User login
What is your diagnosis? - June 2019
Multiple myeloma
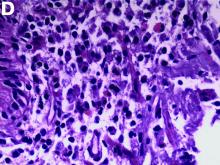
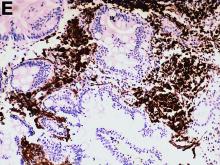
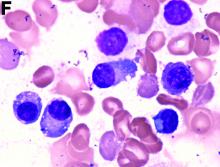
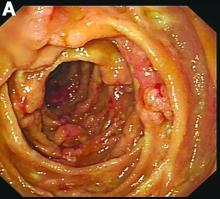
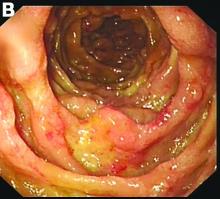
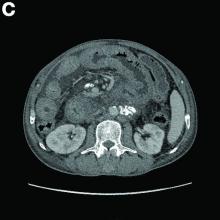
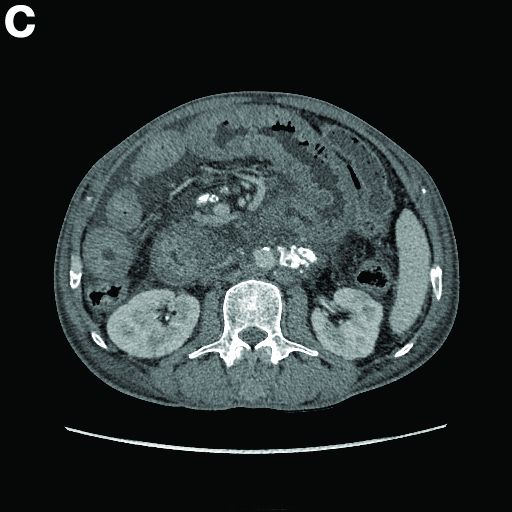
An abdominal CT scan (Figure D) showed gastric and whole intestinal wall thickening of up to 2 cm. Pathology (Figure E) demonstrated that diffuse plasmacytoid cells, eosinophilic granulocytes, and lymphocytes infiltrated into the lamina propria. Immunohistochemically, the plasmacytoid cells were positive for the common plasma cell marker CD38, and in situ hybridization indicated that they were kappa-Ig light-chain restricted (Figure F).
Results of the subsequent bone marrow aspirate revealed 27.5% atypical plasma cells. Serum electrophoresis and immunofixation showed an M spike of IgA-kappa. Together, these findings confirmed a final diagnosis of a multiple myeloma (MM) involving the whole gastrointestinal (GI) duct, which was the cause of his melena.
MM is a malignant hematologic neoplasm, primarily involving the bone marrow, and has a potent tendency to involve other organs and to present with various clinical manifestations.1
The clinical features of MM with GI involvement are uncommon. Patients may present with nausea, vomiting, diarrhea, protein loss, malabsorption, intestinal obstruction, and hemorrhage. Endoscopic findings can manifest as four types: a discrete ulcer, ulcerating mass, thickening of the mucosal fold, and mucosal polyp.2
However, GI bleeding in MM has only been reported in a few patients. A biopsy reaching the submucosal layer and bone marrow biopsy is essential. Diagnosis of MM as a cause of the GI duct wall edema and multiple small intestinal polypoid ulcers is challenging. An interdisciplinary approach is mandatory to establish such a diagnosis.
References
1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;3518:1860-73.
2. Karam AR, Semaan RJ, Buch K, et al. Extramedullary duodenal plasmacytoma presenting with gastric outlet obstruction and painless jaundice. Radiol Cases. 2010;4:22-8.
Multiple myeloma
An abdominal CT scan (Figure D) showed gastric and whole intestinal wall thickening of up to 2 cm. Pathology (Figure E) demonstrated that diffuse plasmacytoid cells, eosinophilic granulocytes, and lymphocytes infiltrated into the lamina propria. Immunohistochemically, the plasmacytoid cells were positive for the common plasma cell marker CD38, and in situ hybridization indicated that they were kappa-Ig light-chain restricted (Figure F).
Results of the subsequent bone marrow aspirate revealed 27.5% atypical plasma cells. Serum electrophoresis and immunofixation showed an M spike of IgA-kappa. Together, these findings confirmed a final diagnosis of a multiple myeloma (MM) involving the whole gastrointestinal (GI) duct, which was the cause of his melena.
