User login
She Needs A-cyst-ance
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.
EXAMINATION
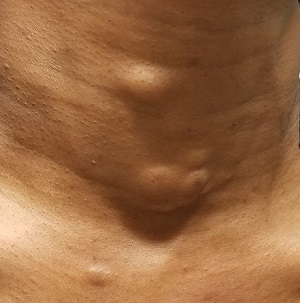
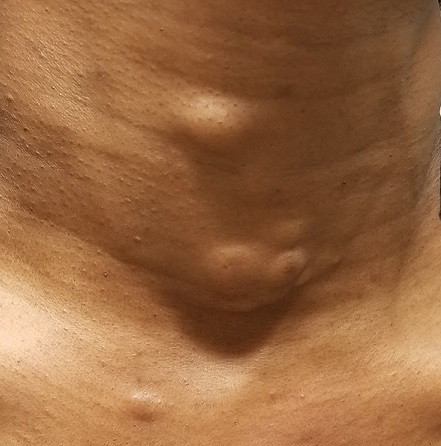
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.

EXAMINATION
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.

EXAMINATION
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
Multiple skin ulcers
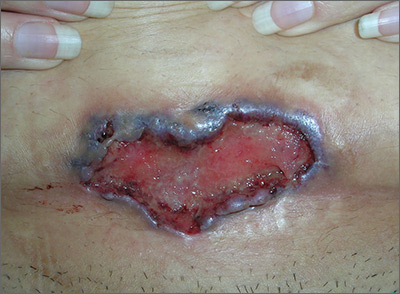
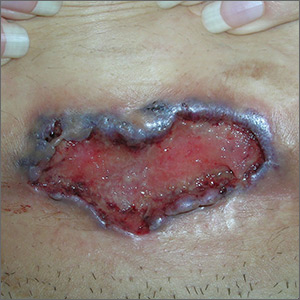
The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Psoriasis Treatment in Patients With Human Immunodeficiency Virus

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8
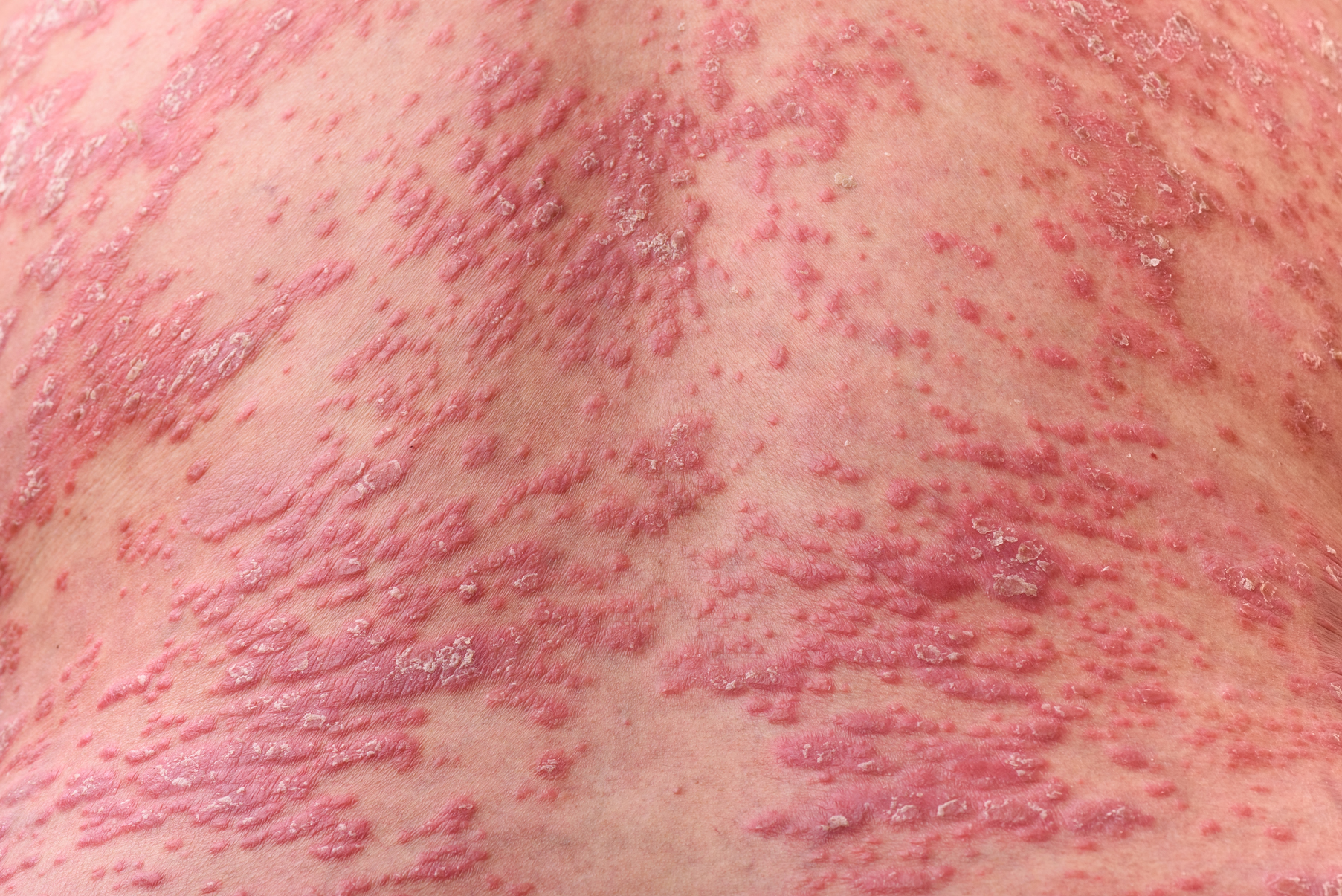
Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
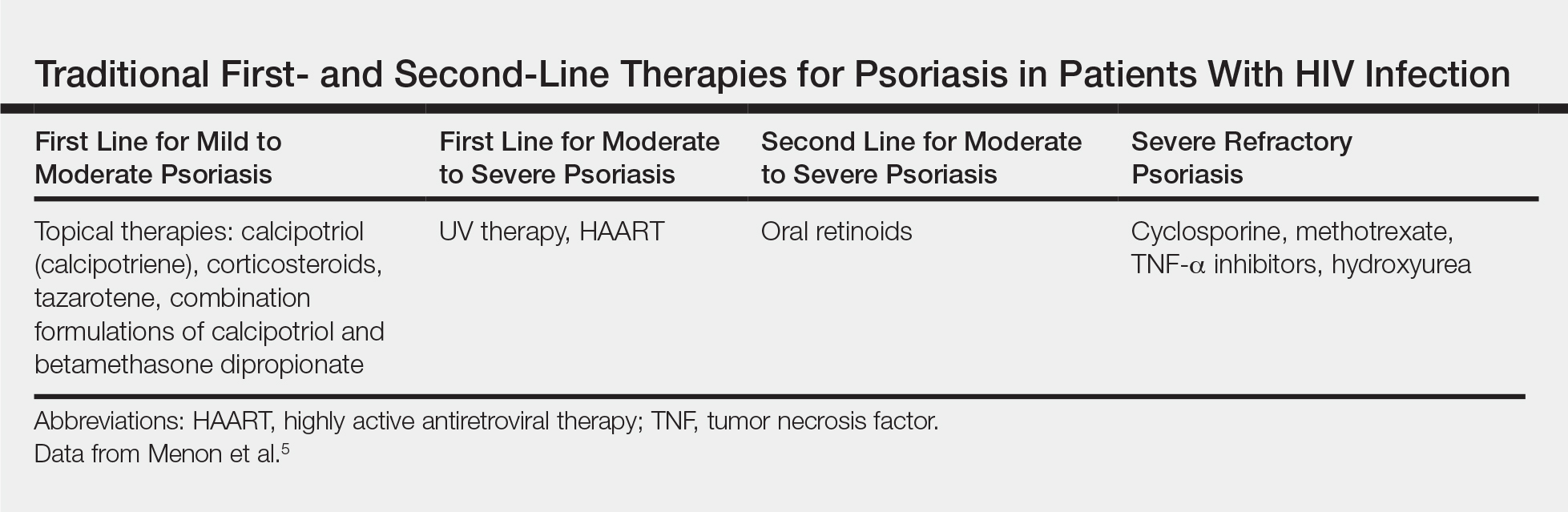
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1
Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16
Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
A 50-year-old man with Fitzpatrick skin type IV presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. The patient’s medical history was positive for human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected), for which clobetasol spray and calcitriol ointment had been prescribed. The patient’s CD4 count was 460 at presentation, and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to presentation. For the last 5 months, the patient had been undergoing phototherapy 3 times weekly for treatment of psoriasis.
An apremilast starter pack was initiated with the dosage titrated from 10 mg to 30 mg over the course of 1 week. The patient was maintained on a dose of 30 mg twice daily after 1 week, while continuing clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the patient’s affected BSA was 0%. Apremilast was continued, and phototherapy was reduced to once weekly. After 7 months of concomitant treatment with apremilast, phototherapy was discontinued after clearance was maintained. Phototherapy was reinitiated twice weekly after a mild flare (3% BSA affected).
The patient continued apremilast for a total of 20 months until it became cost prohibitive. After discontinuing apremilast for 4 months, he presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with intention to apply for an apremilast financial assistance program.
