Program’s data may have been inaccurate. However, they also anticipated that “inaccuracies would not vary between laboratory directors and other clinicians.”
Dr. Nihar R. Desai reported receiving funding from the Centers for Medicare & Medicaid Services, financial support from Johnson & Johnson, and research support from the American College of Cardiology Foundation. Dr. Jeptha P. Curtis reported receiving salary support from the American College of Cardiology, funding from the Centers for Medicare & Medicaid Services, and equity interest in Medtronic. No other authors reported any relevant financial disclosures. No funding for the study was reported.
SOURCE: Annapureddy A et al. JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8775.
Far too much about the purchasing of medical devices – and the costs passed to insurers and ultimately patients – is secretive and requires an overhaul, according to Rita F. Redberg, MD.
Noting that the results from the study by Annapureddy et al. found that nearly one-third of catheterization lab directors and one-half of electrophysiology lab directors receive more than $10,000 annually, she wrote that “the prevalence of financial relationships and the magnitude of the payments are of great concern.” Even picking up a meal can be influential, she added; imagine the impact of such a large payment.
What can be done? “Professional society guidelines should prohibit doctors with relationships with industry from participating in decisions about what devices their hospital chooses to purchase,” she stated, and patients should be made aware of relationships between doctors and device companies. More transparency, across the board, is necessary.
Dr. Redburg is a cardiologist at the University of California, San Francisco; she is interested in promoting high-value health care, which emphasizes delivering appropriate treatment while avoiding tests or therapies without known benefit. These comments are adapted from an editorial accompanying the article by Annapureddy et al. (JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8737). Dr. Redberg reported no conflicts of interest.
Far too much about the purchasing of medical devices – and the costs passed to insurers and ultimately patients – is secretive and requires an overhaul, according to Rita F. Redberg, MD.
Noting that the results from the study by Annapureddy et al. found that nearly one-third of catheterization lab directors and one-half of electrophysiology lab directors receive more than $10,000 annually, she wrote that “the prevalence of financial relationships and the magnitude of the payments are of great concern.” Even picking up a meal can be influential, she added; imagine the impact of such a large payment.
What can be done? “Professional society guidelines should prohibit doctors with relationships with industry from participating in decisions about what devices their hospital chooses to purchase,” she stated, and patients should be made aware of relationships between doctors and device companies. More transparency, across the board, is necessary.
Dr. Redburg is a cardiologist at the University of California, San Francisco; she is interested in promoting high-value health care, which emphasizes delivering appropriate treatment while avoiding tests or therapies without known benefit. These comments are adapted from an editorial accompanying the article by Annapureddy et al. (JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8737). Dr. Redberg reported no conflicts of interest.
Far too much about the purchasing of medical devices – and the costs passed to insurers and ultimately patients – is secretive and requires an overhaul, according to Rita F. Redberg, MD.
Noting that the results from the study by Annapureddy et al. found that nearly one-third of catheterization lab directors and one-half of electrophysiology lab directors receive more than $10,000 annually, she wrote that “the prevalence of financial relationships and the magnitude of the payments are of great concern.” Even picking up a meal can be influential, she added; imagine the impact of such a large payment.
What can be done? “Professional society guidelines should prohibit doctors with relationships with industry from participating in decisions about what devices their hospital chooses to purchase,” she stated, and patients should be made aware of relationships between doctors and device companies. More transparency, across the board, is necessary.
Dr. Redburg is a cardiologist at the University of California, San Francisco; she is interested in promoting high-value health care, which emphasizes delivering appropriate treatment while avoiding tests or therapies without known benefit. These comments are adapted from an editorial accompanying the article by Annapureddy et al. (JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8737). Dr. Redberg reported no conflicts of interest.
according to a new study of payments to lab directors at top hospitals.
“With continued concerns about rising health care costs, it is important to ensure that physician decisions regarding choice of devices and other pharmaceutical therapies be driven by clinical and cost effectiveness, not industry influence,” Amarnath Annapureddy, MD, of the Yale School of Medicine, New Haven, Conn. and coauthors wrote in their study published in JAMA Internal Medicine.