MM is a malignant hematologic neoplasm, primarily involving the bone marrow, and has a potent tendency to involve other organs and to present with various clinical manifestations.1
The clinical features of MM with GI involvement are uncommon. Patients may present with nausea, vomiting, diarrhea, protein loss, malabsorption, intestinal obstruction, and hemorrhage. Endoscopic findings can manifest as four types: a discrete ulcer, ulcerating mass, thickening of the mucosal fold, and mucosal polyp.2
However, GI bleeding in MM has only been reported in a few patients. A biopsy reaching the submucosal layer and bone marrow biopsy is essential. Diagnosis of MM as a cause of the GI duct wall edema and multiple small intestinal polypoid ulcers is challenging. An interdisciplinary approach is mandatory to establish such a diagnosis.
References
1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;3518:1860-73.
2. Karam AR, Semaan RJ, Buch K, et al. Extramedullary duodenal plasmacytoma presenting with gastric outlet obstruction and painless jaundice. Radiol Cases. 2010;4:22-8.
Multiple myeloma
An abdominal CT scan (Figure D) showed gastric and whole intestinal wall thickening of up to 2 cm. Pathology (Figure E) demonstrated that diffuse plasmacytoid cells, eosinophilic granulocytes, and lymphocytes infiltrated into the lamina propria. Immunohistochemically, the plasmacytoid cells were positive for the common plasma cell marker CD38, and in situ hybridization indicated that they were kappa-Ig light-chain restricted (Figure F).
Results of the subsequent bone marrow aspirate revealed 27.5% atypical plasma cells. Serum electrophoresis and immunofixation showed an M spike of IgA-kappa. Together, these findings confirmed a final diagnosis of a multiple myeloma (MM) involving the whole gastrointestinal (GI) duct, which was the cause of his melena.
MM is a malignant hematologic neoplasm, primarily involving the bone marrow, and has a potent tendency to involve other organs and to present with various clinical manifestations.1
The clinical features of MM with GI involvement are uncommon. Patients may present with nausea, vomiting, diarrhea, protein loss, malabsorption, intestinal obstruction, and hemorrhage. Endoscopic findings can manifest as four types: a discrete ulcer, ulcerating mass, thickening of the mucosal fold, and mucosal polyp.2
However, GI bleeding in MM has only been reported in a few patients. A biopsy reaching the submucosal layer and bone marrow biopsy is essential. Diagnosis of MM as a cause of the GI duct wall edema and multiple small intestinal polypoid ulcers is challenging. An interdisciplinary approach is mandatory to establish such a diagnosis.
References
1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;3518:1860-73.
2. Karam AR, Semaan RJ, Buch K, et al. Extramedullary duodenal plasmacytoma presenting with gastric outlet obstruction and painless jaundice. Radiol Cases. 2010;4:22-8.
He denied experiencing hematemesis, abdominal pain, fever, osteodynia, or arthralgia. His medical history included a 6-year history of alcoholic hepatocirrhosis and type 2 diabetes mellitus. Colonoscopy found no evidence of hemorrhage.
What is the underlying condition leading to the endoscopic and CT findings?
Transcatheter pulmonary valve shows 5-year durability in postapproval study
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
REPORTING FROM SCAI 2019
ENZAMET trial: Early enzalutamide delays progression, improves survival in mHSPC
CHICAGO – Adding the oral androgen receptor inhibitor enzalutamide to standard first-line testosterone suppression delays progression and improves survival in men with metastatic hormone-sensitive prostate cancer (mHSPC), according to “practice-informing” interim results from the randomized phase 3 ENZAMET trial.
The survival rate at 3 years in 563 men with mHSPC who were enrolled in the international trial and who received early testosterone suppression and enzalutamide was 80%, compared with 72% among 562 men who received testosterone suppression and standard nonsteroidal antiandrogen therapy with or without docetaxel, study cochair Christopher Sweeney, MBBS, reported at the annual meeting of the American Society of Clinical Oncology (Abstract LBA2).
The findings of the Australian and New Zealand Urogenital and Prostate (ANZUP) Cancer Trials Group study (ANZUP 1304/ENZAMET) were published simultaneously in the New England Journal of Medicine.
“So ... we’re moving forward by going backwards in the disease setting where the disease is more sensitive and responds better to therapy,” Dr. Sweeney, of Dana-Farber Cancer Institute’s Lank Center for Genitourinary Oncology and professor of medicine at Harvard Medical School, Boston, explained in this video interview.
