User login
Is chest radiography routinely needed after thoracentesis?
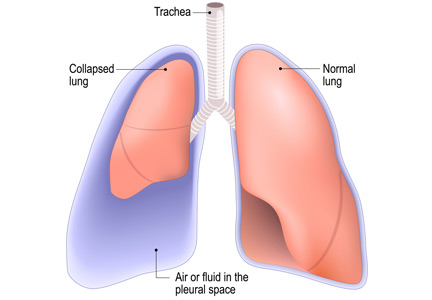
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
A 69-year-old woman with double vision and lower-extremity weakness
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
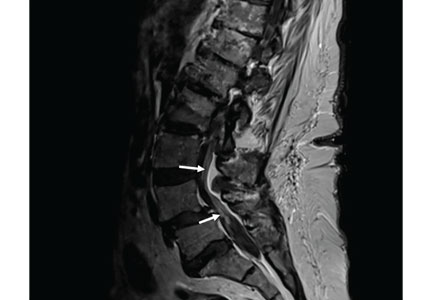
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
A 69-year-old woman was admitted to the hospital with double vision, weakness in the lower extremities, sensory loss, pain, and falls. Her symptoms started with sudden onset of horizontal diplopia 6 weeks before, followed by gradually worsening lower-extremity weakness, as well as ataxia and patchy and bilateral radicular burning leg pain more pronounced on the right. Her medical history included narcolepsy, obstructive sleep apnea, hypertension, hyperlipidemia, and bilateral knee replacements for osteoarthritis.
Neurologic examination showed inability to abduct the right eye, bilateral hip flexion weakness, decreased pinprick response, decreased proprioception, and diminished muscle stretch reflexes in the lower extremities. Magnetic resonance imaging (MRI) of the brain without contrast and magnetic resonance angiography of the brain and carotid arteries showed no evidence of acute stroke. No abnormalities were noted on electrocardiography and echocardiography.
A diagnosis of idiopathic peripheral neuropathy was made, and outpatient physical therapy was recommended. Over the subsequent 2 weeks, her condition declined to the point where she needed a walker. She continued to have worsening leg weakness with falls, prompting hospital readmission.
INITIAL EVALUATION
In addition to her diplopia and weakness, she said she had lost 15 pounds since the onset of symptoms and had experienced symptoms suggesting urinary retention.
Physical examination
Her temperature was 37°C (98.6°F), heart rate 79 beats per minute, blood pressure 117/86 mm Hg, respiratory rate 14 breaths per minute, and oxygen saturation 98% on room air. Examination of the head, neck, heart, lung, abdomen, lymph nodes, and extremities yielded nothing remarkable except for chronic venous changes in the lower extremities.
The neurologic examination showed incomplete lateral gaze bilaterally (cranial nerve VI dysfunction). Strength in the upper extremities was normal. In the legs, the Medical Research Council scale score for proximal muscle strength was 2 to 3 out of 5, and for distal muscles 3 to 4 out of 5, with the right side worse than the left and flexors and extensors affected equally. Muscle stretch reflexes were absent in both lower extremities and the left upper extremity, but intact in the right upper extremity. No abnormal corticospinal tract reflexes were elicited.
Sensory testing revealed diminished pin-prick perception in a length-dependent fashion in the lower extremities, reduced 50% compared with the hands. Gait could not be assessed due to weakness.
Initial laboratory testing
Results of initial laboratory tests—complete blood cell count, complete metabolic panel, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, and hemoglobin A1c—were unremarkable.
FURTHER EVALUATION AND DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis at this point?
- Cerebral infarction
- Guillain-Barré syndrome
- Progressive polyneuropathy
- Transverse myelitis
- Polyradiculopathy
In the absence of definitive diagnostic tests, all of the above options were considered in the differential diagnosis for this patient.
Cerebral infarction
Although acute-onset diplopia can be explained by brainstem stroke involving cranial nerve nuclei or their projections, the onset of diplopia with progressive bilateral lower-extremity weakness makes stroke unlikely. Flaccid paralysis, areflexia of the lower extremities, and sensory involvement can also be caused by acute anterior spinal artery occlusion leading to spinal cord infarction; however, the deficits are usually maximal at onset.
Guillain-Barré syndrome
The combination of acute-subacute progressive ascending weakness, sensory involvement, and diminished or absent reflexes is typical of Guillain-Barré syndrome. Cranial nerve involvement can overlap with the more typical features of the syndrome. However, most patients reach the nadir of their disease by 4 weeks after initial symptom onset, even without treatment.1 This patient’s condition continued to worsen over 8 weeks. In addition, the asymmetric lower-extremity weakness and sparing of the arms are atypical for Guillain-Barré syndrome.