Dr. Annapureddy and fellow researchers analyzed Open Payments Program data from 2017 to determine payments made to cardiac catheterization and electrophysiology laboratory directors the top 100 hospitals based on US News and World Report’s ranking. They compared those payments to payments made to local interventional cardiologists and electrophysiologists, along with payments to interventional cardiologists and electrophysiologists from across the country.
In 2017, the directors of the top cardiac catheterization and electrophysiology labs received $1,416,232 and $2,307,504 from industry, respectively. The median payments to cardiac catheterization lab directors were significantly higher ($3,203 [$388-$14,156]), compared with interventional cardiologists ($1,064 [$206-$4,104]). The results were similar comparing electrophysiology lab directors with electrophysiology physicians practicing in the same zip codes ($10,521 [$1,159-$35,076] versus $2,900 [$549-$13,101]).
Overall, nearly one-third of cardiac catheterization lab directors and nearly half of electrophysiology lab directors received payments of $10,000 or more, an amount the National Academy of Medicine noted as constituting “a significant conflict of interest.”
The coauthors acknowledged their study’s limitations, including the possibility that they misclassified some lab directors and the fact that Open Payments Program’s data may have been inaccurate. However, they also anticipated that “inaccuracies would not vary between laboratory directors and other clinicians.”
Dr. Nihar R. Desai reported receiving funding from the Centers for Medicare & Medicaid Services, financial support from Johnson & Johnson, and research support from the American College of Cardiology Foundation. Dr. Jeptha P. Curtis reported receiving salary support from the American College of Cardiology, funding from the Centers for Medicare & Medicaid Services, and equity interest in Medtronic. No other authors reported any relevant financial disclosures. No funding for the study was reported.
SOURCE: Annapureddy A et al. JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8775.
according to a new study of payments to lab directors at top hospitals.
“With continued concerns about rising health care costs, it is important to ensure that physician decisions regarding choice of devices and other pharmaceutical therapies be driven by clinical and cost effectiveness, not industry influence,” Amarnath Annapureddy, MD, of the Yale School of Medicine, New Haven, Conn. and coauthors wrote in their study published in JAMA Internal Medicine.
Dr. Annapureddy and fellow researchers analyzed Open Payments Program data from 2017 to determine payments made to cardiac catheterization and electrophysiology laboratory directors the top 100 hospitals based on US News and World Report’s ranking. They compared those payments to payments made to local interventional cardiologists and electrophysiologists, along with payments to interventional cardiologists and electrophysiologists from across the country.
In 2017, the directors of the top cardiac catheterization and electrophysiology labs received $1,416,232 and $2,307,504 from industry, respectively. The median payments to cardiac catheterization lab directors were significantly higher ($3,203 [$388-$14,156]), compared with interventional cardiologists ($1,064 [$206-$4,104]). The results were similar comparing electrophysiology lab directors with electrophysiology physicians practicing in the same zip codes ($10,521 [$1,159-$35,076] versus $2,900 [$549-$13,101]).
Overall, nearly one-third of cardiac catheterization lab directors and nearly half of electrophysiology lab directors received payments of $10,000 or more, an amount the National Academy of Medicine noted as constituting “a significant conflict of interest.”
The coauthors acknowledged their study’s limitations, including the possibility that they misclassified some lab directors and the fact that Open Payments Program’s data may have been inaccurate. However, they also anticipated that “inaccuracies would not vary between laboratory directors and other clinicians.”
Dr. Nihar R. Desai reported receiving funding from the Centers for Medicare & Medicaid Services, financial support from Johnson & Johnson, and research support from the American College of Cardiology Foundation. Dr. Jeptha P. Curtis reported receiving salary support from the American College of Cardiology, funding from the Centers for Medicare & Medicaid Services, and equity interest in Medtronic. No other authors reported any relevant financial disclosures. No funding for the study was reported.
SOURCE: Annapureddy A et al. JAMA Intern Med. 2019 Jun 17. doi:10.1001/jamainternmed.2018.8775.
FROM JAMA INTERNAL MEDICINE
New lemborexant efficacy and safety data unveiled
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
REPORTING FROM SLEEP 2019