He also described the future directions for the research – in particular the need for longer follow-up to clarify the effects of docetaxel in this setting – and how the current findings will be reflected in his own management of patients with prostate cancer.
The findings have immediate implications for practice, ASCO expert Neeraj Agarwal, MD, professor of medicine and investigator at the Huntsman Cancer Institute, University of Utah, Salt Lake City, said during a press briefing at the meeting.
“In my view, using enzalutamide early on will allow our patients to avoid chemotherapy and steroids for many years, and thus, hopefully, improve their quality of life,” he said, noting that the findings are particularly exciting when considered in the context of the “equally impressive margin of benefit” seen with the similar drug apalutamide in the TITAN trial, which was presented separately during the ASCO meeting.
“One study is encouraging, but two large studies ... demonstrating similar findings, is even better,” he said. “This increases my confidence that targeting [the androgen receptor] is the optimal approach for newly diagnosed patients with advanced prostate cancer.”
Dr. Sweeney reported relationships (stock and other ownership interests, consulting or advisory roles, research funding to his institution, and/or patents/royalties/other intellectual property) with Leuchemix, Amgen, Astellas Pharma, AstraZeneca, Bayer, Genentech/Roche, Janssen Biotech, Pfizer, Sanofi, Dendreon, Sotio, and Exelixis. Dr. Agarwal reported consultancy or research for Pfizer, Novartis, Exelixis, Eisai, Genentech, Medivation, Clovis, Merck, Bayer, GlaxoSmithKline, AstraZeneca, EMD Serono, and Bristol-Myers Squibb.
CHICAGO – Adding the oral androgen receptor inhibitor enzalutamide to standard first-line testosterone suppression delays progression and improves survival in men with metastatic hormone-sensitive prostate cancer (mHSPC), according to “practice-informing” interim results from the randomized phase 3 ENZAMET trial.
The survival rate at 3 years in 563 men with mHSPC who were enrolled in the international trial and who received early testosterone suppression and enzalutamide was 80%, compared with 72% among 562 men who received testosterone suppression and standard nonsteroidal antiandrogen therapy with or without docetaxel, study cochair Christopher Sweeney, MBBS, reported at the annual meeting of the American Society of Clinical Oncology (Abstract LBA2).
The findings of the Australian and New Zealand Urogenital and Prostate (ANZUP) Cancer Trials Group study (ANZUP 1304/ENZAMET) were published simultaneously in the New England Journal of Medicine.
“So ... we’re moving forward by going backwards in the disease setting where the disease is more sensitive and responds better to therapy,” Dr. Sweeney, of Dana-Farber Cancer Institute’s Lank Center for Genitourinary Oncology and professor of medicine at Harvard Medical School, Boston, explained in this video interview.
He also described the future directions for the research – in particular the need for longer follow-up to clarify the effects of docetaxel in this setting – and how the current findings will be reflected in his own management of patients with prostate cancer.
The findings have immediate implications for practice, ASCO expert Neeraj Agarwal, MD, professor of medicine and investigator at the Huntsman Cancer Institute, University of Utah, Salt Lake City, said during a press briefing at the meeting.
“In my view, using enzalutamide early on will allow our patients to avoid chemotherapy and steroids for many years, and thus, hopefully, improve their quality of life,” he said, noting that the findings are particularly exciting when considered in the context of the “equally impressive margin of benefit” seen with the similar drug apalutamide in the TITAN trial, which was presented separately during the ASCO meeting.
“One study is encouraging, but two large studies ... demonstrating similar findings, is even better,” he said. “This increases my confidence that targeting [the androgen receptor] is the optimal approach for newly diagnosed patients with advanced prostate cancer.”
Dr. Sweeney reported relationships (stock and other ownership interests, consulting or advisory roles, research funding to his institution, and/or patents/royalties/other intellectual property) with Leuchemix, Amgen, Astellas Pharma, AstraZeneca, Bayer, Genentech/Roche, Janssen Biotech, Pfizer, Sanofi, Dendreon, Sotio, and Exelixis. Dr. Agarwal reported consultancy or research for Pfizer, Novartis, Exelixis, Eisai, Genentech, Medivation, Clovis, Merck, Bayer, GlaxoSmithKline, AstraZeneca, EMD Serono, and Bristol-Myers Squibb.