Given the progression of symptoms, chronic inflammatory demyelinating polyneuropathy is also a consideration, typically presenting as a relapsing or progressive neuropathy in proximal and distal muscles and worsening over at least an 8-week period.2
The initial workup for Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy includes lumbar puncture to assess for albuminocytologic dissociation (elevated protein with normal white blood cell count) in cerebrospinal fluid (CSF), and electromyography (EMG) to assess for neurophysiologic evidence of peripheral nerve demyelination. In Miller-Fisher syndrome, a rare variant of Guillain-Barré syndrome characterized by ataxia, ophthalmoparesis, and areflexia, serum ganglioside antibodies to GQ1b are found in over 90% of patients.3,4 Although MRI of the spine is not necessary to diagnose Guillain-Barré syndrome, it is often done to exclude other causes of lower-extremity weakness such as spinal cord or cauda equina compression that would require urgent neurosurgical consultation. MRI can support the diagnosis of Guillain-Barré syndrome when it reveals enhancement of the spinal nerve roots or cauda equina.
Other polyneuropathies
Polyneuropathy is caused by a variety of diseases that affect the function of peripheral motor, sensory, or autonomic nerves. The differential diagnosis is broad and involves inflammatory diseases (including autoimmune and paraneoplastic causes), hereditary disorders, infection, toxicity, and ischemic and nutritional deficiencies.5 Polyneuropathy can present in a distal-predominant, generalized, or asymmetric pattern involving individual nerve trunks termed “mononeuropathy multiplex,” as in our patient’s presentation. The initial workup includes EMG and a battery of serologic tests. In cases of severe and progressive polyneuropathy, nerve biopsy can assess for the presence of vasculitis, amyloidosis, and paraprotein deposition.
Transverse myelitis
Transverse myelitis is an inflammatory myelopathy that usually presents with acute or subacute weakness of the upper extremities or lower extremities, or both, corresponding to the level of the lesion, hyperreflexia, bladder and bowel dysfunction, spinal level of sensory loss, and autonomic involvement.6 The differential diagnosis of acute myelopathy includes:
- Infection (eg, herpes simplex virus, West Nile virus, Lyme disease, Mycoplasma pneumoniae, human immunodeficiency virus)
- Systemic inflammatory disease (systemic lupus erythematosus, sarcoidosis, Sjögren syndrome, scleroderma, paraneoplastic syndrome)
- Central nervous system demyelinating disease (acute disseminated encephalomyelitis, multiple sclerosis, neuromyelitis optica)
- Vascular malformation (dural arteriovenous fistula)
- Compression due to tumor, bleeding, disc herniation, infection, or abscess.
The workup involves laboratory tests to exclude systemic inflammatory and infectious causes, as well as MRI of the spine with and without contrast to identify a causative lesion. Lumbar puncture and CSF analysis may show pleocytosis, elevated protein concentration, and increased intrathecal immunoglobulin G (IgG) index.7
Although our patient’s presentation with subacute lower-extremity weakness, sensory changes, and bladder dysfunction were consistent with transverse myelitis, her cranial nerve abnormalities would be atypical for it.
Polyradiculopathy
Polyradiculopathy has many possible causes. In the United States, the most common causes are lumbar spondylosis, lumbar canal stenosis, and diabetic polyradiculoneuropathy.
When multiple spinal segments are affected, leptomeningeal disease involving the arachnoid and pia mater should be considered. Causes include malignant invasion, inflammatory cell accumulation, and protein deposition, leading to patchy but widespread dysfunction of spinal nerve roots and cranial nerves. Specific causes are myriad and include carcinomatous meningitis,8 syphilis, tuberculosis, sarcoidosis, and paraproteinemias. CSF and MRI changes are often nonspecific, leading to the need for meningeal biopsy for diagnosis.
CASE CONTINUED
During her hospitalization, our patient developed acute right upper and lower facial weakness consistent with peripheral facial mononeuropathy. Bilateral lower-extremity weakness progressed to disabling paraparesis.
She underwent lumbar puncture and CSF analysis (Table 1). The most notable findings were significant pleocytosis (72% lymphocytic predominance), protein elevation, and elevated IgG index (indicative of elevated intrathecal immunoglobulin synthesis in the central nervous system). Viral, bacterial, and fungal studies were negative. Guillain-Barré syndrome, other polyneuropathies, and spinal cord infarction would not be expected with these CSF features.
