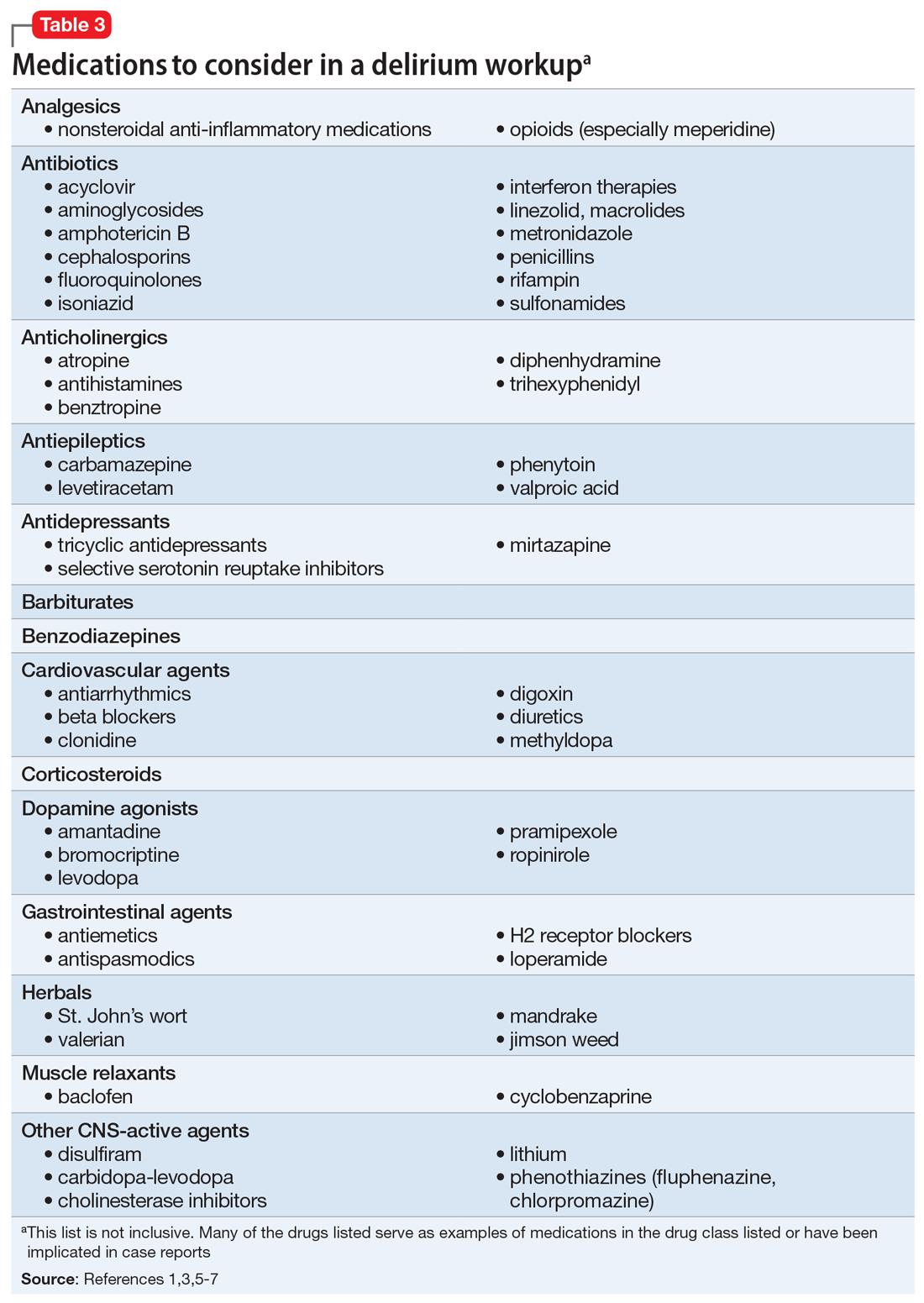
User login
In Reference to: “Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized Patients”
Boughton et al.1 reported a high incidence of hypoglycemia resulting from glucose-with-insulin (GwI) infusion used to treat acute hyperkalemia. This has been reported by other investigators—particularly in subjects without preexisting diabetes2 and resonates with the experiences of clinicians practicing in Internal Medicine or Diabetes.
The authors suggested that patients at risk of hypoglycemia be identified and offered a regimen containing less insulin. However, for subjects without preexisting diagnosis and not at high risk of diabetes, we question the physiological logic and the safety basis for administering insulin.