CHICAGO – Adding the oral androgen receptor inhibitor enzalutamide to standard first-line testosterone suppression delays progression and improves survival in men with metastatic hormone-sensitive prostate cancer (mHSPC), according to “practice-informing” interim results from the randomized phase 3 ENZAMET trial.
The survival rate at 3 years in 563 men with mHSPC who were enrolled in the international trial and who received early testosterone suppression and enzalutamide was 80%, compared with 72% among 562 men who received testosterone suppression and standard nonsteroidal antiandrogen therapy with or without docetaxel, study cochair Christopher Sweeney, MBBS, reported at the annual meeting of the American Society of Clinical Oncology (Abstract LBA2).
The findings of the Australian and New Zealand Urogenital and Prostate (ANZUP) Cancer Trials Group study (ANZUP 1304/ENZAMET) were published simultaneously in the New England Journal of Medicine.
“So ... we’re moving forward by going backwards in the disease setting where the disease is more sensitive and responds better to therapy,” Dr. Sweeney, of Dana-Farber Cancer Institute’s Lank Center for Genitourinary Oncology and professor of medicine at Harvard Medical School, Boston, explained in this video interview.
He also described the future directions for the research – in particular the need for longer follow-up to clarify the effects of docetaxel in this setting – and how the current findings will be reflected in his own management of patients with prostate cancer.
The findings have immediate implications for practice, ASCO expert Neeraj Agarwal, MD, professor of medicine and investigator at the Huntsman Cancer Institute, University of Utah, Salt Lake City, said during a press briefing at the meeting.
“In my view, using enzalutamide early on will allow our patients to avoid chemotherapy and steroids for many years, and thus, hopefully, improve their quality of life,” he said, noting that the findings are particularly exciting when considered in the context of the “equally impressive margin of benefit” seen with the similar drug apalutamide in the TITAN trial, which was presented separately during the ASCO meeting.
“One study is encouraging, but two large studies ... demonstrating similar findings, is even better,” he said. “This increases my confidence that targeting [the androgen receptor] is the optimal approach for newly diagnosed patients with advanced prostate cancer.”
Dr. Sweeney reported relationships (stock and other ownership interests, consulting or advisory roles, research funding to his institution, and/or patents/royalties/other intellectual property) with Leuchemix, Amgen, Astellas Pharma, AstraZeneca, Bayer, Genentech/Roche, Janssen Biotech, Pfizer, Sanofi, Dendreon, Sotio, and Exelixis. Dr. Agarwal reported consultancy or research for Pfizer, Novartis, Exelixis, Eisai, Genentech, Medivation, Clovis, Merck, Bayer, GlaxoSmithKline, AstraZeneca, EMD Serono, and Bristol-Myers Squibb.
REPORTING FROM ASCO 2019
Increasingly violent storms may strain mental health
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
REPORTING FROM APA 2019
Get a Free Head Shot at SVS Booth
Are you in need of a new headshot for your website or institution’s site? Good news for you! Attendees can get a professional head shot taken from 10am to 2pm on Thursday and Friday at the Vascular Annual Meeting. Stop by the SVS Member Booth, #331, in the Exhibit Hall to take advantage of the opportunity! SVS reserves the right to use the photos, but you may use them however you’d like. Still need to register for the meeting? Do so today.
Are you in need of a new headshot for your website or institution’s site? Good news for you! Attendees can get a professional head shot taken from 10am to 2pm on Thursday and Friday at the Vascular Annual Meeting. Stop by the SVS Member Booth, #331, in the Exhibit Hall to take advantage of the opportunity! SVS reserves the right to use the photos, but you may use them however you’d like. Still need to register for the meeting? Do so today.
Are you in need of a new headshot for your website or institution’s site? Good news for you! Attendees can get a professional head shot taken from 10am to 2pm on Thursday and Friday at the Vascular Annual Meeting. Stop by the SVS Member Booth, #331, in the Exhibit Hall to take advantage of the opportunity! SVS reserves the right to use the photos, but you may use them however you’d like. Still need to register for the meeting? Do so today.
Bidding for Silent Auction Now Open
Many fabulous prizes are now available for bidding. The Society for Vascular Surgery has compiled nearly 70 packages for items graciously donated for the silent auction portion of the ‘Vascular Spectacular’ gala. The event takes place at the Vascular Annual Meeting on Friday, June 14, in National Harbor, MD. Everyone, including non-attendees, may participate in the silent auction until it closes during the gala itself. Sign up to participate in the auction here.