Surface EMG demonstrated normal sensory responses, and needle EMG showed chronic and active motor axon loss in the L3 and S1 root distributions, suggesting polyradiculopathy without polyneuropathy. These findings would not be expected in typical acute transverse myelitis but could be seen with spinal cord infarction.
MRI of the entire spine with and without contrast showed cauda equina nerve root thickening and enhancement, especially involving the L5 and S1 roots (Figure 1). The spinal cord appeared normal. These findings further supported polyradiculopathy and a leptomeningeal process.
Further evaluation included chest radiography, erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, human immunodeficiency virus testing, antinuclear antibody, antineutrophil cytoplasmic antibody, extractable nuclear antibody, GQ1b antibody, serum and CSF paraneoplastic panels, levels of vitamin B1, B12, and B6, copper, and ceruloplasmin, and a screen for heavy metals. All results were within normal ranges.
ESTABLISHING THE DIAGNOSIS
Serum monoclonal protein analysis with immunofixation revealed IgM kappa monoclonal gammopathy with an IgM level of 1,570 (reference range 53–334 mg/dL) and M-spike 0.75 (0.00 mg/dL), serum free kappa light chains 61.1 (3.30–19.40 mg/L), lambda 9.3 (5.7–26.3 mg/L), and kappa-lambda ratio 6.57 (0.26–1.65).
2. Which is the best next step in this patient’s neurologic evaluation?
- Test CSF angiotensin-converting enzyme level
- CSF cytology
- Meningeal biopsy
- Peripheral nerve biopsy
Given the high suspicion for malignancy, CSF cytology was performed and showed increased numbers of mononuclear chronic inflammatory cells, including a mixture of lymphocytes and monocytes, favoring a reactive lymphoid pleocytosis. Flow cytometry indicated the presence of a monoclonal, CD5- and CD10- negative, B-cell lymphoproliferative disorder. The immunophenotypic findings were not specific for a single diagnosis. The differential diagnosis included marginal zone lymphoma and lymphoplasmacytic lymphoma.
3. Given the presence of serum IgM monoclonal gammopathy in this patient, which is the most likely diagnosis?
- Neurosarcoidosis
- Multiple myeloma
- Waldenström macroglobulinemia
- Carcinomatous meningitis
Study of bone marrow biopsy demonstrated limited bone marrow involvement (1%) by a lymphoproliferative disorder with plasmacytoid features, and DNA testing detected an MYD88 L265P mutation, reported to be present in 90% of patients with Waldenström macroglobulinemia.9 This finding confirmed the diagnosis of Waldenström macroglobulinemia with central nervous system involvement. Our patient began therapy with rituximab and methotrexate, which resulted in some improvement in strength, gait, and vision.
WALDENSTRÖM MACROGLOBULINEMIA AND BING-NEEL SYNDROME
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma associated with a monoclonal IgM protein.10 It is considered a paraproteinemic disorder, similar to multiple myeloma. The presenting symptoms and complications are related to direct tumor infiltration, hyperviscosity syndrome, and deposition of IgM in various tissues.11,12
Waldenström macroglobulinemia is usually indolent, and treatment is reserved for patients with symptoms.13,14 It includes rituximab, usually in combination with chemotherapy or other targeted agents.15,16
Paraneoplastic antibody-mediated polyneuropathy may occur in these patients. However, the pattern is usually symmetrical clinically, with demyelination on EMG, and is not associated with cranial nerve or meningeal involvement. Management with plasmapheresis, corticosteroids, and intravenous immunoglobulin has not been shown to be effective.17
Involvement of the central nervous system as a complication of Waldenström macroglobulinemia has been described as Bing-Neel syndrome. It can present as diffuse malignant cell infiltration of the leptomeningeal space, white matter, or spinal cord, or in a tumoral form presenting as intraparenchymal masses or nodular lesions. The distinction between the tumoral and diffuse forms is based primarily on imaging findings.18
In a report of 44 patients with Bing-Neel syndrome, 36% presented with the disorder as the initial manifestation of Waldenström macroglobulinemia.18 The primary presenting symptoms were imbalance and gait difficulty (48%) and cranial nerve involvement (36%), which presented as predominantly facial or oculomotor nerve palsy. Cauda equina syndrome with motor involvement (seen in our patient) occurred in 14% of patients. Other presenting symptoms included cognitive impairment, sensory deficits, headache, dysarthria, aphasia, and seizures.