Infusion of glucose only (GO) to subjects with intact pancreatic function and insulin sensitivity stimulates endogenous insulin secretion in a dose-dependent manner, resulting in a reduction in extracellular fluid potassium with no risk of hypoglycemia.3,4
It is unclear why GwI historically entered mainstream practice rather than GO, but the rationale may have been based on the potential risks of paradoxical hyperglycemia-mediated hyperkalemia (HMK) being induced by GO. In practice, HMK was only observed in subjects with diabetes.5
As there is an ongoing need to reduce the impact of iatrogenic hypoglycemia, revisiting of the prematurely abandoned GO regimen in hyperkalemia management is warranted. Such approach may offer a safe and physiological alternative to GwI in nondiabetic patients with hyperkalemia.
We advocate that GO be prospectively evaluated against GwI for the treatment of hyperkalemia in subjects without diabetes, against the endpoints being noninferiority in respect of efficacy and maintenance of euglycemia in respect of safety.
Disclosures
Nothing to declare.
1. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. doi: 10.12788/jhm.3145. PubMed
2. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
3. Chothia MY, Halperin ML, Rensburg MA, Hassan MS, Davids MR. Bolus administration of intravenous glucose in the treatment of hyperkalemia: a randomized controlled trial. Nephron Physiol. 2014;126(1):1-8. doi: 10.1159/000358836. PubMed
4. Groen J, Willebrands AF, Kamminga CE, Van Schothorst HK, Godfried EG. Effects of glucose administration on the potassium and inorganic phosphate content of the blood serum and the electrocardiogram in normal individuals and in non-diabetic patients. Acta Med Scand. 1952;141(5):352-366. doi: 10.1111/j.0954-6820.1952.tb14227.x. PubMed
5. Nicolis GL, Kahn T, Sanchez A, Gabrilove JL. Glucose-induced hyperkalemia in diabetic subjects. Arch Intern Med. 1981;141(1):49-53. doi:10.1001/archinte.1981.00340010045012. PubMed
Boughton et al.1 reported a high incidence of hypoglycemia resulting from glucose-with-insulin (GwI) infusion used to treat acute hyperkalemia. This has been reported by other investigators—particularly in subjects without preexisting diabetes2 and resonates with the experiences of clinicians practicing in Internal Medicine or Diabetes.
The authors suggested that patients at risk of hypoglycemia be identified and offered a regimen containing less insulin. However, for subjects without preexisting diagnosis and not at high risk of diabetes, we question the physiological logic and the safety basis for administering insulin.
Infusion of glucose only (GO) to subjects with intact pancreatic function and insulin sensitivity stimulates endogenous insulin secretion in a dose-dependent manner, resulting in a reduction in extracellular fluid potassium with no risk of hypoglycemia.3,4
It is unclear why GwI historically entered mainstream practice rather than GO, but the rationale may have been based on the potential risks of paradoxical hyperglycemia-mediated hyperkalemia (HMK) being induced by GO. In practice, HMK was only observed in subjects with diabetes.5
As there is an ongoing need to reduce the impact of iatrogenic hypoglycemia, revisiting of the prematurely abandoned GO regimen in hyperkalemia management is warranted. Such approach may offer a safe and physiological alternative to GwI in nondiabetic patients with hyperkalemia.
We advocate that GO be prospectively evaluated against GwI for the treatment of hyperkalemia in subjects without diabetes, against the endpoints being noninferiority in respect of efficacy and maintenance of euglycemia in respect of safety.
Disclosures
Nothing to declare.
Boughton et al.1 reported a high incidence of hypoglycemia resulting from glucose-with-insulin (GwI) infusion used to treat acute hyperkalemia. This has been reported by other investigators—particularly in subjects without preexisting diabetes2 and resonates with the experiences of clinicians practicing in Internal Medicine or Diabetes.
The authors suggested that patients at risk of hypoglycemia be identified and offered a regimen containing less insulin. However, for subjects without preexisting diagnosis and not at high risk of diabetes, we question the physiological logic and the safety basis for administering insulin.
Infusion of glucose only (GO) to subjects with intact pancreatic function and insulin sensitivity stimulates endogenous insulin secretion in a dose-dependent manner, resulting in a reduction in extracellular fluid potassium with no risk of hypoglycemia.3,4
It is unclear why GwI historically entered mainstream practice rather than GO, but the rationale may have been based on the potential risks of paradoxical hyperglycemia-mediated hyperkalemia (HMK) being induced by GO. In practice, HMK was only observed in subjects with diabetes.5
As there is an ongoing need to reduce the impact of iatrogenic hypoglycemia, revisiting of the prematurely abandoned GO regimen in hyperkalemia management is warranted. Such approach may offer a safe and physiological alternative to GwI in nondiabetic patients with hyperkalemia.
