User login
These Hips Don’t Lie
ANSWER
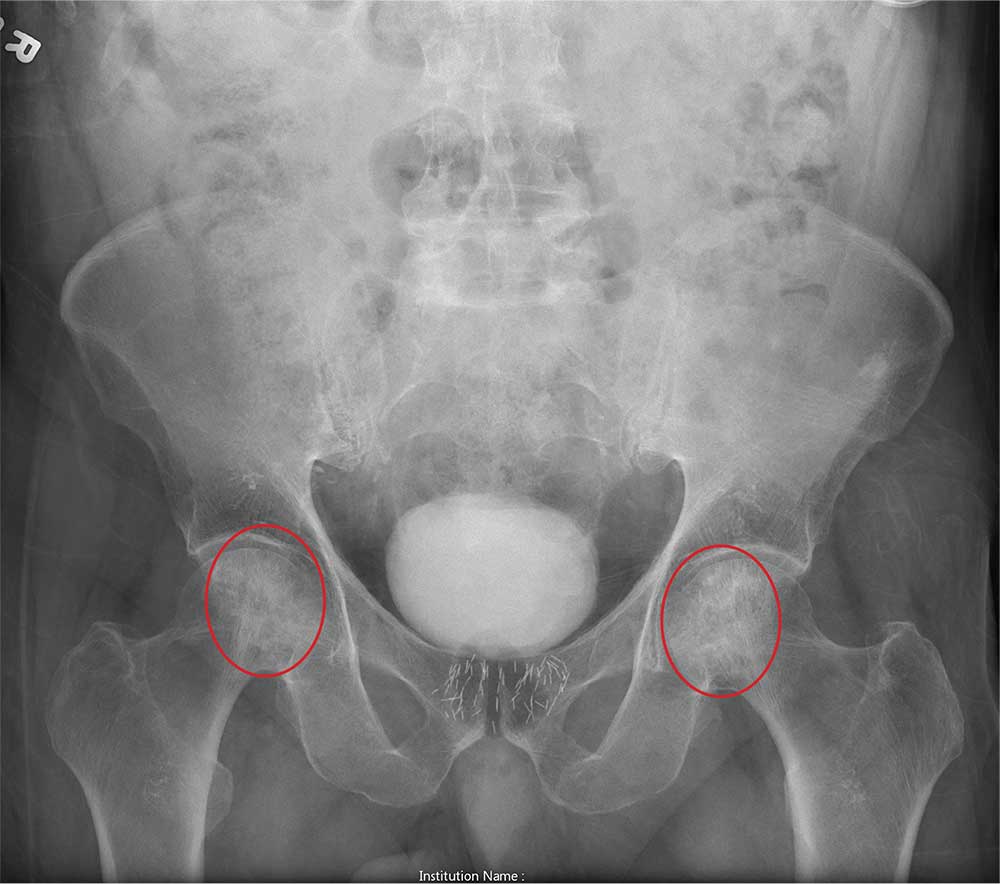
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.
ANSWER
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.
ANSWER
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.
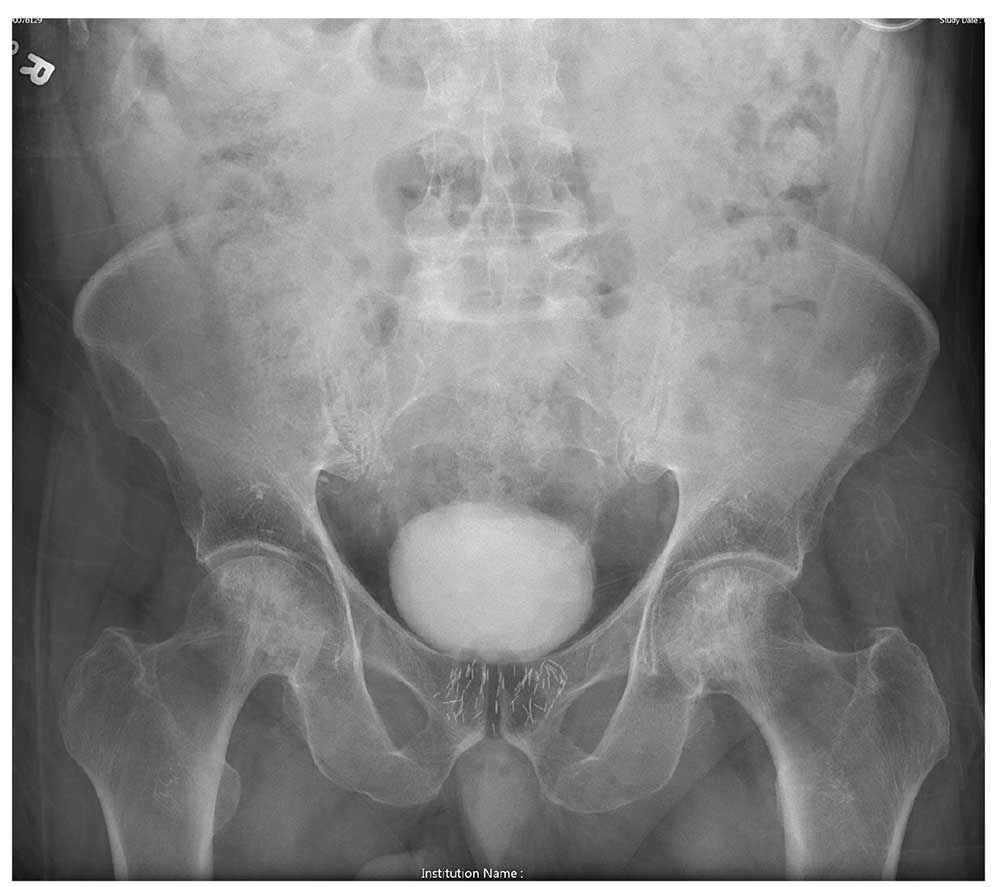
An 80-year-old man is transferred to your facility for evaluation of a lumbar compression fracture he sustained from a motor vehicle collision. The patient was a restrained driver in a vehicle that was broadsided at an unknown speed. His airbags deployed. In addition to mild back discomfort, he complains of severe right hip pain.
His medical history is significant for prostate cancer and coronary artery disease. Surgical history includes remote cardiac bypass surgery and recent revascularization with stents.
On examination, you note an elderly male who is awake and alert. His vital signs are stable. He is able to move all extremities
A portable pelvis radiograph is obtained (shown). What is your impression?
Study finds differences for HCC in women
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
REPORTING FROM DDW 2019
CSF neurofilament light level could aid in diagnosis
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
FROM JAMA NEUROLOGY
FDA approves pembrolizumab for advanced SCLC
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to pembrolizumab for patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
Approval was based on an overall response rate of 19% among 83 patients with SCLC who had disease progression on or after two or more prior lines of therapy enrolled in two nonrandomized trials, according to the FDA.
SCLC cohorts in KEYNOTE-028 and KEYNOTE-158 received either pembrolizumab 200 mg intravenously every 3 weeks (n = 64) or 10 mg/kg intravenously every 2 weeks (n = 19). Treatment continued until documented disease progression, unacceptable toxicity, or for a maximum of 24 months.
The ORR was 19% (95% confidence interval, 11%-29%), while the complete response rate was 2%. Responses were durable for 6 months or longer in 94% of the 16 responding patients.
Common adverse reactions included fatigue, decreased appetite, cough, nausea, and constipation. The most frequent serious adverse reactions were pneumonia and pleural effusion.