LEARNING POINTS
The differential diagnosis for patients presenting with multifocal neurologic symptoms can be broad, and a systematic approach to the diagnosis is necessary. Localizing the lesion is important in determining the diagnosis for patients presenting with neurologic symptoms. The process of localization begins with taking the history, is further refined during the examination, and is confirmed with diagnostic studies. Atypical presentations of relatively common neurologic diseases such as Guillain-Barré syndrome, transverse myelitis, and peripheral polyneuropathy do occur, but uncommon diagnoses need to be considered when support for the initial diagnosis is lacking.
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
- Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain 2014; 137(Pt 1):33–43. doi:10.1093/brain/awt285
- Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015; 86(9):973–985. doi:10.1136/jnnp-2014-309697
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993; 43(10):1911–1917. pmid:8413947
- Teener J. Miller Fisher’s syndrome. Semin Neurol 2012; 32(5):512–516. doi:10.1055/s-0033-1334470
- Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc 2015; 90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
- Greenberg BM. Treatment of acute transverse myelitis and its early complications. Continuum (Minneap Minn) 2011; 17(4):733–743. doi:10.1212/01.CON.0000403792.36161.f5
- West TW. Transverse myelitis—a review of the presentation, diagnosis, and initial management. Discov Med 2013; 16(88):167–177. pmid:24099672
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013; 4(suppl 4):S265–S288. doi:10.4103/2152-7806.111304
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012; 367(9):826–833. doi:10.1056/NEJMoa1200710
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30(2):110–115. doi:10.1053/sonc.2003.50082
- Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30(2):226–230. doi:10.1053/sonc.2003.50054
- Rison RA, Beydoun SR. Paraproteinemic neuropathy: a practical review. BMC Neurol 2016; 16:13. doi:10.1186/s12883-016-0532-4
- Kyle RA, Benson J, Larson D, et al. IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma 2009; 9(1):17–18. doi:10.3816/CLM.2009.n.002
- Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood 2012; 119(19):4462–4466. doi:10.1182/blood-2011-10-384768
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s macroglobulinemia. Blood 2016; 128(10):1321–1328. doi:10.1182/blood-2016-04-711234
- Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines 2016. JAMA Oncol 2017; 3(9):1257–1265. doi:10.1001/jamaoncol.2016.5763
- D’Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenström-associated peripheral neuropathies: recommendations from the IWWM-8 consensus panel. Br J Haematol 2017; 176(5):728–742. doi:10.1111/bjh.14492
- Simon L, Fitsiori A, Lemal R, et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: analysis of 44 cases and review of the literature. A study on behalf of the French Innovative Leukemia Organization (FILO). Haematologica 2015; 100(12):1587–1594. doi:10.3324/haematol.2015.133744
Complete blood cell count
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
To the Editor: The review by May et al1 of 3 neglected numbers in the complete blood cell count (CBC) was a good reminder to look more closely at the results of the CBCs we often order in primary care. I was surprised to see no mention of the red cell distribution width in relation to another cardiovascular disorder—obstructive sleep apnea.2,3 I wonder if the authors would comment on this association?
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- May JE, Marques MB, Reddy VVB, Gangaraju R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Cleve Clin J Med 2019; 86(3):167–172. doi:10.3949/ccjm.86a.18072
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
In reply: Complete blood cell count
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
In Reply: We thank Dr. Homler for his question and for highlighting another important disease state, obstructive sleep apnea, in which a high red cell distribution width (RDW) has correlated with disease severity.1,2 The 2 retrospective studies he mentioned indicated that RDW is negatively correlated with metrics such as oxygen saturation, sleep time, and sleep quality. Interestingly, another retrospective study showed that RDW was significantly higher in patients with concurrent obstructive sleep apnea and cardiovascular disease than in patients with obstructive sleep apnea alone, suggesting that the presence of anisocytosis in obstructive sleep apnea may be due to its link to cardiovascular disease.3
Although we focused on cardiovascular disease in our review, RDW has also shown prognostic significance in many other disorders including ischemic stroke,4 pneumonia,5,6 chronic kidney disease,7 and gastrointestinal disorders.8 Collectively, these studies indicate that RDW may serve as a red flag for clinicians, raising concern for increased disease severity and potential adverse outcomes. However, further research is needed to determine if and how RDW monitoring should be used to prompt interventions to improve patient outcomes.