We advocate that GO be prospectively evaluated against GwI for the treatment of hyperkalemia in subjects without diabetes, against the endpoints being noninferiority in respect of efficacy and maintenance of euglycemia in respect of safety.
Disclosures
Nothing to declare.
1. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. doi: 10.12788/jhm.3145. PubMed
2. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
3. Chothia MY, Halperin ML, Rensburg MA, Hassan MS, Davids MR. Bolus administration of intravenous glucose in the treatment of hyperkalemia: a randomized controlled trial. Nephron Physiol. 2014;126(1):1-8. doi: 10.1159/000358836. PubMed
4. Groen J, Willebrands AF, Kamminga CE, Van Schothorst HK, Godfried EG. Effects of glucose administration on the potassium and inorganic phosphate content of the blood serum and the electrocardiogram in normal individuals and in non-diabetic patients. Acta Med Scand. 1952;141(5):352-366. doi: 10.1111/j.0954-6820.1952.tb14227.x. PubMed
5. Nicolis GL, Kahn T, Sanchez A, Gabrilove JL. Glucose-induced hyperkalemia in diabetic subjects. Arch Intern Med. 1981;141(1):49-53. doi:10.1001/archinte.1981.00340010045012. PubMed
1. Boughton CK, Dixon D, Goble E, et al. Preventing hypoglycemia following treatment of hyperkalemia in hospitalized patients. J Hosp Med. 2019;14:E1-E4. doi: 10.12788/jhm.3145. PubMed
2. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
3. Chothia MY, Halperin ML, Rensburg MA, Hassan MS, Davids MR. Bolus administration of intravenous glucose in the treatment of hyperkalemia: a randomized controlled trial. Nephron Physiol. 2014;126(1):1-8. doi: 10.1159/000358836. PubMed
4. Groen J, Willebrands AF, Kamminga CE, Van Schothorst HK, Godfried EG. Effects of glucose administration on the potassium and inorganic phosphate content of the blood serum and the electrocardiogram in normal individuals and in non-diabetic patients. Acta Med Scand. 1952;141(5):352-366. doi: 10.1111/j.0954-6820.1952.tb14227.x. PubMed
5. Nicolis GL, Kahn T, Sanchez A, Gabrilove JL. Glucose-induced hyperkalemia in diabetic subjects. Arch Intern Med. 1981;141(1):49-53. doi:10.1001/archinte.1981.00340010045012. PubMed
© 2019 Society of Hospital Medicine
Transitions of Care with Incidental Pulmonary Nodules
With advancement in imaging techniques, incidental pulmonary nodules (IPNs) are routinely found on imaging studies. Depending on the size, an IPN has diagnostic uncertainty. Is it a benign finding? Will it progress to cancer? These questions have the potential to create anxiety for our patients. Between 2012 and 2014, 19,739 patients were discharged from hospitals in the United States with a diagnosis of a solitary pulmonary nodule.1 Roughly 7,500 were discharged after an inpatient stay; the remainder from the emergency room. Aggregate costs for these visits totaled $49 million. The exact number of nodules receiving follow-up is unknown.
The Fleischner guidelines, updated in 2017, outline management for IPNs.2 Depending on nodule size and patient risk factors, repeat imaging is either not indicated or one to two follow-up scans could be recommended. In this issue of the Journal of Hospital Medicine®, two reports assess provider awareness of the Fleischner guidelines and examine the proportion of patients receiving follow-up.
Umscheid et al. surveyed hospitalists to understand their approach IPN management. Of 174 respondents, 42% were unfamiliar with the Fleischner guidelines.3 The authors proposed methods for improving provider awareness, including better communication between hospitalists and primary care providers, better documentation, and in the case of their institution, the development of an IPN consult team. The IPN consult team is composed of a nurse practitioner and pulmonologist. They inform primary care providers of patient findings and need for follow-up. If no follow-up is made, the team will see the patients in an IPN ambulatory clinic to ensure follow-up imaging is obtained.