Many fabulous prizes are now available for bidding. The Society for Vascular Surgery has compiled nearly 70 packages for items graciously donated for the silent auction portion of the ‘Vascular Spectacular’ gala. The event takes place at the Vascular Annual Meeting on Friday, June 14, in National Harbor, MD. Everyone, including non-attendees, may participate in the silent auction until it closes during the gala itself. Sign up to participate in the auction here.
Many fabulous prizes are now available for bidding. The Society for Vascular Surgery has compiled nearly 70 packages for items graciously donated for the silent auction portion of the ‘Vascular Spectacular’ gala. The event takes place at the Vascular Annual Meeting on Friday, June 14, in National Harbor, MD. Everyone, including non-attendees, may participate in the silent auction until it closes during the gala itself. Sign up to participate in the auction here.
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.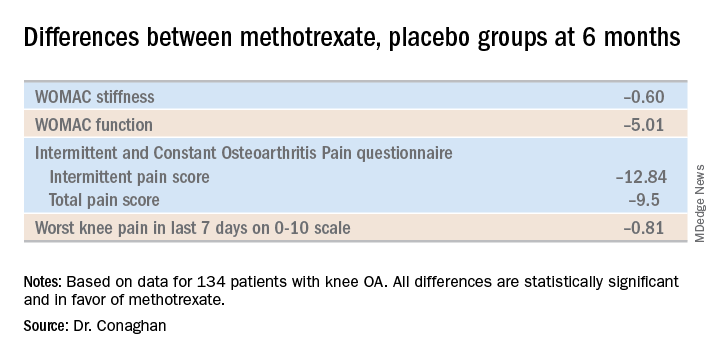
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Switching from interferon beta-1a to alemtuzumab improves MS outcomes
SEATTLE – , according to an analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. These outcomes may be maintained for 6 years without continuous treatment, said the investigators.
The CARE-MS II study demonstrated alemtuzumab’s superior efficacy, compared with subcutaneous interferon beta-1a, over 2 years in patients with active relapsing-remitting MS who had had an inadequate response to previous therapy. The trial was followed by a 4-year extension, during which patients who had received interferon beta-1a were given the option of discontinuing that therapy and initiating alemtuzumab. The alemtuzumab regimen for these patients was 12 mg/day for 5 consecutive days at baseline, and the same dose for 3 consecutive days at 1 year. Additional annual alemtuzumab as needed for disease activity was allowed. At investigators’ discretion, patients could receive other disease-modifying therapy (DMT) at any time. After the 4-year extension, patients could continue in the 5-year TOPAZ extension.
Carolina Ionete, MD, a neurologist at University of Massachusetts Memorial Medical Center in Worcester, and colleagues examined data from the TOPAZ extension study to evaluate the efficacy and safety of alemtuzumab over 6 years in patients with relapsing-remitting MS from CARE-MS II who discontinued interferon beta-1a. In TOPAZ, patients can receive additional alemtuzumab (12 mg/day on 3 consecutive days at 12 or more months after the most recent course) or other DMTs at any time at investigators’ discretion.
In all, 143 patients started alemtuzumab in the extension study. Of this group 117 patients (82%) completed year 2 of TOPAZ (i.e., year 6 after initiating alemtuzumab). The annualized relapse rate at year 6 was 0.19, and the annual rate of freedom from relapse ranged from 83% to 90% during years 1 through 6. At year 6, Expanded Disability Status Scale (EDSS) scores were stable (51%) or improved (17%) in 68% of patients, compared with the baseline of the main study. At year 6, the mean EDSS score change was 0.43. Over 6 years, 69% of patients were free from 6-month confirmed disability worsening, and 23% achieved 6-month confirmed disability improvement.
In addition, 69% of patients were free of MRI disease activity in year 6. The median percent cumulative brain volume loss from alemtuzumab initiation through year 6 was 0.53%, compared with 0.81% over 2 years with interferon beta-1a. Brain volume loss was 0.32% or less annually during years 2 through 6 after initiating alemtuzumab (0.04% at year 2, 0.15% at year 3, 0.14% at year 4, 0.07% at year 5, and 0.32% at year 6). These efficacy outcomes were observed as 57% of patients received neither additional alemtuzumab nor another DMT through year 6. Safety results were consistent with those for the alemtuzumab-treated patients in the core and extension studies.