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
- Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012; 8(5):521–525. doi:10.5664/jcsm.2146
- Yousef AM, Alkhiary W. The severity of obstructive sleep apnea syndrome is related to red cell distribution width and hematocrit values. J Sleep Disord Ther 2015; 4(2):1000192. doi:10.4172/2167-0277.1000192
- Sunnetcioglu A, Gunbatar H, Yildiz H. Red cell distribution width and uric acid in patients with obstructive sleep apnea. Clin Respir J 2018; 12(3):1046–1052. doi:10.1111/crj.12626
- Feng G-H, Li H-P, Li Q-L, Fu Y, Huang R-B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017; 2(3):172-175. doi:10.1136/svn-2017-000071
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013; 31:72–79. doi:10.1016/j.ajem.2012.06.004
- Miranda SJ. Validity of red cell distribution width as a predictor of clinical outcomes in pediatric patients diagnosed with pneumonia [abstract]. Chest 2017; 152(4 suppl):A843. doi:10.1016/j.chest.2017.08.877
- Kor CT, Hsieh YP, Chang CC, Chiu PF. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci Rep 2018; 8(1):11870. doi:10.1038/s41598-018-19881-2
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27):4879–4891. doi:10.3748/wjg.v23.i27.4879
Correction: Subclinical hypothyroidism
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
In Azim S, Nasr C, “Subclinical hypothyroidism: When to treat,” Cleve Clin J Med 2019; 86(2):101–110, on page 103, in the section “Subclinical hypothyroidism can resolve or progress,” the sentence “The rate of progression to overt hypothyroidism is estimated to be 33% to 35% over 10 to 20 years of follow-up” contained an error. The correct rate of progression is 33% to 55%. This error has been corrected online.
Steroids May Not Benefit Patients With Mild Asthma
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
Click for Credit: Biomarkers for VTE risk; Exercise & concussion recovery; more
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
STIs may be overlooked if you fail to ask this question
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Ado-trastuzumab highly efficacious for rare HER2-amplified SGCs
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
CHICAGO – Ado-trastuzumab emtansine, previously known as T-DM1, is highly efficacious in patients with HER2-amplified salivary gland cancers, according to findings from an ongoing phase 2 multi-histology basket trial.
In fact, nine of 10 patients with this rare tumor responded to treatment with the HER2-targeted antibody drug conjugate after prior trastuzumab, pertuzumab, and anti-androgen therapy, and 5 of those had a complete response, Bob T. Li, MD, said at the annual meeting of the American Society of Clinical Oncology.
“We are reporting this study early because it has already met its primary endpoint,” said Dr. Li of Memorial Sloan Kettering Cancer Center, N.Y.
The 90% response rate was based on either Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 or Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria; the latter was used because many patients with HER-amplified salivary gland cancer aren’t “RECIST measurable” due to presentation with only lymph node- and bone-only metastasis, he explained, adding that “many of the responses were quite durable, with some lasting 2 years.”
Even at a median of 12 months, neither duration of response nor median progression-free survival have been reached, he said.
Study subjects included nine men and one woman with salivary gland cancers (SGCs) and HER2 amplification identified by next-generation sequencing (NGS). They had a median age of 65 years and a median of 2 prior lines of systemic therapy.
Dr. Li described one patient who had bone and vertebral metastases.
“After just two doses, he had a complete metabolic response,” he said. “His symptoms improved, his pain went away, he feels well, and just recently he celebrated his 92nd birthday.”
Treatment, which included 3.6 mg/kg delivered intravenously every 3 weeks until disease progression or unacceptable toxicity, was well tolerated; toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and transaminitis. Two dose reductions were required, but no treatment-related deaths occurred, Dr. Li said.
SGCs are rare tumors accounting for only about 0.8% of malignancies. There is no approved therapy for metastatic disease, and due to the rarity of the disease there is no established standard of care, he said, noting, however, that chemotherapy and anti-androgen therapy are considered treatment options based on some retrospective case series.
“Now, coming in from a molecular angle, HER2 amplification turns out to be very common in this rare tumor,” he said.
In fact, NGS of more than 40,000 tumors using the MSK-IMPACT 468-gene oncopanel showed that HER2 amplification occurs in 8% of all SGC histologies, and additional published data show that it occurs in about 30% of those with “the very aggressive salivary duct carcinoma histologic subtype,” he said, adding that case reports and a phase 2 study reported at ASCO 2018 showed encouraging response rates with chemotherapy plus trastuzumab.
Ado-trastuzumab emtansine is a Food and Drug Administration-approved agent for the treatment HER2-positive breast cancer.
“It’s got the trastuzumab antibody, it has a linker which attaches the highly toxic DM1 chemotherapy to it, and ... it binds to the over-expressed HER2 receptor and uses that receptor to internalize the drug into the cancer cell and by lysosome deregulation release the highly toxic DM1 into the cell to cause cancer cell kill,” he explained. “We hypothesized that this drug, as a single agent, would be efficacious in HER2-amplified SGC tumors, and it turns out [that] recently there was a nice case series published from the University of Pennsylvania supporting this hypothesis in a group of patients.”