The recommended dosage for SCLC treatment is 200 mg, administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression, the FDA said.
Pembrolizumab is marketed as Keytruda by Merck.
Gepant Safety & Lack of Liver Toxicity: Highlights from AAN 2019
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
The gepants and monoclonal antibodies (mAbs) against calcitonin gene-related peptide (CGRP) and its receptor appear to be effective, tolerable and safe, according to several posters recently presented at the 2019 American Academy of Neurology (AAN) Annual Meeting in Philadelphia. Information was presented on the 3 gepants being studied for US Food and Drug Administration (FDA) approval: 1 for acute care of migraine, 1 for prevention and 1 for both (though the presented data for rimegepant only covers acute care).
All drugs cause a small degree of adverse events (AEs), somewhat more than placebo. Based on the presented data, it seems that those associated with 3 times higher than normal elevation of liver enzymes, were usually not found to be the cause of that elevation. At no time was bilirubin elevated. This shows that all of these gepants appear to be effective and safe, despite the fact that some were found to have cause liver toxicity many years ago.
The first gepant study to be published was on olcegepant in 2004, in the New England Journal of Medicine. Professor Jes Olesen, MD, was the lead author of the study, which detailed the efficacy and safety of this small molecule CGRP receptor antagonist. Olcegepant was in an intravenous formulation and the plan was to convert it to a tablet, which never happened. Another company then produced telcagepant as a tablet and it was shown to be safe and effective in 2 large, multicenter, double-blind trials. Before receiving FDA approval for the acute care of migraine, it was studied on a daily basis for migraine prevention. It was found to cause some liver toxicity, so development was stopped. At that time several other gepants in development were placed on the shelf, partially for the fear of liver toxicity. The FDA is unlikely to approve a drug with significant liver toxicity, which can cause a range of symptoms including jaundice, itching, abdominal pain, fatigue, loss of appetite, nausea, vomiting, rash, and weight loss.