Kwan et al. found that fewer than 50% of patients with high-risk new pulmonary nodules received follow-up.4 Although a single-site study, the study is consistent with prior work on tests pending at discharge, which essentially show that there are poor follow-up rates.5,6 Follow-up was more likely when the IPN was mentioned in the discharge summary. This conclusion builds on previous work showing that IPNs are more likely to be included in a discharge summary if the nodule is noted in the report heading, the radiologist recommends further imaging, and the patient is discharged from a medicine service as opposed to a surgical service.7 IPN follow-up is less likely if results are mentioned in the findings section alone.5
IPN follow-up is a piece of a larger issue of how best to ensure appropriate follow-up of any tests pending after discharge. A systematic review of discharge interventions found improvement in follow-up when discharge summaries are combined with e-mail alerts.6 A study of the effects of integrated electronic health records (EHR) web modules with discharge specific instructions showed an increase in follow-up from 18% to 27%.8 Studies also consider provider-to-patient communication. One intervention uses the patient portal to remind patients to pick up their medications,9 finding a decrease in nonadherence from 65.5% to 22.2%. Engaging patients by way of patient portals and reminders are an effective way to hold both the physician and the patient accountable for follow-up. Mobile technologies studied in the emergency department show patient preferences toward texting to receive medication and appointment reminders.10 Given wide-spread adoption of mobile technologies,11 notification systems could leverage applications or texting modalities to keep patients informed of discharge appointments and follow-up imaging studies. Similar interventions could be designed for IPNs using the Fleischner guidelines, generating alerts when patients have not received follow-up imaging.
The number of IPNs identified in the hospital will likely remain in the tens of thousands. From the hospitalist perspective, the findings presented in this month’s Journal of Hospital Medicine suggest that patients be educated about their findings and recommended follow-up, that follow-up be arranged before discharge, and that findings are clearly documented for patients and primary care providers to review. More study into how to implement these enhancements is needed to guide how we focus educational, systems, and technological interventions. Further study is also needed to help understand the complexities of communication channels between hospitalists and primary care physicians. As hospitalist workflow is more integrated with the EHR and mobile technology, future interventions can facilitate follow-up, keeping all providers and, most importantly, the patient aware of the next steps in care.
Acknowledgments
Author support is provided by the South Texas Veterans Health Care System. The views expressed are those of the authors and do not reflect the position or policy of the Department of Veterans Affairs.
Disclosures
The authors report no financial conflicts of interest.
1. HCUPNet: A tool for identifying, tracking and analyzing national hospital statistics (2018). Retrieved from https://hcupnet.ahrq.gov/#setup on 10/25/2019
2. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT Images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
3. Umscheid CA, Wilen J, Garin M, et al. National Survey of Hospitalists’ experiences with incidental pulmonary nodules. J Hosp Med. 2019;14(6):353-356. doi: 10.12788/jhm.3115. PubMed
4. Kwan JL, Yermak D, Markell L, Paul NS, Shojania KG, Cram P. Follow-up of incidental high-risk pulmonary nodules on computed tomography pulmonary angiography at care transitions. J Hosp Med. 2019;14(6):349-352. doi: 10.12788/jhm.3128. PubMed
5. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
7. Darragh PJ, Bodley T, Orchanian-cheff A, Shojania KG, Kwan JL, Cram P. A systematic review of interventions to follow-up test results pending at discharge. J Gen Intern Med. 2018;33(5):750-758. doi: 10.1007/s11606-017-4290-9. PubMed
8. Bates R, Plooster C, Croghan I, Schroeder D, Mccoy C. Incidental pulmonary nodules reported on CT abdominal imaging: frequency and factors affecting inclusion in the hospital discharge summary. J Hosp Med. 2017;12(6):454-457. doi: 10.12788/jhm.2757. PubMed
9. Lacson R, Desai S, Landman A, Proctor R, Sumption S, Khorasani R. Impact of a health information technology intervention on the follow-up management of pulmonary nodules. J Digit Imaging. 2018;31(1):19-25. doi: 10.1007/s10278-017-9989-y. PubMed
10. Kerner DE, Knezevich EL. Use of communication tool within electronic medical record to improve primary nonadherence. J Am Pharm Assoc (2003). 2017;57(3S):S270-S273.e2. doi: 10.1016/j.japh.2017.03.009. PubMed
11. Ray M, Dayan PS, Pahalyants V, Chernick LS. Mobile health technology to communicate discharge and follow-up information to adolescents from the emergency department. Pediatr Emerg Care. 2016;32(12):900-905. doi: 10.1097/PEC.0000000000000970. PubMed
12. Gallagher R, Roach K, Sadler L, et al. Mobile technology use across age groups in patients eligible for cardiac rehabilitation: survey study. JMIR mHealth uhealth. 2017;5(10):e161. doi: 10.2196/mhealth.8352. PubMed
With advancement in imaging techniques, incidental pulmonary nodules (IPNs) are routinely found on imaging studies. Depending on the size, an IPN has diagnostic uncertainty. Is it a benign finding? Will it progress to cancer? These questions have the potential to create anxiety for our patients. Between 2012 and 2014, 19,739 patients were discharged from hospitals in the United States with a diagnosis of a solitary pulmonary nodule.1 Roughly 7,500 were discharged after an inpatient stay; the remainder from the emergency room. Aggregate costs for these visits totaled $49 million. The exact number of nodules receiving follow-up is unknown.