Indeed, the findings are encouraging and warrant cohort expansion to confirm the results, he said.
Of note, HER2 amplification by NGS (fold change 2.8 to 22.8) correlated with findings on fluorescence in situ hybridization (FISH) in 8 of 8 patients tested, and with immunohistochemistry (IHC) 3+ in 10 of 10 patients tested, thereby confirming the validity of this testing method for the biomarker and as a study entry criterion, he said, adding that ongoing correlative analyses are focusing on cell-free DNA NGS to look for acquired resistance, quantitative HER2 protein analysis by mass spectrometry, and also a dimerization assay looking at the degree of HER2-HER3 dimerization, which leads to receptor internalization that may predict response to HER2 antibody drug conjugates.
“We wanted to see why [HER2-amplified SGC patients] respond so well in contrast to the other diseases in the basket trial,” Dr. Li said, explaining that the trial also includes lung, bladder and urinary tract, endometrial, and colorectal cancer cohorts.
“However, to me as an oncologist, the most pressing thing is that with these kind of results and with this kind of response rate and progression-free survival ... there are patients in need of this treatment, so that is certainly the priority–to further accrue patients, complete the trial, publish the data, and hopefully have this new treatment approved to benefit all patients,” he concluded.
Discussant Vanita Noronha, MD, noted that the survival data are immature but “very clinically relevant and clinically significant,” and that they fulfill an unmet need.
“As much as we would like to have randomized trial, this is really a challenge in these kind of rare tumors,” said Dr. Noronha, a professor in the Department of Medical Oncology at Tata Memorial Hospital in Mumbai, India. “So my take-home message ... is that HER2neu is an important molecule driver in salivary gland tumors [and] all patients with salivary gland cancers should be tested for HER2neu amplification.”
Ado trastuzumab emtansine appears to be a good treatment option in those with HER2 amplified SGC, she added.
“Is this a practice changing study? Yes, potentially it is,” she said, noting that in patients with recurrent/metastatic SGC not amenable to radical therapy who are found to have HER2neu amplification, treatment options include either ado trastuzumab emtansine or the combination of trastuzumab and chemotherapy.
Dr. Li reported consulting or advisory roles with Biosceptre International, Guardant Health, Hengrui Therapeutics, Mersana, Roche, and Thermo Fisher Scientific. He reported research funding to his institution from AstraZeneca, BioMed Valley Discoveries, Daiichi Sankyo, GRAIL, Guardant Health, Hengrui Therapeutics, Illumina, and Roche/Genentech. Dr. Noronha has received research funding (to her institution) from Amgen,and Sanofi Aventis.
SOURCE: Li B et al., ASCO 2019: Abstract 6001.
REPORTING FROM ASCO 2019
Maintenance olaparib extends PFS in pancreatic cancer with BRCA mutation
CHICAGO – In patients with metastatic pancreatic cancer and a germline BRCA mutation, maintenance treatment with olaparib resulted in significant and clinically meaningful improvements in progression-free survival in a phase 3 trial, an investigator reported.
For patients who completed chemotherapy and went on to receive the PARP inhibitor, median progression-free survival was 7.4 months, versus just 3.8 months for placebo-treated patients in the phase 3 POLO study, said Hedy L. Kindler, MD, professor of medicine with University of Chicago Medicine.
“A strategic approach of first-line platinum based chemo followed by maintenance olaparib treatment should become a new standard of care for patients with metastatic pancreatic cancer who have a germ line BRCA mutation,” Dr. Kindler said here in a press conference at the annual meeting of the American Society of Clinical Oncology.
This phase 3 study is the first to show that treatment of metastatic pancreatic cancer can be tailored based on a biomarker, highlights the importance of germline BRCA mutation testing, according to ASCO expert Suzanne Cole, MD.
“I think this is practice-changing for people who have BRCA mutations,” Dr. Cole said in the press conference. “I can’t wait to go back to clinic on Tuesday and look for it in my own patients.”
Four to seven percent of patients with pancreatic cancer harbor a germline BRCA1 or BRCA2 mutation, according to Dr. Kindler, lead author of the POLO trial.
The phase 3 study by Dr. Kindler and colleagues included 247 patients with metastatic pancreatic cancer and germline BRCA1/BRCA2 mutations who received at least 16 weeks of platinum-based chemotherapy.
Thirty-eight percent of enrolled patients had disease progression, declined randomization, or were ineligible to continue, Dr. Kindler said. The remaining 154 patients were randomized 3:2 to receive olaparib 300 mg twice daily or placebo.