This case was adapted from Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7
Mindfulness-based stress reduction reduces migraine frequency
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
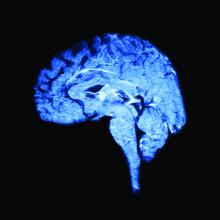
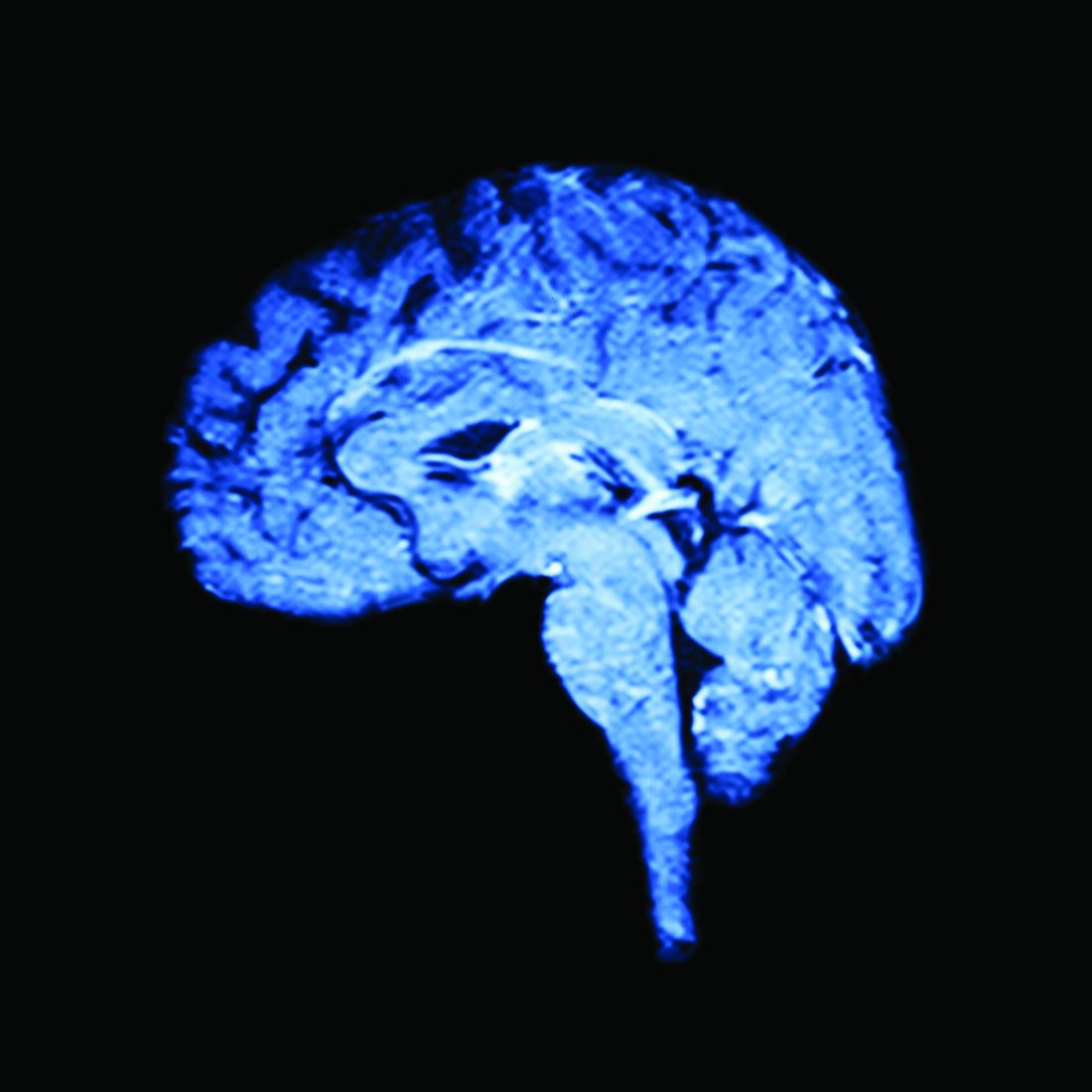
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
SAN ANTONIO – Episodic migraine patients benefit from mindfulness-based stress reduction training, according to new research. The intervention reduced headache frequency, slightly increased whole-brain gray matter volume, and reduced symptoms of anxiety, depression, and stress.
The gray matter findings may indicate opportunities for therapeutic targets, while the psychosocial findings are important in understanding migraine burden, treatment response, and personalized medicine opportunities, Shana Burrowes, PhD, a postdoctoral associate at Boston University, said at the annual meeting of the College on Problems of Drug Dependence.
In a session focused on exploring alternatives to opioids for pain treatment, Dr. Burrowes described interim results of a randomized, controlled trial testing the effectiveness of mindfulness-based stress reduction (MBSR) training for managing migraine.
In discussing the rationale for study endpoints, she explained a three-pronged model for understanding migraine. Those elements include the symptoms themselves – unilateral throbbing pain, nausea, and photophobia – and the psychosocial symptoms and comorbidities, including anxiety, depression, stress, and catastrophizing. Up to 30%* of migraine patients have comorbid depression.
Those two prongs have a bidirectional relationship, since each increases the risk of the other. For example, frequent migraine can leave people feeling anxious about when their next migraine will occur, and that anxiety can increase the risk of it occurring.
Both elements lead to the third prong, which is change in gray matter volume. “If you’re a patient with migraine, an MRI on your brain is going to look different from somebody who does not have migraine,” Dr. Burrowes said. “With all these things going on in a patient, a migraine patient is actually pretty difficult to treat.”
Therefore, the researchers focused on outcomes from each of these three domains: gray matter volume in MRI; headache frequency as a clinical outcome; and the psychosocial comorbidities of anxiety, stress, and depression.