In the next few years we will have 4 mAbs for the prevention of migraine, and 3 gepants if all studies are positive. Below are the key takeaways from the presented posters on ubrogepant and atogepant, as information that is currently available on rimegepant.
Key Takeaways:
- Ubrogepant – Ailani J, Hutchinson S, Lipton R, et al.
- Intermittent use of ubrogepant for the acute treatment of migraine over 1 year was well-tolerated with no identified safety concerns. Throughout the 1-year, Phase III study of 1254 participants, 22,454 migraine attacks were treated with 31,968 doses of ubrogepant.
- Twenty cases of ALT/AST ≥3x ULN were reported and adjudicated by an independent panel of liver experts blinded to treatment.
- Of the 20 cases, 17 (4 usual care, 3 ubrogepant 50-mg, and 10 ubrogepant 100-mg) were determined to be unlikely related based on plausible alternative etiology/confounding factors.
- Just 2 cases (both ubrogepant 50-mg) were described as possibly related to study medication and 1 case (ubrogepant 100-mg) was adjudicated as probably related; however, confounding factors were noted.
- All cases were asymptomatic with no concurrent bilirubin elevation. ALT/AST elevations resolved in those who continued dosing.
- Atogepant – Goadsby PJ, Dodick DW, Trugman JM, et al.
- In a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial of adults with a history of migraine, with or without aura, atogepant was well tolerated with no treatment-related serious AEs.
- Of the 834 randomized subjects, 825 were evaluated in the safety population. Treatment-emergent AEs were reported by 480 subjects (58.2%), and for 170 (20.6%), the AEs were considered treatment-related. Seven subjects (0.8%) reported serious AEs, but none were determined as treatment related.
- There were 10 cases of treatment-emergent ALT/AST elevations >3x the upper limit of normal, and this was balanced across the treatment dosage groups (10 mg QD, 30 mg QD, 30 mg BID, 60 mg QD, and 60 mg BID).
- Rimegepant – See Biogen press release for more information
- In December 2019, Biohaven announced initial positive results from an ongoing long-term, open-label safety study for rimegepant.
- The interim results included hepatic safety and tolerability data of rimegepant 75 mg in study participants based on a review of adverse events and regularly scheduled liver function tests.
- A panel of external independent liver experts provided a consensus based on the Drug-Induced Liver Injury Network (DILIN) causality assessment, determining that there were no liver cases probably related to the study drug and that there were no Hy’s Law cases identified.
- The panel also concluded that there were no liver safety signals detected and that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver abnormalities.
Patients with mood disorders may have altered microbiome
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM FOCUS ON NEUROPSYCHIATRY 2019
Durable transfusion independence in MDS with imetelstat
AMSTERDAM – For patients with low-risk myelodysplastic syndrome (MDS) for whom erythropoietin therapy has failed, the novel telomerase inhibitor imetelstat may provide long-lasting independence from transfusion, investigators reported.
Among 38 patients with low-risk MDS who had relapsed or were refractory to treatment with an erythropoiesis stimulating agent (ESA) who received imetelstat, 16 (42%) were free from the need for transfusion for at least 8 weeks, with one patient being transfusion free for up to 141 weeks, reported Pierre Fenaux, MD, of Hôpital Saint-Louis in Paris.
Patients with a generally worse prognosis “tended to respond better to imetelstat in terms of transfusion independence, which suggests that the drug is promising for higher-risk MDS,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
Imetelstat is a first-in-class telomerase inhibitor targeting cells with short telomere lengths and active telomerase, the enzyme that maintains telomere length. Higher telomerase activity and shorter telomeres are predictive of shorter overall survival in patients with MDS, Dr. Fenaux explained.
He and colleagues enrolled 38 patients, median age 71.5 years, with low-risk MDS, with an International Prognostic Scoring System (IPSS) score of low or intermediate-1, whose disease was relapsed or refractory to ESA or to erythropoietin at a dose of more than 500 mU/mL. Of this group, 24 patients had IPSS low disease, 14 had intermediate-1 disease.
The median transfusion burden was 8 units per 8 weeks (range 4-14). The majority of patients (34, or 89%) had received prior ESAs.
The patients were transfusion dependent, defined as the need for 4 or more units of red blood cells within 8 weeks over the 16 weeks prior to study entry.