The Fleischner guidelines, updated in 2017, outline management for IPNs.2 Depending on nodule size and patient risk factors, repeat imaging is either not indicated or one to two follow-up scans could be recommended. In this issue of the Journal of Hospital Medicine®, two reports assess provider awareness of the Fleischner guidelines and examine the proportion of patients receiving follow-up.
Umscheid et al. surveyed hospitalists to understand their approach IPN management. Of 174 respondents, 42% were unfamiliar with the Fleischner guidelines.3 The authors proposed methods for improving provider awareness, including better communication between hospitalists and primary care providers, better documentation, and in the case of their institution, the development of an IPN consult team. The IPN consult team is composed of a nurse practitioner and pulmonologist. They inform primary care providers of patient findings and need for follow-up. If no follow-up is made, the team will see the patients in an IPN ambulatory clinic to ensure follow-up imaging is obtained.
Kwan et al. found that fewer than 50% of patients with high-risk new pulmonary nodules received follow-up.4 Although a single-site study, the study is consistent with prior work on tests pending at discharge, which essentially show that there are poor follow-up rates.5,6 Follow-up was more likely when the IPN was mentioned in the discharge summary. This conclusion builds on previous work showing that IPNs are more likely to be included in a discharge summary if the nodule is noted in the report heading, the radiologist recommends further imaging, and the patient is discharged from a medicine service as opposed to a surgical service.7 IPN follow-up is less likely if results are mentioned in the findings section alone.5
IPN follow-up is a piece of a larger issue of how best to ensure appropriate follow-up of any tests pending after discharge. A systematic review of discharge interventions found improvement in follow-up when discharge summaries are combined with e-mail alerts.6 A study of the effects of integrated electronic health records (EHR) web modules with discharge specific instructions showed an increase in follow-up from 18% to 27%.8 Studies also consider provider-to-patient communication. One intervention uses the patient portal to remind patients to pick up their medications,9 finding a decrease in nonadherence from 65.5% to 22.2%. Engaging patients by way of patient portals and reminders are an effective way to hold both the physician and the patient accountable for follow-up. Mobile technologies studied in the emergency department show patient preferences toward texting to receive medication and appointment reminders.10 Given wide-spread adoption of mobile technologies,11 notification systems could leverage applications or texting modalities to keep patients informed of discharge appointments and follow-up imaging studies. Similar interventions could be designed for IPNs using the Fleischner guidelines, generating alerts when patients have not received follow-up imaging.
The number of IPNs identified in the hospital will likely remain in the tens of thousands. From the hospitalist perspective, the findings presented in this month’s Journal of Hospital Medicine suggest that patients be educated about their findings and recommended follow-up, that follow-up be arranged before discharge, and that findings are clearly documented for patients and primary care providers to review. More study into how to implement these enhancements is needed to guide how we focus educational, systems, and technological interventions. Further study is also needed to help understand the complexities of communication channels between hospitalists and primary care physicians. As hospitalist workflow is more integrated with the EHR and mobile technology, future interventions can facilitate follow-up, keeping all providers and, most importantly, the patient aware of the next steps in care.
Acknowledgments
Author support is provided by the South Texas Veterans Health Care System. The views expressed are those of the authors and do not reflect the position or policy of the Department of Veterans Affairs.
Disclosures
The authors report no financial conflicts of interest.
With advancement in imaging techniques, incidental pulmonary nodules (IPNs) are routinely found on imaging studies. Depending on the size, an IPN has diagnostic uncertainty. Is it a benign finding? Will it progress to cancer? These questions have the potential to create anxiety for our patients. Between 2012 and 2014, 19,739 patients were discharged from hospitals in the United States with a diagnosis of a solitary pulmonary nodule.1 Roughly 7,500 were discharged after an inpatient stay; the remainder from the emergency room. Aggregate costs for these visits totaled $49 million. The exact number of nodules receiving follow-up is unknown.