Study participants included 98 patients with episodic migraine, defined as fewer than 15 headache days a month, and 27 controls* matched by demographics to the patients and without any chronic pain conditions. The groups were 92% female and had similar ratios of whites (75% and 77%) and college graduates (95% and 96%).
Only the patients were randomized to the two interventions, one a training on MBSR and the other focusing on stress management for headache (SMH).
The MBSR training involved group sessions, eight 2.5-hour meditation sessions, at-home practice, a half-day retreat, and then an additional four biweekly sessions. The mindfulness training specifically focused on intentionally paying attention in the moment without judgment. The SMH arm focused on education for managing headache symptoms, stress, sleep hygiene, and diet, but it did not involve any specific skills training, such as relaxation training.
All participants, including healthy controls, underwent clinical assessment and baseline MRI and psychosocial questionnaires, followed by MRI and psychosocial questionnaire follow-ups at 3 and 6 months. MRI imaging focused on the whole brain and on the bilateral insula, dorsolateral prefrontal cortex, anterior cingulate cortex, and superior frontal gyrus. Patients also kept headache diaries throughout the trial.
Both intervention groups showed an increase in gray matter volume over 6 months, compared with healthy controls: 1.3% in the whole brain for SMH participants and 1.01% in the MBSR patients, compared with –1.37% in healthy participants. In the right superior frontal gyrus, gray matter volume also increased 2.62% in SMH participants and 1.25% in MBSR patients but decreased 0.19% in healthy participants.
Dr. Burrowes said she could not share specific findings on headache frequency and psychosocial outcomes because her team’s research is currently under review. Overall, however, headache frequency declined more than 50% post intervention, and 39% of migraine patients responded to the therapy.
In addition, anxiety, stress, and depression symptoms all saw improvements from MBSR and slightly but significantly mediated the effect of MBSR on migraine reduction.