Sanofi and Bayer HealthCare Pharmaceuticals supported this study. Dr. Ionete received research support from Biogen, Roche, and Sanofi. She reported receiving compensation for advisory board participation from Sanofi.
SEATTLE – , according to an analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. These outcomes may be maintained for 6 years without continuous treatment, said the investigators.
The CARE-MS II study demonstrated alemtuzumab’s superior efficacy, compared with subcutaneous interferon beta-1a, over 2 years in patients with active relapsing-remitting MS who had had an inadequate response to previous therapy. The trial was followed by a 4-year extension, during which patients who had received interferon beta-1a were given the option of discontinuing that therapy and initiating alemtuzumab. The alemtuzumab regimen for these patients was 12 mg/day for 5 consecutive days at baseline, and the same dose for 3 consecutive days at 1 year. Additional annual alemtuzumab as needed for disease activity was allowed. At investigators’ discretion, patients could receive other disease-modifying therapy (DMT) at any time. After the 4-year extension, patients could continue in the 5-year TOPAZ extension.
Carolina Ionete, MD, a neurologist at University of Massachusetts Memorial Medical Center in Worcester, and colleagues examined data from the TOPAZ extension study to evaluate the efficacy and safety of alemtuzumab over 6 years in patients with relapsing-remitting MS from CARE-MS II who discontinued interferon beta-1a. In TOPAZ, patients can receive additional alemtuzumab (12 mg/day on 3 consecutive days at 12 or more months after the most recent course) or other DMTs at any time at investigators’ discretion.
In all, 143 patients started alemtuzumab in the extension study. Of this group 117 patients (82%) completed year 2 of TOPAZ (i.e., year 6 after initiating alemtuzumab). The annualized relapse rate at year 6 was 0.19, and the annual rate of freedom from relapse ranged from 83% to 90% during years 1 through 6. At year 6, Expanded Disability Status Scale (EDSS) scores were stable (51%) or improved (17%) in 68% of patients, compared with the baseline of the main study. At year 6, the mean EDSS score change was 0.43. Over 6 years, 69% of patients were free from 6-month confirmed disability worsening, and 23% achieved 6-month confirmed disability improvement.
In addition, 69% of patients were free of MRI disease activity in year 6. The median percent cumulative brain volume loss from alemtuzumab initiation through year 6 was 0.53%, compared with 0.81% over 2 years with interferon beta-1a. Brain volume loss was 0.32% or less annually during years 2 through 6 after initiating alemtuzumab (0.04% at year 2, 0.15% at year 3, 0.14% at year 4, 0.07% at year 5, and 0.32% at year 6). These efficacy outcomes were observed as 57% of patients received neither additional alemtuzumab nor another DMT through year 6. Safety results were consistent with those for the alemtuzumab-treated patients in the core and extension studies.
Sanofi and Bayer HealthCare Pharmaceuticals supported this study. Dr. Ionete received research support from Biogen, Roche, and Sanofi. She reported receiving compensation for advisory board participation from Sanofi.
SEATTLE – , according to an analysis presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. These outcomes may be maintained for 6 years without continuous treatment, said the investigators.
The CARE-MS II study demonstrated alemtuzumab’s superior efficacy, compared with subcutaneous interferon beta-1a, over 2 years in patients with active relapsing-remitting MS who had had an inadequate response to previous therapy. The trial was followed by a 4-year extension, during which patients who had received interferon beta-1a were given the option of discontinuing that therapy and initiating alemtuzumab. The alemtuzumab regimen for these patients was 12 mg/day for 5 consecutive days at baseline, and the same dose for 3 consecutive days at 1 year. Additional annual alemtuzumab as needed for disease activity was allowed. At investigators’ discretion, patients could receive other disease-modifying therapy (DMT) at any time. After the 4-year extension, patients could continue in the 5-year TOPAZ extension.
Carolina Ionete, MD, a neurologist at University of Massachusetts Memorial Medical Center in Worcester, and colleagues examined data from the TOPAZ extension study to evaluate the efficacy and safety of alemtuzumab over 6 years in patients with relapsing-remitting MS from CARE-MS II who discontinued interferon beta-1a. In TOPAZ, patients can receive additional alemtuzumab (12 mg/day on 3 consecutive days at 12 or more months after the most recent course) or other DMTs at any time at investigators’ discretion.