No patients had the 5q deletion, and no patients had received either a hypomethylating agent or lenolidamide (Revlimid), neither of which are approved for this indication in Europe.
The patients received imetelstat 7.5 mg/kg intravenously every 4 weeks.
As noted earlier, 16 patients (42%) achieved the primary endpoint of 8-week transfusion independence, with a median duration of 85.9 weeks (range 8-141 weeks).
Eleven patients (29%) had transfusion independence lasting at least 24 weeks – a secondary endpoint – and 26 (68%) met International Working Group 2006 criteria for a HI-E (erythroid) response, with 12 of these patients having an increase in hemoglobin of 1.5 g/dL or greater lasting for at least 8 weeks, and all 26 having a reduction in transfusions of 4 or more units over 8 weeks.
There was evidence to suggest a disease-modifying effect of imetelstat, with five patients achieving a complete response (CR), and five having a marrow CR.
The most frequent adverse events were manageable and reversible grade 3 or greater cytopenias, but there were no new safety signals seen. Two patients were hospitalized for febrile neutropenia, but there were no treatment-related deaths.
Based on these results, investigators are planning a phase 3 study comparing imetelstat with placebo in a 2:1 ratio. The trial is scheduled to begin in the late summer or fall of 2019.
When asked if imetelstat might have off-target effects by inhibiting telomerase in other cells, Dr. Fenaux replied that the mechanism of action is unclear, and that its potential effects on erythropoiesis are still unknown.
Briefing moderator Anton Hagenbeek, MD, of Amsterdam University Medical Center, commented on the drug’s potential for treating MDS, and asked whether investigators are considering combining it with other therapies for MDS.
“I think the first step will be to study it in high-risk MDS as a single agent before combining it, including with hypomethylating agents, et cetera,” Dr. Fenaux replied.
SOURCE: Fenaux P et al. EHA 2019, Abstract S837.
AMSTERDAM – For patients with low-risk myelodysplastic syndrome (MDS) for whom erythropoietin therapy has failed, the novel telomerase inhibitor imetelstat may provide long-lasting independence from transfusion, investigators reported.
Among 38 patients with low-risk MDS who had relapsed or were refractory to treatment with an erythropoiesis stimulating agent (ESA) who received imetelstat, 16 (42%) were free from the need for transfusion for at least 8 weeks, with one patient being transfusion free for up to 141 weeks, reported Pierre Fenaux, MD, of Hôpital Saint-Louis in Paris.
Patients with a generally worse prognosis “tended to respond better to imetelstat in terms of transfusion independence, which suggests that the drug is promising for higher-risk MDS,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
Imetelstat is a first-in-class telomerase inhibitor targeting cells with short telomere lengths and active telomerase, the enzyme that maintains telomere length. Higher telomerase activity and shorter telomeres are predictive of shorter overall survival in patients with MDS, Dr. Fenaux explained.
He and colleagues enrolled 38 patients, median age 71.5 years, with low-risk MDS, with an International Prognostic Scoring System (IPSS) score of low or intermediate-1, whose disease was relapsed or refractory to ESA or to erythropoietin at a dose of more than 500 mU/mL. Of this group, 24 patients had IPSS low disease, 14 had intermediate-1 disease.
The median transfusion burden was 8 units per 8 weeks (range 4-14). The majority of patients (34, or 89%) had received prior ESAs.
The patients were transfusion dependent, defined as the need for 4 or more units of red blood cells within 8 weeks over the 16 weeks prior to study entry.
No patients had the 5q deletion, and no patients had received either a hypomethylating agent or lenolidamide (Revlimid), neither of which are approved for this indication in Europe.
The patients received imetelstat 7.5 mg/kg intravenously every 4 weeks.
As noted earlier, 16 patients (42%) achieved the primary endpoint of 8-week transfusion independence, with a median duration of 85.9 weeks (range 8-141 weeks).
Eleven patients (29%) had transfusion independence lasting at least 24 weeks – a secondary endpoint – and 26 (68%) met International Working Group 2006 criteria for a HI-E (erythroid) response, with 12 of these patients having an increase in hemoglobin of 1.5 g/dL or greater lasting for at least 8 weeks, and all 26 having a reduction in transfusions of 4 or more units over 8 weeks.
There was evidence to suggest a disease-modifying effect of imetelstat, with five patients achieving a complete response (CR), and five having a marrow CR.
The most frequent adverse events were manageable and reversible grade 3 or greater cytopenias, but there were no new safety signals seen. Two patients were hospitalized for febrile neutropenia, but there were no treatment-related deaths.