Dr. Burrowes reported having no disclosures.
*The story was updated 6/20/2019.
REPORTING FROM CPDD 2019
Nicotine replacement therapy beats varenicline for smokers with OUD
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
REPORTING FROM CPDD 2019
Prophylactic rudeness, surgical barbecue, and MRI-ectomy
Stay rude, stay alive
Middle fingers up! Did you know there’s a biological theory that proposes rudeness as a mechanism for health?
The theory proposes that the single most important factor of determining human behavior is disease. Where disease flourishes, humans are meaner to strangers. This is a self-preservation tactic – strangers could carry sickness, so it’s best to steer clear. As people continue to avoid strangers (and potential new diseases), larger divides develop between language and culture.
Researchers looked at countries and cultures around the world and found that their theory held true: Locations that had less disease tended to have less diversity in language and culture, while higher disease rates coincided with more cultural diversity.
Can disease really be the sole factor for all human behavior, however? Tough to say, but in the meantime we’ll endorse never talking to strangers – just in case.
Facebook fires
If you’re a health care provider, you’ve probably had a difficult patient experience or two … or 7,000. In the olden days before the Internet, perhaps you turned to a trusted friend to vent your frustrations. Maybe you were an avid journaler, furiously sharing your problems with the page. With the advent of social media, however, you can publicly exorcise your doctor demons for your whole network to share!
In case you weren’t sure, this is a terrible idea – and now there are the data to prove it.
Participants in a recent study rated (fake) Facebook profiles of medical professionals who posted comments about their workday. Some comments were ambiguous, such as, “Started with new electronic patient charts today ... interesting experience for sure.” Other comments were blatantly frustrated, saying things like “What is it with some people?? I know I only went through 9 years of university ... but really, I know what I’m talking about ... yeesh!!!”
Unsurprisingly, the Facebook profiles with the obviously frustrated comments were rated “significantly less credible” than profiles with ambiguous comments, and they negatively affected willingness to become a patient of the fake doctor.
All this to say, when you get the urge to angrily post on Facebook about that overprotective parent in your office, perhaps turn to your diary instead.
Saying goodbye to a 12,000-lb friend
Lots of physicians have ordered MRI scans for their patients, but how many have performed an MRI-ectomy?
What? No, no, NO! We’re not talking about removing one from a patient! How would that even work? You do know that the patient goes inside the machine, right?
Okay, let’s try again.
How do you remove an MRI machine from a medical center? Verrrry carefully … with a forklift … after you’ve cut a big hole in the side of the building. That’s what they did at OSF HealthCare’s Center for Health – Glen Park in Peoria, Ill., on June 10. They had a party first, though, and someone brought one of those giant cookies, which said, “We will miss you, Open MRI,” the Pekin Daily Times reported.
Photos were taken, cookie was eaten, and tears were shed. “It’s definitely kind of bittersweet that it’s going away,” said Jamie White, manager of CT and MRI outpatient diagnostics.
On the day of the actual removal, a small crowd gathered outside to watch the experts who were brought in to extract the 6-ton machine. “I just took it off life support,” said Eddie Rivera, an engineer with ATI-Advanced Technologies, Miami, when he disconnected the electricity.
We’re tearing up a bit ourselves, actually, but there is some good news. The machine is not headed to that big imaging center in the sky just yet. Like the saying goes: Old MRIs never die, they just get hauled off to Arizona for refurbishment.
A bad case of heartburn
You would think that your day couldn’t get much worse than having to undergo emergency surgery. That’s a pretty rough time, no matter what. But for a 60-year-old man receiving a repair of an ascending aortic dissection, his doctors managed to add insult to a very serious injury: They lit a fire inside their patient.
According to a case study presented at the 2019 Euroanaesthesia Congress in Vienna, it all started with the patient’s history of chronic obstructive pulmonary disease. Bullae in the lungs caused by the disease were stuck to the sternum, and during an attempt to separate the lung from the sternum, a bulla was punctured, causing an air leak. To compensate, the surgeons boosted the proportion of oxygen to 100%.
In retrospect, what happened next almost seems predictable. A spark from the electrocautery device ignited a dry surgical pack, and with the assist of that extra oxygen, the doctors immediately had a fire on their hands, localized within their patient’s chest cavity. We believe this is what the medical community calls a “complication.”
To the surgeons’ credit, the fire was extinguished immediately, and after they presumably took a break to change into clean underwear, the rest of the operation went without incident. Though we imagine the patient was a bit confused when he woke up to the smell of barbecue.

Stay rude, stay alive
Middle fingers up! Did you know there’s a biological theory that proposes rudeness as a mechanism for health?
The theory proposes that the single most important factor of determining human behavior is disease. Where disease flourishes, humans are meaner to strangers. This is a self-preservation tactic – strangers could carry sickness, so it’s best to steer clear. As people continue to avoid strangers (and potential new diseases), larger divides develop between language and culture.
Researchers looked at countries and cultures around the world and found that their theory held true: Locations that had less disease tended to have less diversity in language and culture, while higher disease rates coincided with more cultural diversity.
Can disease really be the sole factor for all human behavior, however? Tough to say, but in the meantime we’ll endorse never talking to strangers – just in case.
Facebook fires
If you’re a health care provider, you’ve probably had a difficult patient experience or two … or 7,000. In the olden days before the Internet, perhaps you turned to a trusted friend to vent your frustrations. Maybe you were an avid journaler, furiously sharing your problems with the page. With the advent of social media, however, you can publicly exorcise your doctor demons for your whole network to share!
In case you weren’t sure, this is a terrible idea – and now there are the data to prove it.