The Fleischner guidelines, updated in 2017, outline management for IPNs.2 Depending on nodule size and patient risk factors, repeat imaging is either not indicated or one to two follow-up scans could be recommended. In this issue of the Journal of Hospital Medicine®, two reports assess provider awareness of the Fleischner guidelines and examine the proportion of patients receiving follow-up.
Umscheid et al. surveyed hospitalists to understand their approach IPN management. Of 174 respondents, 42% were unfamiliar with the Fleischner guidelines.3 The authors proposed methods for improving provider awareness, including better communication between hospitalists and primary care providers, better documentation, and in the case of their institution, the development of an IPN consult team. The IPN consult team is composed of a nurse practitioner and pulmonologist. They inform primary care providers of patient findings and need for follow-up. If no follow-up is made, the team will see the patients in an IPN ambulatory clinic to ensure follow-up imaging is obtained.
Kwan et al. found that fewer than 50% of patients with high-risk new pulmonary nodules received follow-up.4 Although a single-site study, the study is consistent with prior work on tests pending at discharge, which essentially show that there are poor follow-up rates.5,6 Follow-up was more likely when the IPN was mentioned in the discharge summary. This conclusion builds on previous work showing that IPNs are more likely to be included in a discharge summary if the nodule is noted in the report heading, the radiologist recommends further imaging, and the patient is discharged from a medicine service as opposed to a surgical service.7 IPN follow-up is less likely if results are mentioned in the findings section alone.5
IPN follow-up is a piece of a larger issue of how best to ensure appropriate follow-up of any tests pending after discharge. A systematic review of discharge interventions found improvement in follow-up when discharge summaries are combined with e-mail alerts.6 A study of the effects of integrated electronic health records (EHR) web modules with discharge specific instructions showed an increase in follow-up from 18% to 27%.8 Studies also consider provider-to-patient communication. One intervention uses the patient portal to remind patients to pick up their medications,9 finding a decrease in nonadherence from 65.5% to 22.2%. Engaging patients by way of patient portals and reminders are an effective way to hold both the physician and the patient accountable for follow-up. Mobile technologies studied in the emergency department show patient preferences toward texting to receive medication and appointment reminders.10 Given wide-spread adoption of mobile technologies,11 notification systems could leverage applications or texting modalities to keep patients informed of discharge appointments and follow-up imaging studies. Similar interventions could be designed for IPNs using the Fleischner guidelines, generating alerts when patients have not received follow-up imaging.
The number of IPNs identified in the hospital will likely remain in the tens of thousands. From the hospitalist perspective, the findings presented in this month’s Journal of Hospital Medicine suggest that patients be educated about their findings and recommended follow-up, that follow-up be arranged before discharge, and that findings are clearly documented for patients and primary care providers to review. More study into how to implement these enhancements is needed to guide how we focus educational, systems, and technological interventions. Further study is also needed to help understand the complexities of communication channels between hospitalists and primary care physicians. As hospitalist workflow is more integrated with the EHR and mobile technology, future interventions can facilitate follow-up, keeping all providers and, most importantly, the patient aware of the next steps in care.
Acknowledgments
Author support is provided by the South Texas Veterans Health Care System. The views expressed are those of the authors and do not reflect the position or policy of the Department of Veterans Affairs.
Disclosures
The authors report no financial conflicts of interest.