The primary end point of the study was progression-free survival measured from the time of randomization. The median progression-free survival of 7.4 versus 3.8 months for olaparib and placebo, respectively, represented a 47% decrease in risk of progression or death (HR, 0.53; P = .0038), Dr. Kindler reported at the meeting.
“What is truly remarkable is that the median duration of response to olaparib in these patients who had metastatic pancreatic cancer was more than 2 years,” she said in the press conference. Specifically, median duration of response was 24.9 months and 3.7 months for olaparib and placebo arms, respectively.
There was no difference in overall survival between olaparib and placebo arms in the interim analysis. Final overall survival results will be evaluated when the data are more mature, Dr. Kindler said.
Olaparib treatment was well tolerated and had an adverse event profile similar to what has been observed in other tumor types, according to Dr. Kindler, who added that health-related quality of life was not different between the PARP inhibitor and placebo arms of the trial.
While longer-term data are awaited to understand the full impact of the results, the POLO study already represents a “huge step forward” in the treatment of metastatic pancreatic cancer, said Dr. Cole, the ASCO expert.
“It is our duty to search for this genetic mutation in all patients with metastatic pancreatic cancer, so we can identify those people who have the BRCA mutation and can benefit from being treated with an oral agent that can extend their life,” she said at the press conference.
Funding for the study came from AstraZeneca and Merck Sharp & Dohme Corp. Dr. Kindler provided disclosures related to Aduro Biotech, Aldeyra Therapeutics, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Deciphera, ERYTECH Pharma, Five Prime Therapeutics, GlaxoSmithKline, Inhibrx, ipsen, Kyowa Hakko Kirin, Lilly, MedImmune, Merck, Paradox Therapeutics, Polaris, Roche/Genentech, and Verastem.
SOURCE: Kindler HL, et al. ASCO 2019. Abstract LBA4.
CHICAGO – In patients with metastatic pancreatic cancer and a germline BRCA mutation, maintenance treatment with olaparib resulted in significant and clinically meaningful improvements in progression-free survival in a phase 3 trial, an investigator reported.
For patients who completed chemotherapy and went on to receive the PARP inhibitor, median progression-free survival was 7.4 months, versus just 3.8 months for placebo-treated patients in the phase 3 POLO study, said Hedy L. Kindler, MD, professor of medicine with University of Chicago Medicine.
“A strategic approach of first-line platinum based chemo followed by maintenance olaparib treatment should become a new standard of care for patients with metastatic pancreatic cancer who have a germ line BRCA mutation,” Dr. Kindler said here in a press conference at the annual meeting of the American Society of Clinical Oncology.
This phase 3 study is the first to show that treatment of metastatic pancreatic cancer can be tailored based on a biomarker, highlights the importance of germline BRCA mutation testing, according to ASCO expert Suzanne Cole, MD.
“I think this is practice-changing for people who have BRCA mutations,” Dr. Cole said in the press conference. “I can’t wait to go back to clinic on Tuesday and look for it in my own patients.”
Four to seven percent of patients with pancreatic cancer harbor a germline BRCA1 or BRCA2 mutation, according to Dr. Kindler, lead author of the POLO trial.
The phase 3 study by Dr. Kindler and colleagues included 247 patients with metastatic pancreatic cancer and germline BRCA1/BRCA2 mutations who received at least 16 weeks of platinum-based chemotherapy.
Thirty-eight percent of enrolled patients had disease progression, declined randomization, or were ineligible to continue, Dr. Kindler said. The remaining 154 patients were randomized 3:2 to receive olaparib 300 mg twice daily or placebo.
The primary end point of the study was progression-free survival measured from the time of randomization. The median progression-free survival of 7.4 versus 3.8 months for olaparib and placebo, respectively, represented a 47% decrease in risk of progression or death (HR, 0.53; P = .0038), Dr. Kindler reported at the meeting.
“What is truly remarkable is that the median duration of response to olaparib in these patients who had metastatic pancreatic cancer was more than 2 years,” she said in the press conference. Specifically, median duration of response was 24.9 months and 3.7 months for olaparib and placebo arms, respectively.
There was no difference in overall survival between olaparib and placebo arms in the interim analysis. Final overall survival results will be evaluated when the data are more mature, Dr. Kindler said.
Olaparib treatment was well tolerated and had an adverse event profile similar to what has been observed in other tumor types, according to Dr. Kindler, who added that health-related quality of life was not different between the PARP inhibitor and placebo arms of the trial.