Participants in a recent study rated (fake) Facebook profiles of medical professionals who posted comments about their workday. Some comments were ambiguous, such as, “Started with new electronic patient charts today ... interesting experience for sure.” Other comments were blatantly frustrated, saying things like “What is it with some people?? I know I only went through 9 years of university ... but really, I know what I’m talking about ... yeesh!!!”
Unsurprisingly, the Facebook profiles with the obviously frustrated comments were rated “significantly less credible” than profiles with ambiguous comments, and they negatively affected willingness to become a patient of the fake doctor.
All this to say, when you get the urge to angrily post on Facebook about that overprotective parent in your office, perhaps turn to your diary instead.
Saying goodbye to a 12,000-lb friend
Lots of physicians have ordered MRI scans for their patients, but how many have performed an MRI-ectomy?
What? No, no, NO! We’re not talking about removing one from a patient! How would that even work? You do know that the patient goes inside the machine, right?
Okay, let’s try again.
How do you remove an MRI machine from a medical center? Verrrry carefully … with a forklift … after you’ve cut a big hole in the side of the building. That’s what they did at OSF HealthCare’s Center for Health – Glen Park in Peoria, Ill., on June 10. They had a party first, though, and someone brought one of those giant cookies, which said, “We will miss you, Open MRI,” the Pekin Daily Times reported.
Photos were taken, cookie was eaten, and tears were shed. “It’s definitely kind of bittersweet that it’s going away,” said Jamie White, manager of CT and MRI outpatient diagnostics.
On the day of the actual removal, a small crowd gathered outside to watch the experts who were brought in to extract the 6-ton machine. “I just took it off life support,” said Eddie Rivera, an engineer with ATI-Advanced Technologies, Miami, when he disconnected the electricity.
We’re tearing up a bit ourselves, actually, but there is some good news. The machine is not headed to that big imaging center in the sky just yet. Like the saying goes: Old MRIs never die, they just get hauled off to Arizona for refurbishment.
A bad case of heartburn
You would think that your day couldn’t get much worse than having to undergo emergency surgery. That’s a pretty rough time, no matter what. But for a 60-year-old man receiving a repair of an ascending aortic dissection, his doctors managed to add insult to a very serious injury: They lit a fire inside their patient.
According to a case study presented at the 2019 Euroanaesthesia Congress in Vienna, it all started with the patient’s history of chronic obstructive pulmonary disease. Bullae in the lungs caused by the disease were stuck to the sternum, and during an attempt to separate the lung from the sternum, a bulla was punctured, causing an air leak. To compensate, the surgeons boosted the proportion of oxygen to 100%.
In retrospect, what happened next almost seems predictable. A spark from the electrocautery device ignited a dry surgical pack, and with the assist of that extra oxygen, the doctors immediately had a fire on their hands, localized within their patient’s chest cavity. We believe this is what the medical community calls a “complication.”
To the surgeons’ credit, the fire was extinguished immediately, and after they presumably took a break to change into clean underwear, the rest of the operation went without incident. Though we imagine the patient was a bit confused when he woke up to the smell of barbecue.

Stay rude, stay alive
Middle fingers up! Did you know there’s a biological theory that proposes rudeness as a mechanism for health?
The theory proposes that the single most important factor of determining human behavior is disease. Where disease flourishes, humans are meaner to strangers. This is a self-preservation tactic – strangers could carry sickness, so it’s best to steer clear. As people continue to avoid strangers (and potential new diseases), larger divides develop between language and culture.
Researchers looked at countries and cultures around the world and found that their theory held true: Locations that had less disease tended to have less diversity in language and culture, while higher disease rates coincided with more cultural diversity.
Can disease really be the sole factor for all human behavior, however? Tough to say, but in the meantime we’ll endorse never talking to strangers – just in case.
Facebook fires
If you’re a health care provider, you’ve probably had a difficult patient experience or two … or 7,000. In the olden days before the Internet, perhaps you turned to a trusted friend to vent your frustrations. Maybe you were an avid journaler, furiously sharing your problems with the page. With the advent of social media, however, you can publicly exorcise your doctor demons for your whole network to share!
In case you weren’t sure, this is a terrible idea – and now there are the data to prove it.
Participants in a recent study rated (fake) Facebook profiles of medical professionals who posted comments about their workday. Some comments were ambiguous, such as, “Started with new electronic patient charts today ... interesting experience for sure.” Other comments were blatantly frustrated, saying things like “What is it with some people?? I know I only went through 9 years of university ... but really, I know what I’m talking about ... yeesh!!!”
Unsurprisingly, the Facebook profiles with the obviously frustrated comments were rated “significantly less credible” than profiles with ambiguous comments, and they negatively affected willingness to become a patient of the fake doctor.
All this to say, when you get the urge to angrily post on Facebook about that overprotective parent in your office, perhaps turn to your diary instead.
Saying goodbye to a 12,000-lb friend
Lots of physicians have ordered MRI scans for their patients, but how many have performed an MRI-ectomy?
What? No, no, NO! We’re not talking about removing one from a patient! How would that even work? You do know that the patient goes inside the machine, right?
Okay, let’s try again.
How do you remove an MRI machine from a medical center? Verrrry carefully … with a forklift … after you’ve cut a big hole in the side of the building. That’s what they did at OSF HealthCare’s Center for Health – Glen Park in Peoria, Ill., on June 10. They had a party first, though, and someone brought one of those giant cookies, which said, “We will miss you, Open MRI,” the Pekin Daily Times reported.
Photos were taken, cookie was eaten, and tears were shed. “It’s definitely kind of bittersweet that it’s going away,” said Jamie White, manager of CT and MRI outpatient diagnostics.