In all, 143 patients started alemtuzumab in the extension study. Of this group 117 patients (82%) completed year 2 of TOPAZ (i.e., year 6 after initiating alemtuzumab). The annualized relapse rate at year 6 was 0.19, and the annual rate of freedom from relapse ranged from 83% to 90% during years 1 through 6. At year 6, Expanded Disability Status Scale (EDSS) scores were stable (51%) or improved (17%) in 68% of patients, compared with the baseline of the main study. At year 6, the mean EDSS score change was 0.43. Over 6 years, 69% of patients were free from 6-month confirmed disability worsening, and 23% achieved 6-month confirmed disability improvement.
In addition, 69% of patients were free of MRI disease activity in year 6. The median percent cumulative brain volume loss from alemtuzumab initiation through year 6 was 0.53%, compared with 0.81% over 2 years with interferon beta-1a. Brain volume loss was 0.32% or less annually during years 2 through 6 after initiating alemtuzumab (0.04% at year 2, 0.15% at year 3, 0.14% at year 4, 0.07% at year 5, and 0.32% at year 6). These efficacy outcomes were observed as 57% of patients received neither additional alemtuzumab nor another DMT through year 6. Safety results were consistent with those for the alemtuzumab-treated patients in the core and extension studies.
Sanofi and Bayer HealthCare Pharmaceuticals supported this study. Dr. Ionete received research support from Biogen, Roche, and Sanofi. She reported receiving compensation for advisory board participation from Sanofi.
REPORTING FROM CMSC 2019
Racial disparities in time to cancer care erased with Medicaid expansion
CHICAGO – For decades investigators have documented racial disparities in access to cancer care and in clinical outcomes, with socioeconomic factors suspected – but not conclusively proven – to play a role
Now a new study based on electronic health record (EHR) data shows that after Medicaid expansion under the Affordable Care Act (ACA), racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, African Americans were 4.8% less likely than whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
The findings suggest that the expanded availability of health insurance has had a salutary effect on cancer care.
In this video interview, co-authors Amy J. Davidoff, PhD, MS, from the Yale Cancer Center in New Haven Connecticut, and Blythe J.S. Adamson, PhD, from Flatiron Health in New York, New York, discuss the study findings and the possible implications for health care policy in the United States.
The study was funded by Flatiron Health. Dr. Adamson is an employee of the company. Dr. Davidoff disclosed consulting or advisory roles with and honoraria from several pharmaceutical companies.
CHICAGO – For decades investigators have documented racial disparities in access to cancer care and in clinical outcomes, with socioeconomic factors suspected – but not conclusively proven – to play a role
Now a new study based on electronic health record (EHR) data shows that after Medicaid expansion under the Affordable Care Act (ACA), racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, African Americans were 4.8% less likely than whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
The findings suggest that the expanded availability of health insurance has had a salutary effect on cancer care.
In this video interview, co-authors Amy J. Davidoff, PhD, MS, from the Yale Cancer Center in New Haven Connecticut, and Blythe J.S. Adamson, PhD, from Flatiron Health in New York, New York, discuss the study findings and the possible implications for health care policy in the United States.
The study was funded by Flatiron Health. Dr. Adamson is an employee of the company. Dr. Davidoff disclosed consulting or advisory roles with and honoraria from several pharmaceutical companies.
CHICAGO – For decades investigators have documented racial disparities in access to cancer care and in clinical outcomes, with socioeconomic factors suspected – but not conclusively proven – to play a role
Now a new study based on electronic health record (EHR) data shows that after Medicaid expansion under the Affordable Care Act (ACA), racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, African Americans were 4.8% less likely than whites to receive timely cancer treatment, defined as treatment starting within 30 days of diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups had dwindled to just 0.8% and was no longer statistically significant.
The findings suggest that the expanded availability of health insurance has had a salutary effect on cancer care.
In this video interview, co-authors Amy J. Davidoff, PhD, MS, from the Yale Cancer Center in New Haven Connecticut, and Blythe J.S. Adamson, PhD, from Flatiron Health in New York, New York, discuss the study findings and the possible implications for health care policy in the United States.
The study was funded by Flatiron Health. Dr. Adamson is an employee of the company. Dr. Davidoff disclosed consulting or advisory roles with and honoraria from several pharmaceutical companies.
REPORTING FROM ASCO 2019
Cleveland Clinic targets time to treat in cancer
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
FROM ASCO 2019