Based on these results, investigators are planning a phase 3 study comparing imetelstat with placebo in a 2:1 ratio. The trial is scheduled to begin in the late summer or fall of 2019.
When asked if imetelstat might have off-target effects by inhibiting telomerase in other cells, Dr. Fenaux replied that the mechanism of action is unclear, and that its potential effects on erythropoiesis are still unknown.
Briefing moderator Anton Hagenbeek, MD, of Amsterdam University Medical Center, commented on the drug’s potential for treating MDS, and asked whether investigators are considering combining it with other therapies for MDS.
“I think the first step will be to study it in high-risk MDS as a single agent before combining it, including with hypomethylating agents, et cetera,” Dr. Fenaux replied.
SOURCE: Fenaux P et al. EHA 2019, Abstract S837.
AMSTERDAM – For patients with low-risk myelodysplastic syndrome (MDS) for whom erythropoietin therapy has failed, the novel telomerase inhibitor imetelstat may provide long-lasting independence from transfusion, investigators reported.
Among 38 patients with low-risk MDS who had relapsed or were refractory to treatment with an erythropoiesis stimulating agent (ESA) who received imetelstat, 16 (42%) were free from the need for transfusion for at least 8 weeks, with one patient being transfusion free for up to 141 weeks, reported Pierre Fenaux, MD, of Hôpital Saint-Louis in Paris.
Patients with a generally worse prognosis “tended to respond better to imetelstat in terms of transfusion independence, which suggests that the drug is promising for higher-risk MDS,” he said at a briefing prior to his presentation of the data at the annual congress of the European Hematology Association.
Imetelstat is a first-in-class telomerase inhibitor targeting cells with short telomere lengths and active telomerase, the enzyme that maintains telomere length. Higher telomerase activity and shorter telomeres are predictive of shorter overall survival in patients with MDS, Dr. Fenaux explained.
He and colleagues enrolled 38 patients, median age 71.5 years, with low-risk MDS, with an International Prognostic Scoring System (IPSS) score of low or intermediate-1, whose disease was relapsed or refractory to ESA or to erythropoietin at a dose of more than 500 mU/mL. Of this group, 24 patients had IPSS low disease, 14 had intermediate-1 disease.
The median transfusion burden was 8 units per 8 weeks (range 4-14). The majority of patients (34, or 89%) had received prior ESAs.
The patients were transfusion dependent, defined as the need for 4 or more units of red blood cells within 8 weeks over the 16 weeks prior to study entry.
No patients had the 5q deletion, and no patients had received either a hypomethylating agent or lenolidamide (Revlimid), neither of which are approved for this indication in Europe.
The patients received imetelstat 7.5 mg/kg intravenously every 4 weeks.
As noted earlier, 16 patients (42%) achieved the primary endpoint of 8-week transfusion independence, with a median duration of 85.9 weeks (range 8-141 weeks).
Eleven patients (29%) had transfusion independence lasting at least 24 weeks – a secondary endpoint – and 26 (68%) met International Working Group 2006 criteria for a HI-E (erythroid) response, with 12 of these patients having an increase in hemoglobin of 1.5 g/dL or greater lasting for at least 8 weeks, and all 26 having a reduction in transfusions of 4 or more units over 8 weeks.
There was evidence to suggest a disease-modifying effect of imetelstat, with five patients achieving a complete response (CR), and five having a marrow CR.
The most frequent adverse events were manageable and reversible grade 3 or greater cytopenias, but there were no new safety signals seen. Two patients were hospitalized for febrile neutropenia, but there were no treatment-related deaths.
Based on these results, investigators are planning a phase 3 study comparing imetelstat with placebo in a 2:1 ratio. The trial is scheduled to begin in the late summer or fall of 2019.
When asked if imetelstat might have off-target effects by inhibiting telomerase in other cells, Dr. Fenaux replied that the mechanism of action is unclear, and that its potential effects on erythropoiesis are still unknown.
Briefing moderator Anton Hagenbeek, MD, of Amsterdam University Medical Center, commented on the drug’s potential for treating MDS, and asked whether investigators are considering combining it with other therapies for MDS.
“I think the first step will be to study it in high-risk MDS as a single agent before combining it, including with hypomethylating agents, et cetera,” Dr. Fenaux replied.
SOURCE: Fenaux P et al. EHA 2019, Abstract S837.
REPORTING FROM EHA CONGRESS
Adjuvant corticosteroids in hospitalized patients with CAP
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
When is it appropriate to treat?
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
Obesity and overweight declined among lower-income kids
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
FROM JAMA