On the day of the actual removal, a small crowd gathered outside to watch the experts who were brought in to extract the 6-ton machine. “I just took it off life support,” said Eddie Rivera, an engineer with ATI-Advanced Technologies, Miami, when he disconnected the electricity.
We’re tearing up a bit ourselves, actually, but there is some good news. The machine is not headed to that big imaging center in the sky just yet. Like the saying goes: Old MRIs never die, they just get hauled off to Arizona for refurbishment.
A bad case of heartburn
You would think that your day couldn’t get much worse than having to undergo emergency surgery. That’s a pretty rough time, no matter what. But for a 60-year-old man receiving a repair of an ascending aortic dissection, his doctors managed to add insult to a very serious injury: They lit a fire inside their patient.
According to a case study presented at the 2019 Euroanaesthesia Congress in Vienna, it all started with the patient’s history of chronic obstructive pulmonary disease. Bullae in the lungs caused by the disease were stuck to the sternum, and during an attempt to separate the lung from the sternum, a bulla was punctured, causing an air leak. To compensate, the surgeons boosted the proportion of oxygen to 100%.
In retrospect, what happened next almost seems predictable. A spark from the electrocautery device ignited a dry surgical pack, and with the assist of that extra oxygen, the doctors immediately had a fire on their hands, localized within their patient’s chest cavity. We believe this is what the medical community calls a “complication.”
To the surgeons’ credit, the fire was extinguished immediately, and after they presumably took a break to change into clean underwear, the rest of the operation went without incident. Though we imagine the patient was a bit confused when he woke up to the smell of barbecue.

Novel chip system could improve preclinical drug studies
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of anticancer therapies.
Major finding: Overall, results support the utility of the system to assess both off-target toxicity and on-target efficacy for various anticancer drugs.
Study details: A study exploring the utility of a multi-organ-on-a-chip system to assess safety and effectiveness of anticancer therapies in the preclinical setting.
Disclosures: The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
Source: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
Senators agree surprise medical bills must go. But how?
Two years, 16 hearings, and one massive bipartisan package of legislation later, a key Senate committee says it is ready to start marking up a bill the week of June 24 designed to contain health care costs. But it might not be easy since lawmakers and stakeholders at a final hearing June 18 showed they are still far apart on one simple aspect of the proposal.
That sticking point: a formula for paying for surprise medical bills, those unexpected and often high charges patients face when they get care from a doctor or hospital that isn’t in their insurance network.
“People get health insurance precisely so they won’t be surprised by health care bills,” said Sen. Maggie Hassan (D-N.H.), the coauthor of a separate proposal to tamp down surprise bills. “So it is completely unacceptable that people do everything that they’re supposed to do to ensure that their care is in their insurance network and then still end up with large, unexpected bills from an out-of-network provider.”
It’s a cause that has been taken up by President Donald Trump and various bipartisan groups of lawmakers on Capitol Hill.
The wide-ranging legislative package on curbing health care costs is sponsored by Sen. Lamar Alexander (R-Tenn.) and Sen. Patty Murray (D-Wash.), the chairman and ranking member, respectively, of the Health, Education, Labor and Pensions (HELP) Committee. Given the committee’s influence, and because this legislation has bipartisan support in the Senate where not many bills are moving, industry observers are taking the HELP panel’s proposal very seriously.
The Alexander/Murray bill lays out three options for paying surprise medical bills but does not specify which path the final legislation should take. Advocates for each of the choices were among the five witnesses June 18.
Their positions fell along familiar fault lines. Everyone acknowledged that patients who stumble into a surprise bill because their emergency care was handled at a facility not in their insurance network or because a doctor at their in-network hospital doesn’t take the patient’s plan should not have to pay more than they would for an inpatient service. But they differ on how much doctors, hospitals, and other providers should be compensated and how the disputes should be resolved.
Tom Nickels, an executive vice president of the American Hospital Association, cautioned against using benchmarks to set pay levels, such as local customary averages or a price set in relation to Medicare. He said such a plan might underpay providers and hospitals could lose their leverage to negotiate with insurers.
Elizabeth Mitchell, president and CEO of the Pacific Business Group on Health – a group that represents employers, including some who are self-insured who pay their workers’ health costs – said doctors should be paid 125% of what Medicare pays. She told senators that an independent arbitration process like the one Nickels advocates would add unnecessary costs to the system.
Benedic Ippolito, a researcher with the American Enterprise Institute, said requiring all providers in a hospital to be in-network was the cleanest solution.
“On surprise billing, all three approaches are equal in that first and foremost they protect the consumer,” said Sean Cavanaugh, chief administrative officer for Aledade, a company that matches primary care physicians with accountable care organizations.
There was also broad support among the witnesses for some of the legislation’s transparency measures, especially the creation of a nongovernmental nonprofit organization to collect claims data from private health plans, Medicare, and some states to create what’s called an all-payer claims database. That could help policymakers better understand the true cost of care, these experts told the committee.
Sen. Susan Collins (R-Maine) expressed trepidation about the all-payer claims database, noting that increased transparency could hurt rural hospitals, which typically charge higher prices than those in cities because their patient base is small and they need to bring in enough revenue to cover fixed costs.
The witnesses also offered support for eliminating “gag clauses” between doctors and health plans. These stipulations often prevent providers from telling patients the cost of a procedure or service.
“Patients and families absolutely have skin in the game ... but they are in a completely untenable and unfair situation. They have no information,” said Ms. Mitchell, from the Pacific Business Group on Health. “We’re talking about providers not being allowed to share information. ... Transparency is necessary so people can have active involvement.”
If one thing is clear, it’s that Sen. Alexander doesn’t want this summer to be a rehash of last year, when it appeared he had a bipartisan deal to address problems in the federal health law’s marketplaces before the effort fell apart.