1. HCUPNet: A tool for identifying, tracking and analyzing national hospital statistics (2018). Retrieved from https://hcupnet.ahrq.gov/#setup on 10/25/2019
2. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT Images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
3. Umscheid CA, Wilen J, Garin M, et al. National Survey of Hospitalists’ experiences with incidental pulmonary nodules. J Hosp Med. 2019;14(6):353-356. doi: 10.12788/jhm.3115. PubMed
4. Kwan JL, Yermak D, Markell L, Paul NS, Shojania KG, Cram P. Follow-up of incidental high-risk pulmonary nodules on computed tomography pulmonary angiography at care transitions. J Hosp Med. 2019;14(6):349-352. doi: 10.12788/jhm.3128. PubMed
5. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
7. Darragh PJ, Bodley T, Orchanian-cheff A, Shojania KG, Kwan JL, Cram P. A systematic review of interventions to follow-up test results pending at discharge. J Gen Intern Med. 2018;33(5):750-758. doi: 10.1007/s11606-017-4290-9. PubMed
8. Bates R, Plooster C, Croghan I, Schroeder D, Mccoy C. Incidental pulmonary nodules reported on CT abdominal imaging: frequency and factors affecting inclusion in the hospital discharge summary. J Hosp Med. 2017;12(6):454-457. doi: 10.12788/jhm.2757. PubMed
9. Lacson R, Desai S, Landman A, Proctor R, Sumption S, Khorasani R. Impact of a health information technology intervention on the follow-up management of pulmonary nodules. J Digit Imaging. 2018;31(1):19-25. doi: 10.1007/s10278-017-9989-y. PubMed
10. Kerner DE, Knezevich EL. Use of communication tool within electronic medical record to improve primary nonadherence. J Am Pharm Assoc (2003). 2017;57(3S):S270-S273.e2. doi: 10.1016/j.japh.2017.03.009. PubMed
11. Ray M, Dayan PS, Pahalyants V, Chernick LS. Mobile health technology to communicate discharge and follow-up information to adolescents from the emergency department. Pediatr Emerg Care. 2016;32(12):900-905. doi: 10.1097/PEC.0000000000000970. PubMed
12. Gallagher R, Roach K, Sadler L, et al. Mobile technology use across age groups in patients eligible for cardiac rehabilitation: survey study. JMIR mHealth uhealth. 2017;5(10):e161. doi: 10.2196/mhealth.8352. PubMed
1. HCUPNet: A tool for identifying, tracking and analyzing national hospital statistics (2018). Retrieved from https://hcupnet.ahrq.gov/#setup on 10/25/2019
2. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT Images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
3. Umscheid CA, Wilen J, Garin M, et al. National Survey of Hospitalists’ experiences with incidental pulmonary nodules. J Hosp Med. 2019;14(6):353-356. doi: 10.12788/jhm.3115. PubMed
4. Kwan JL, Yermak D, Markell L, Paul NS, Shojania KG, Cram P. Follow-up of incidental high-risk pulmonary nodules on computed tomography pulmonary angiography at care transitions. J Hosp Med. 2019;14(6):349-352. doi: 10.12788/jhm.3128. PubMed
5. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
7. Darragh PJ, Bodley T, Orchanian-cheff A, Shojania KG, Kwan JL, Cram P. A systematic review of interventions to follow-up test results pending at discharge. J Gen Intern Med. 2018;33(5):750-758. doi: 10.1007/s11606-017-4290-9. PubMed
8. Bates R, Plooster C, Croghan I, Schroeder D, Mccoy C. Incidental pulmonary nodules reported on CT abdominal imaging: frequency and factors affecting inclusion in the hospital discharge summary. J Hosp Med. 2017;12(6):454-457. doi: 10.12788/jhm.2757. PubMed