While longer-term data are awaited to understand the full impact of the results, the POLO study already represents a “huge step forward” in the treatment of metastatic pancreatic cancer, said Dr. Cole, the ASCO expert.
“It is our duty to search for this genetic mutation in all patients with metastatic pancreatic cancer, so we can identify those people who have the BRCA mutation and can benefit from being treated with an oral agent that can extend their life,” she said at the press conference.
Funding for the study came from AstraZeneca and Merck Sharp & Dohme Corp. Dr. Kindler provided disclosures related to Aduro Biotech, Aldeyra Therapeutics, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Deciphera, ERYTECH Pharma, Five Prime Therapeutics, GlaxoSmithKline, Inhibrx, ipsen, Kyowa Hakko Kirin, Lilly, MedImmune, Merck, Paradox Therapeutics, Polaris, Roche/Genentech, and Verastem.
SOURCE: Kindler HL, et al. ASCO 2019. Abstract LBA4.
CHICAGO – In patients with metastatic pancreatic cancer and a germline BRCA mutation, maintenance treatment with olaparib resulted in significant and clinically meaningful improvements in progression-free survival in a phase 3 trial, an investigator reported.
For patients who completed chemotherapy and went on to receive the PARP inhibitor, median progression-free survival was 7.4 months, versus just 3.8 months for placebo-treated patients in the phase 3 POLO study, said Hedy L. Kindler, MD, professor of medicine with University of Chicago Medicine.
“A strategic approach of first-line platinum based chemo followed by maintenance olaparib treatment should become a new standard of care for patients with metastatic pancreatic cancer who have a germ line BRCA mutation,” Dr. Kindler said here in a press conference at the annual meeting of the American Society of Clinical Oncology.
This phase 3 study is the first to show that treatment of metastatic pancreatic cancer can be tailored based on a biomarker, highlights the importance of germline BRCA mutation testing, according to ASCO expert Suzanne Cole, MD.
“I think this is practice-changing for people who have BRCA mutations,” Dr. Cole said in the press conference. “I can’t wait to go back to clinic on Tuesday and look for it in my own patients.”
Four to seven percent of patients with pancreatic cancer harbor a germline BRCA1 or BRCA2 mutation, according to Dr. Kindler, lead author of the POLO trial.
The phase 3 study by Dr. Kindler and colleagues included 247 patients with metastatic pancreatic cancer and germline BRCA1/BRCA2 mutations who received at least 16 weeks of platinum-based chemotherapy.
Thirty-eight percent of enrolled patients had disease progression, declined randomization, or were ineligible to continue, Dr. Kindler said. The remaining 154 patients were randomized 3:2 to receive olaparib 300 mg twice daily or placebo.
The primary end point of the study was progression-free survival measured from the time of randomization. The median progression-free survival of 7.4 versus 3.8 months for olaparib and placebo, respectively, represented a 47% decrease in risk of progression or death (HR, 0.53; P = .0038), Dr. Kindler reported at the meeting.
“What is truly remarkable is that the median duration of response to olaparib in these patients who had metastatic pancreatic cancer was more than 2 years,” she said in the press conference. Specifically, median duration of response was 24.9 months and 3.7 months for olaparib and placebo arms, respectively.
There was no difference in overall survival between olaparib and placebo arms in the interim analysis. Final overall survival results will be evaluated when the data are more mature, Dr. Kindler said.
Olaparib treatment was well tolerated and had an adverse event profile similar to what has been observed in other tumor types, according to Dr. Kindler, who added that health-related quality of life was not different between the PARP inhibitor and placebo arms of the trial.
While longer-term data are awaited to understand the full impact of the results, the POLO study already represents a “huge step forward” in the treatment of metastatic pancreatic cancer, said Dr. Cole, the ASCO expert.
“It is our duty to search for this genetic mutation in all patients with metastatic pancreatic cancer, so we can identify those people who have the BRCA mutation and can benefit from being treated with an oral agent that can extend their life,” she said at the press conference.
Funding for the study came from AstraZeneca and Merck Sharp & Dohme Corp. Dr. Kindler provided disclosures related to Aduro Biotech, Aldeyra Therapeutics, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Deciphera, ERYTECH Pharma, Five Prime Therapeutics, GlaxoSmithKline, Inhibrx, ipsen, Kyowa Hakko Kirin, Lilly, MedImmune, Merck, Paradox Therapeutics, Polaris, Roche/Genentech, and Verastem.
SOURCE: Kindler HL, et al. ASCO 2019. Abstract LBA4.
REPORTING FROM ASCO 2019