“For the last decade, Congress had been locked in an argument about the individual health care market,” said Sen. Alexander at the hearing. “That is not this discussion. This is a different discussion. We’ll never lower the cost of health insurance until we lower the cost of health care.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Two years, 16 hearings, and one massive bipartisan package of legislation later, a key Senate committee says it is ready to start marking up a bill the week of June 24 designed to contain health care costs. But it might not be easy since lawmakers and stakeholders at a final hearing June 18 showed they are still far apart on one simple aspect of the proposal.
That sticking point: a formula for paying for surprise medical bills, those unexpected and often high charges patients face when they get care from a doctor or hospital that isn’t in their insurance network.
“People get health insurance precisely so they won’t be surprised by health care bills,” said Sen. Maggie Hassan (D-N.H.), the coauthor of a separate proposal to tamp down surprise bills. “So it is completely unacceptable that people do everything that they’re supposed to do to ensure that their care is in their insurance network and then still end up with large, unexpected bills from an out-of-network provider.”
It’s a cause that has been taken up by President Donald Trump and various bipartisan groups of lawmakers on Capitol Hill.
The wide-ranging legislative package on curbing health care costs is sponsored by Sen. Lamar Alexander (R-Tenn.) and Sen. Patty Murray (D-Wash.), the chairman and ranking member, respectively, of the Health, Education, Labor and Pensions (HELP) Committee. Given the committee’s influence, and because this legislation has bipartisan support in the Senate where not many bills are moving, industry observers are taking the HELP panel’s proposal very seriously.
The Alexander/Murray bill lays out three options for paying surprise medical bills but does not specify which path the final legislation should take. Advocates for each of the choices were among the five witnesses June 18.
Their positions fell along familiar fault lines. Everyone acknowledged that patients who stumble into a surprise bill because their emergency care was handled at a facility not in their insurance network or because a doctor at their in-network hospital doesn’t take the patient’s plan should not have to pay more than they would for an inpatient service. But they differ on how much doctors, hospitals, and other providers should be compensated and how the disputes should be resolved.
Tom Nickels, an executive vice president of the American Hospital Association, cautioned against using benchmarks to set pay levels, such as local customary averages or a price set in relation to Medicare. He said such a plan might underpay providers and hospitals could lose their leverage to negotiate with insurers.
Elizabeth Mitchell, president and CEO of the Pacific Business Group on Health – a group that represents employers, including some who are self-insured who pay their workers’ health costs – said doctors should be paid 125% of what Medicare pays. She told senators that an independent arbitration process like the one Nickels advocates would add unnecessary costs to the system.
Benedic Ippolito, a researcher with the American Enterprise Institute, said requiring all providers in a hospital to be in-network was the cleanest solution.
“On surprise billing, all three approaches are equal in that first and foremost they protect the consumer,” said Sean Cavanaugh, chief administrative officer for Aledade, a company that matches primary care physicians with accountable care organizations.
There was also broad support among the witnesses for some of the legislation’s transparency measures, especially the creation of a nongovernmental nonprofit organization to collect claims data from private health plans, Medicare, and some states to create what’s called an all-payer claims database. That could help policymakers better understand the true cost of care, these experts told the committee.
Sen. Susan Collins (R-Maine) expressed trepidation about the all-payer claims database, noting that increased transparency could hurt rural hospitals, which typically charge higher prices than those in cities because their patient base is small and they need to bring in enough revenue to cover fixed costs.
The witnesses also offered support for eliminating “gag clauses” between doctors and health plans. These stipulations often prevent providers from telling patients the cost of a procedure or service.
“Patients and families absolutely have skin in the game ... but they are in a completely untenable and unfair situation. They have no information,” said Ms. Mitchell, from the Pacific Business Group on Health. “We’re talking about providers not being allowed to share information. ... Transparency is necessary so people can have active involvement.”
If one thing is clear, it’s that Sen. Alexander doesn’t want this summer to be a rehash of last year, when it appeared he had a bipartisan deal to address problems in the federal health law’s marketplaces before the effort fell apart.
“For the last decade, Congress had been locked in an argument about the individual health care market,” said Sen. Alexander at the hearing. “That is not this discussion. This is a different discussion. We’ll never lower the cost of health insurance until we lower the cost of health care.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Two years, 16 hearings, and one massive bipartisan package of legislation later, a key Senate committee says it is ready to start marking up a bill the week of June 24 designed to contain health care costs. But it might not be easy since lawmakers and stakeholders at a final hearing June 18 showed they are still far apart on one simple aspect of the proposal.
That sticking point: a formula for paying for surprise medical bills, those unexpected and often high charges patients face when they get care from a doctor or hospital that isn’t in their insurance network.
“People get health insurance precisely so they won’t be surprised by health care bills,” said Sen. Maggie Hassan (D-N.H.), the coauthor of a separate proposal to tamp down surprise bills. “So it is completely unacceptable that people do everything that they’re supposed to do to ensure that their care is in their insurance network and then still end up with large, unexpected bills from an out-of-network provider.”
It’s a cause that has been taken up by President Donald Trump and various bipartisan groups of lawmakers on Capitol Hill.
The wide-ranging legislative package on curbing health care costs is sponsored by Sen. Lamar Alexander (R-Tenn.) and Sen. Patty Murray (D-Wash.), the chairman and ranking member, respectively, of the Health, Education, Labor and Pensions (HELP) Committee. Given the committee’s influence, and because this legislation has bipartisan support in the Senate where not many bills are moving, industry observers are taking the HELP panel’s proposal very seriously.
The Alexander/Murray bill lays out three options for paying surprise medical bills but does not specify which path the final legislation should take. Advocates for each of the choices were among the five witnesses June 18.