9. Lacson R, Desai S, Landman A, Proctor R, Sumption S, Khorasani R. Impact of a health information technology intervention on the follow-up management of pulmonary nodules. J Digit Imaging. 2018;31(1):19-25. doi: 10.1007/s10278-017-9989-y. PubMed
10. Kerner DE, Knezevich EL. Use of communication tool within electronic medical record to improve primary nonadherence. J Am Pharm Assoc (2003). 2017;57(3S):S270-S273.e2. doi: 10.1016/j.japh.2017.03.009. PubMed
11. Ray M, Dayan PS, Pahalyants V, Chernick LS. Mobile health technology to communicate discharge and follow-up information to adolescents from the emergency department. Pediatr Emerg Care. 2016;32(12):900-905. doi: 10.1097/PEC.0000000000000970. PubMed
12. Gallagher R, Roach K, Sadler L, et al. Mobile technology use across age groups in patients eligible for cardiac rehabilitation: survey study. JMIR mHealth uhealth. 2017;5(10):e161. doi: 10.2196/mhealth.8352. PubMed
© 2019 Society of Hospital Medicine
June 2019 Management of Neurologic Disorders in Federal Health Care
Click here to access June 2019 Management of Neurologic Disorders in Federal Health Care
Table of Contents
- Early and Accurate Identification of Parkinson Disease Among US Veterans
- Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population
- Understanding Psychosis in a Veteran With a History of Combat and Multiple Sclerosis
- Hemolytic Uremic Syndrome With Severe Neurologic Complications in an Adult
- Research News: Neurologic Disorders
Click here to access June 2019 Management of Neurologic Disorders in Federal Health Care
Table of Contents
- Early and Accurate Identification of Parkinson Disease Among US Veterans
- Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population
- Understanding Psychosis in a Veteran With a History of Combat and Multiple Sclerosis
- Hemolytic Uremic Syndrome With Severe Neurologic Complications in an Adult
- Research News: Neurologic Disorders

Click here to access June 2019 Management of Neurologic Disorders in Federal Health Care
Table of Contents
- Early and Accurate Identification of Parkinson Disease Among US Veterans
- Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population
- Understanding Psychosis in a Veteran With a History of Combat and Multiple Sclerosis
- Hemolytic Uremic Syndrome With Severe Neurologic Complications in an Adult
- Research News: Neurologic Disorders

Between a rock and a hard place
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
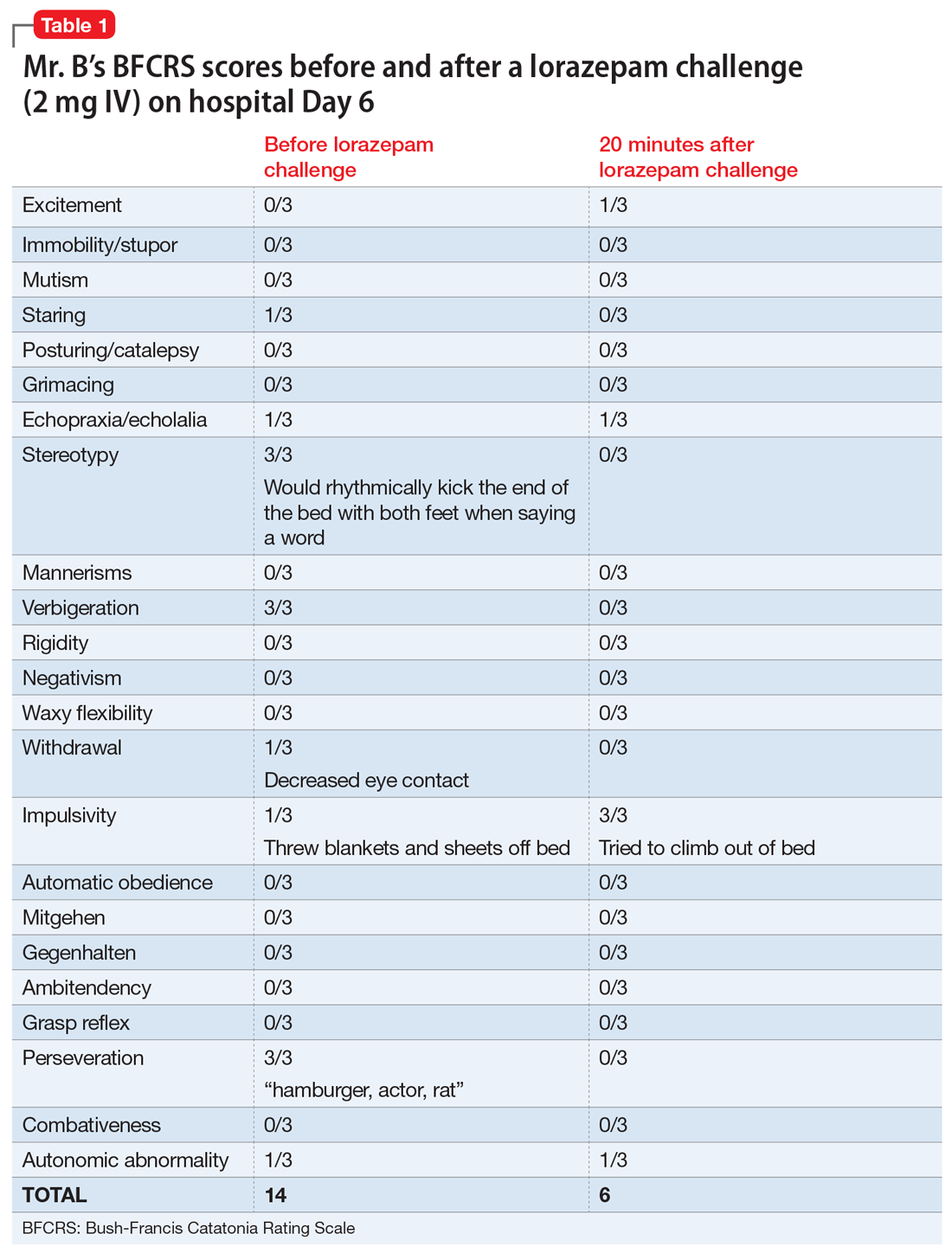
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
Psychiatry and neurology, more
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
SPIRITT: What does ‘spirituality’ mean?
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
When your patient is a physician: Overcoming the challenges
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Mobile apps and mental health: Using technology to quantify real-time clinical risk
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
It’s time to implement measurement-based care in psychiatric practice
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
From sweet to belligerent in the blink of an eye
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.