Their positions fell along familiar fault lines. Everyone acknowledged that patients who stumble into a surprise bill because their emergency care was handled at a facility not in their insurance network or because a doctor at their in-network hospital doesn’t take the patient’s plan should not have to pay more than they would for an inpatient service. But they differ on how much doctors, hospitals, and other providers should be compensated and how the disputes should be resolved.
Tom Nickels, an executive vice president of the American Hospital Association, cautioned against using benchmarks to set pay levels, such as local customary averages or a price set in relation to Medicare. He said such a plan might underpay providers and hospitals could lose their leverage to negotiate with insurers.
Elizabeth Mitchell, president and CEO of the Pacific Business Group on Health – a group that represents employers, including some who are self-insured who pay their workers’ health costs – said doctors should be paid 125% of what Medicare pays. She told senators that an independent arbitration process like the one Nickels advocates would add unnecessary costs to the system.
Benedic Ippolito, a researcher with the American Enterprise Institute, said requiring all providers in a hospital to be in-network was the cleanest solution.
“On surprise billing, all three approaches are equal in that first and foremost they protect the consumer,” said Sean Cavanaugh, chief administrative officer for Aledade, a company that matches primary care physicians with accountable care organizations.
There was also broad support among the witnesses for some of the legislation’s transparency measures, especially the creation of a nongovernmental nonprofit organization to collect claims data from private health plans, Medicare, and some states to create what’s called an all-payer claims database. That could help policymakers better understand the true cost of care, these experts told the committee.
Sen. Susan Collins (R-Maine) expressed trepidation about the all-payer claims database, noting that increased transparency could hurt rural hospitals, which typically charge higher prices than those in cities because their patient base is small and they need to bring in enough revenue to cover fixed costs.
The witnesses also offered support for eliminating “gag clauses” between doctors and health plans. These stipulations often prevent providers from telling patients the cost of a procedure or service.
“Patients and families absolutely have skin in the game ... but they are in a completely untenable and unfair situation. They have no information,” said Ms. Mitchell, from the Pacific Business Group on Health. “We’re talking about providers not being allowed to share information. ... Transparency is necessary so people can have active involvement.”
If one thing is clear, it’s that Sen. Alexander doesn’t want this summer to be a rehash of last year, when it appeared he had a bipartisan deal to address problems in the federal health law’s marketplaces before the effort fell apart.
“For the last decade, Congress had been locked in an argument about the individual health care market,” said Sen. Alexander at the hearing. “That is not this discussion. This is a different discussion. We’ll never lower the cost of health insurance until we lower the cost of health care.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Benefits of Medicare Shared Savings Program ACOs lacking
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
or improving quality, according to new research.
“Our conclusion that the MSSP was not associated with improvements in spending, quality, or most measures of hospital use differ from that of previous evaluations of Medicare ACOs,” Adam Markovitz, of the University of Michigan, Ann Arbor, and colleagues wrote in a new research report published in Annals of Internal Medicine.
“Our instrumental variable model addresses selection effects not directly captured in previous evaluations,” the researchers continued.
To illustrate the point, the researchers found an association between MSSP and spending when using an adjusted longitudinal model (change, –$118; 95% confidence interval, –$151 to –$85 per beneficiary per quarter), with savings coming from reductions in inpatient services, outpatient services, and skilled nursing facility charges.
However, when employing an instrumental variable model, there was not an association with changes in total spending (change, $5; 95%CI, –$51 to $62 per beneficiary per quarter.
“The instrumental variable estimate for spending differed significantly from the adjusted estimate,” Mr. Markovitz and colleagues noted. “Estimated savings were smaller in instrumental variable models than in adjusted models across each ACO cohort.”
Similar patterns were observed in quality observations.
“The MSSP was associated with improvements in all four clinical quality indicators in the adjusted longitudinal model but not in the instrumental variable model,” the authors wrote. “The MSSP was associated with modest decreases in all-cause hospitalizations and preventable hospitalizations in the longitudinal model but not in the instrumental variable model.”
Overall, the authors noted that the results “challenge the view that MSSP ACOs have lowered spending and improved quality; they indicate that savings by MSSP ACOs may be driven by nonrandom exit of high-cost clinicians and their patient panels from this voluntary program.”
Indeed, the report states that removing “high-cost clinicians from ACO contracts could have large effects on spending estimates and may contribute to reported findings that MSSP savings grow over time.”
Primary sources of funding for the research included the Horowitz Foundation for Social Policy, Agency for Healthcare Research and Quality, and the National Institute on Aging. No relevant disclosures were made by the authors.
SOURCE: Markovitz A et al. Ann Intern Med. 2019 Jun 18. doi: 10.7326/M18-2539.
FROM ANNALS OF INTERNAL MEDICINE
A healthy 8-year-old boy presents with several skin-colored, round 1-3 mm papules on the nose, forehead, and cheeks
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A white 8-year-old boy comes to our pediatric dermatology clinic with his mother for evaluation of acne. The lesions started about a year ago on his nose and now have spread to his cheeks. The bumps are not symptomatic. He has been applying over the counter salicylic acid and benzoyl peroxide gels with no help. The mother reports he has been growing well, denies any growth spurt, no axillary or genital hair or body odor noted.
None of the family members have a history of acne. The mother cannot recall any family members with similar lesions on the face. He has had some warts on his fingers for years and has been treated with over the counter salicylic acid. There is no family history of skin cancer.
On physical exam, he is a healthy young boy with several skin color, round papules 1-3 mm on the nose, forehead, and cheeks. There are no lesions on the scalp. He has abundant brown hair. He has few verrucous papules on the fingers. Axillary and genital hair is not noted. There is no body odor and he is Tanner stage I.